Abstract
A bioluminescent assay which employs the luciferin-luciferase ATP-dependent reaction was used to evaluate the viability of populations of Pneumocystis carinii derived from infected rat lungs. Contamination with host cells was reduced by a purification method which involved a combination of low- and high-speed centrifugations resulting in a 1,000-fold reduction of the rat cells while enriching for the trophic form of P. carinii. A linear correlation for the number of P. carinii nuclei versus the amount of ATP was observed. The ATP content of the organism populations could be maintained at inoculum levels for one week, although the number of organisms did not increase. Addition of respiratory chain inhibitors dramatically decreased the ATP content of the P. carinii after 24 h of incubation, with the exception of the antibiotic oligomycin B. Low concentrations of trimethoprim-sulfamethoxazole and pentamidine isethionate reduced the organism ATP content by over 50% after 24 h of exposure, while no effect was observed with 100-fold greater concentrations of ampicillin. The bioluminescent assay was found to be a more sensitive indicator of viability than a dual fluorescent staining technique. This assay does not require replication of P. carinii and should be a useful method for in vitro drug screening and viability assessment of P. carinii populations.
Full text
PDF

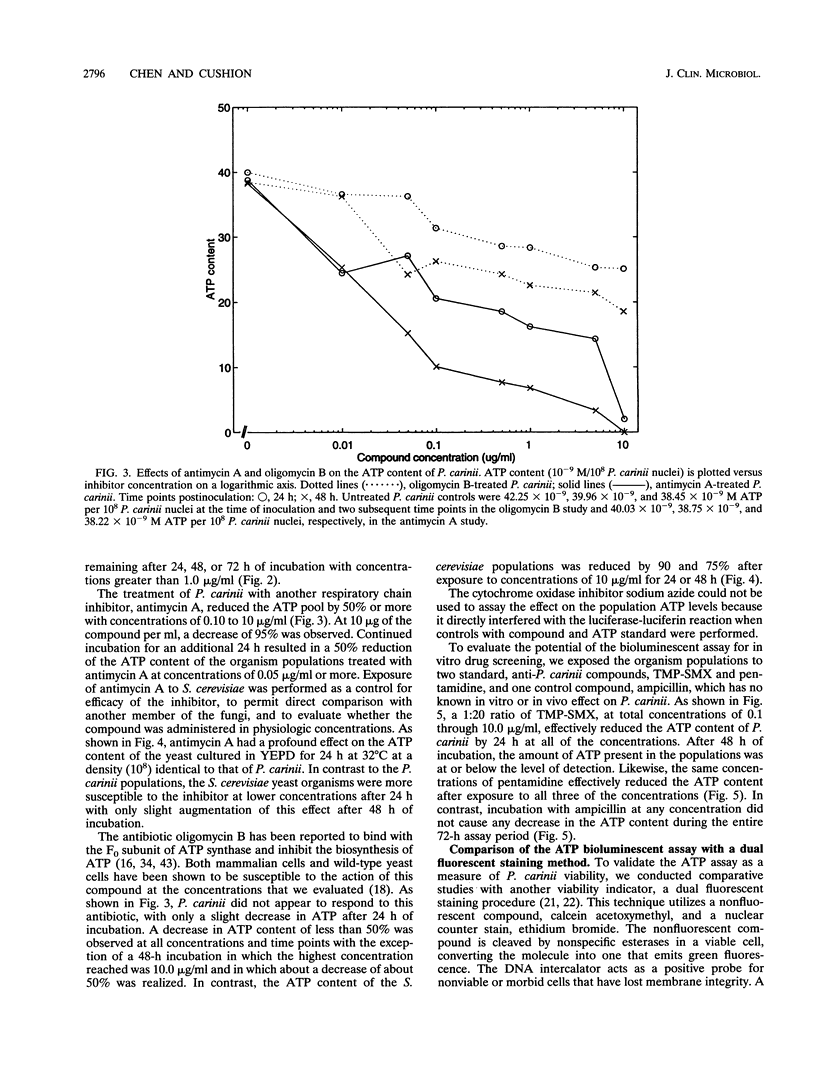
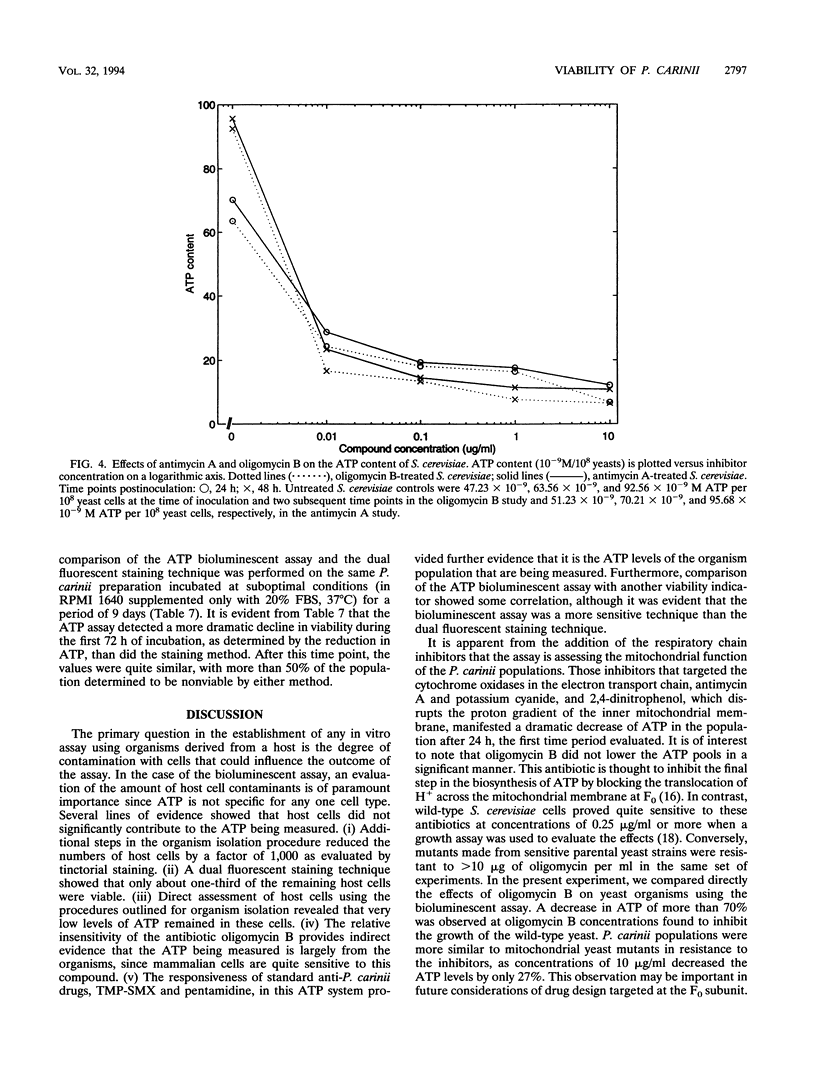
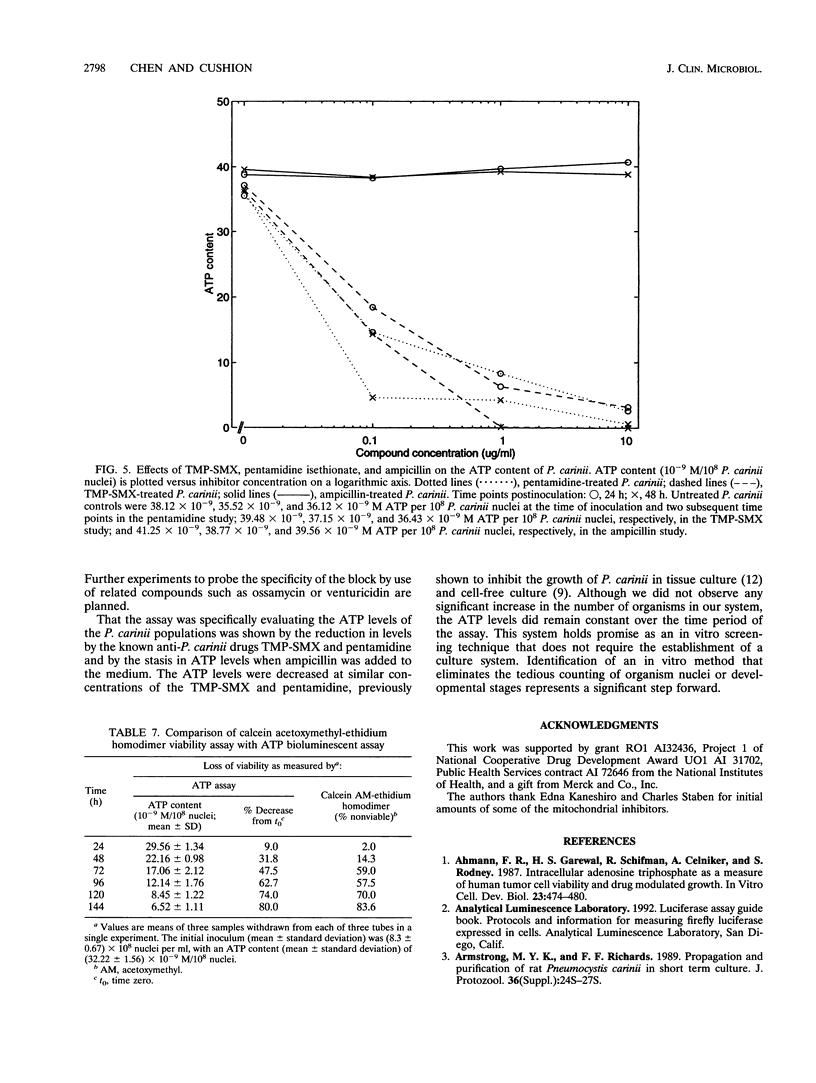


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmann F. R., Garewal H. S., Schifman R., Celniker A., Rodney S. Intracellular adenosine triphosphate as a measure of human tumor cell viability and drug modulated growth. In Vitro Cell Dev Biol. 1987 Jul;23(7):474–480. doi: 10.1007/BF02628417. [DOI] [PubMed] [Google Scholar]
- Armstrong M. Y., Richards F. F. Propagation and purification of rat Pneumocystis carinii in short-term cell culture. J Protozool. 1989 Jan-Feb;36(1):24S–27S. doi: 10.1111/j.1550-7408.1989.tb02677.x. [DOI] [PubMed] [Google Scholar]
- Bartlett M. S., Verbanac P. A., Smith J. W. Cultivation of Pneumocystis carinii with WI-38 cells. J Clin Microbiol. 1979 Dec;10(6):796–799. doi: 10.1128/jcm.10.6.796-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comley J. C., Mullin R. J., Wolfe L. A., Hanlon M. H., Ferone R. Microculture screening assay for primary in vitro evaluation of drugs against Pneumocystis carinii. Antimicrob Agents Chemother. 1991 Oct;35(10):1965–1974. doi: 10.1128/aac.35.10.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T., Ebbets D. Growth and metabolism of Pneumocystis carinii in axenic culture. J Clin Microbiol. 1990 Jun;28(6):1385–1394. doi: 10.1128/jcm.28.6.1385-1394.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T. In vitro studies of Pneumocystis carinii. J Protozool. 1989 Jan-Feb;36(1):45–52. doi: 10.1111/j.1550-7408.1989.tb02691.x. [DOI] [PubMed] [Google Scholar]
- Cushion M. T., Kaselis M., Stringer S. L., Stringer J. R. Genetic stability and diversity of Pneumocystis carinii infecting rat colonies. Infect Immun. 1993 Nov;61(11):4801–4813. doi: 10.1128/iai.61.11.4801-4813.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T., Ruffolo J. J., Linke M. J., Walzer P. D. Pneumocystis carinii: growth variables and estimates in the A549 and WI-38 VA13 human cell lines. Exp Parasitol. 1985 Aug;60(1):43–54. doi: 10.1016/s0014-4894(85)80021-7. [DOI] [PubMed] [Google Scholar]
- Cushion M. T., Stanforth D., Linke M. J., Walzer P. D. Method of testing the susceptibility of Pneumocystis carinii to antimicrobial agents in vitro. Antimicrob Agents Chemother. 1985 Dec;28(6):796–801. doi: 10.1128/aac.28.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T., Walzer P. D. Growth and serial passage of Pneumocystis carinii in the A549 cell line. Infect Immun. 1984 May;44(2):245–251. doi: 10.1128/iai.44.2.245-251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushion M. T., Zhang J., Kaselis M., Giuntoli D., Stringer S. L., Stringer J. R. Evidence for two genetic variants of Pneumocystis carinii coinfecting laboratory rats. J Clin Microbiol. 1993 May;31(5):1217–1223. doi: 10.1128/jcm.31.5.1217-1223.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Regulation of cellular energy metabolism. J Membr Biol. 1982;70(1):1–14. doi: 10.1007/BF01871584. [DOI] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN A., MCELROY W. D. Function of adenosine triphosphate in the activation of luciferin. Arch Biochem Biophys. 1956 Oct;64(2):257–271. doi: 10.1016/0003-9861(56)90268-5. [DOI] [PubMed] [Google Scholar]
- Garewal H. S., Ahmann F. R., Schifman R. B., Celniker A. ATP assay: ability to distinguish cytostatic from cytocidal anticancer drug effects. J Natl Cancer Inst. 1986 Nov;77(5):1039–1045. [PubMed] [Google Scholar]
- Griffiths D. E., Houghton R. L. Studies on energy-linked reactions: modified mitochondrial ATPase of oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1974 Jul 1;46(1):157–167. doi: 10.1111/j.1432-1033.1974.tb03608.x. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., Gray V. L., Gutteridge W. E., Latter V. S., Pudney M. Efficacy of a hydroxynaphthoquinone, 566C80, in experimental Pneumocystis carinii pneumonitis. Antimicrob Agents Chemother. 1990 Feb;34(2):225–228. doi: 10.1128/aac.34.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshiro E. S., Wu Y. P., Cushion M. T. Assays for testing Pneumocystis carinii viability. J Protozool. 1991 Nov-Dec;38(6):85S–87S. [PubMed] [Google Scholar]
- Kaneshiro E. S., Wyder M. A., Zhou L. H., Ellis J. E., Voelker D. R., Langreth S. G. Characterization of Pneumocystis carinii preparations developed for lipid analysis. J Eukaryot Microbiol. 1993 Nov-Dec;40(6):805–815. doi: 10.1111/j.1550-7408.1993.tb04479.x. [DOI] [PubMed] [Google Scholar]
- Kangas L., Grönroos M., Nieminen A. L. Bioluminescence of cellular ATP: a new method for evaluating cytotoxic agents in vitro. Med Biol. 1984;62(6):338–343. [PubMed] [Google Scholar]
- Kuzmits R., Rumpold H., Müller M. M., Schopf G. The use of bioluminescence to evaluate the influence of chemotherapeutic drugs on ATP-levels of malignant cell lines. J Clin Chem Clin Biochem. 1986 May;24(5):293–298. doi: 10.1515/cclm.1986.24.5.293. [DOI] [PubMed] [Google Scholar]
- Lapinsky S. E., Glencross D., Car N. G., Kallenbach J. M., Zwi S. Quantification and assessment of viability of Pneumocystis carinii organisms by flow cytometry. J Clin Microbiol. 1991 May;29(5):911–915. doi: 10.1128/jcm.29.5.911-915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre C. R., Sulzer A. J., Norman L. G. Serial propagation of Pneumocystis carinii in cell line cultures. Appl Environ Microbiol. 1977 May;33(5):1204–1206. doi: 10.1128/aem.33.5.1204-1206.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett D., Kessock-Philip S., Bascomb S., Easmon C. S. Evaluation of the Lumac kit for the detection of bacteriuria by bioluminescence. J Clin Pathol. 1982 Jan;35(1):107–110. doi: 10.1136/jcp.35.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazer M. A., Kovacs J. A., Swan J. C., Parrillo J. E., Masur H. Histoenzymological study of selected dehydrogenase enzymes in Pneumocystis carinii. Infect Immun. 1987 Mar;55(3):727–730. doi: 10.1128/iai.55.3.727-730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWalter P. W. Determination of susceptibility of Staphylococcus aureus to methicillin by luciferin-luciferase assay of bacterial adenosine triphosphate. J Appl Bacteriol. 1984 Feb;56(1):145–150. doi: 10.1111/j.1365-2672.1984.tb04706.x. [DOI] [PubMed] [Google Scholar]
- Mirovsky P., Fishman J. A. An improved method for the prolonged maintenance of Pneumocystis carinii in vitro. J Infect Dis. 1993 Jun;167(6):1470–1473. doi: 10.1093/infdis/167.6.1470. [DOI] [PubMed] [Google Scholar]
- Miyahira Y., Takeuchi T. Application of ATP measurement to evaluation of the growth of parasitic protozoa in vitro with a special reference to Pneumocystis carinii. Comp Biochem Physiol A Comp Physiol. 1991;100(4):1031–1034. doi: 10.1016/0300-9629(91)90332-7. [DOI] [PubMed] [Google Scholar]
- Perlin D. S., Latchney L. R., Senior A. E. Inhibition of Escherichia coli H+-ATPase by venturicidin, oligomycin and ossamycin. Biochim Biophys Acta. 1985 May 31;807(3):238–244. doi: 10.1016/0005-2728(85)90254-3. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L., Cox C. Metabolic and synthetic activities of Pneumocystis carinii in vitro. Infect Immun. 1981 Dec;34(3):908–914. doi: 10.1128/iai.34.3.908-914.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesanti E. L. Enzymes of Pneumocystis carinii: electrophoretic mobility on starch gels. J Protozool. 1989 Jan-Feb;36(1):2S–3S. doi: 10.1111/j.1550-7408.1989.tb02662.x. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L. Pneumocystis carinii: oxygen uptake, antioxidant enzymes, and susceptibility to oxygen-mediated damage. Infect Immun. 1984 Apr;44(1):7–11. doi: 10.1128/iai.44.1.7-11.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifer L. L., Hughes W. T., Murphy M. J., Jr Propagation of Pneumocystis carinii in vitro. Pediatr Res. 1977 Apr;11(4):305–316. doi: 10.1203/00006450-197704000-00010. [DOI] [PubMed] [Google Scholar]
- Pifer L. L., Woods D., Hughes W. T. Propagation of Pneumocystis carinii in Vero cell culture. Infect Immun. 1978 Apr;20(1):66–68. doi: 10.1128/iai.20.1.66-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prioli R. P., Tanna A., Brown I. N. Rapid methods for counting mycobacteria--comparison of methods for extraction of mycobacterial adenosine triphosphate (ATP) determined by firefly luciferase assay. Tubercle. 1985 Jun;66(2):99–108. doi: 10.1016/0041-3879(85)90074-1. [DOI] [PubMed] [Google Scholar]
- Ruffolo J. J., Cushion M. T., Walzer P. D. Techniques for examining Pneumocystis carinii in fresh specimens. J Clin Microbiol. 1986 Jan;23(1):17–21. doi: 10.1128/jcm.23.1.17-21.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz D. M., Romancheck M. A., Pittarelli L. A., Schwartz R. E., Fromtling R. A., Nollstadt K. H., Vanmiddlesworth F. L., Wilson K. E., Turner M. J. Treatment of Pneumocystis carinii pneumonia with 1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5950–5954. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. Bacterial adenosine 5'-triphosphate synthase (F1F0): purification and reconstitution of F0 complexes and biochemical and functional characterization of their subunits. Microbiol Rev. 1987 Dec;51(4):477–497. doi: 10.1128/mr.51.4.477-497.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selan L., Berlutti F., Passariello C., Thaller M. C., Renzini G. Reliability of a bioluminescence ATP assay for detection of bacteria. J Clin Microbiol. 1992 Jul;30(7):1739–1742. doi: 10.1128/jcm.30.7.1739-1742.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevin B. U., Peng Z. L., Perras J. P., Ganjei P., Penalver M., Averette H. E. Application of an ATP-bioluminescence assay in human tumor chemosensitivity testing. Gynecol Oncol. 1988 Sep;31(1):191–204. doi: 10.1016/0090-8258(88)90293-4. [DOI] [PubMed] [Google Scholar]
- Stringer J. R., Stringer S. L., Zhang J., Baughman R., Smulian A. G., Cushion M. T. Molecular genetic distinction of Pneumocystis carinii from rats and humans. J Eukaryot Microbiol. 1993 Nov-Dec;40(6):733–741. doi: 10.1111/j.1550-7408.1993.tb04468.x. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. W., Simpson-Haidaris P. J., Gigliotti F., Harmsen A. G., Haidaris C. G. Conserved sequence homology of cysteine-rich regions in genes encoding glycoprotein A in Pneumocystis carinii derived from different host species. Infect Immun. 1994 May;62(5):1513–1519. doi: 10.1128/iai.62.5.1513-1519.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y. Ultrastructural studies of Pneumocystis carinii. J Protozool. 1989 Jan-Feb;36(1):53–60. doi: 10.1111/j.1550-7408.1989.tb02696.x. [DOI] [PubMed] [Google Scholar]