Abstract
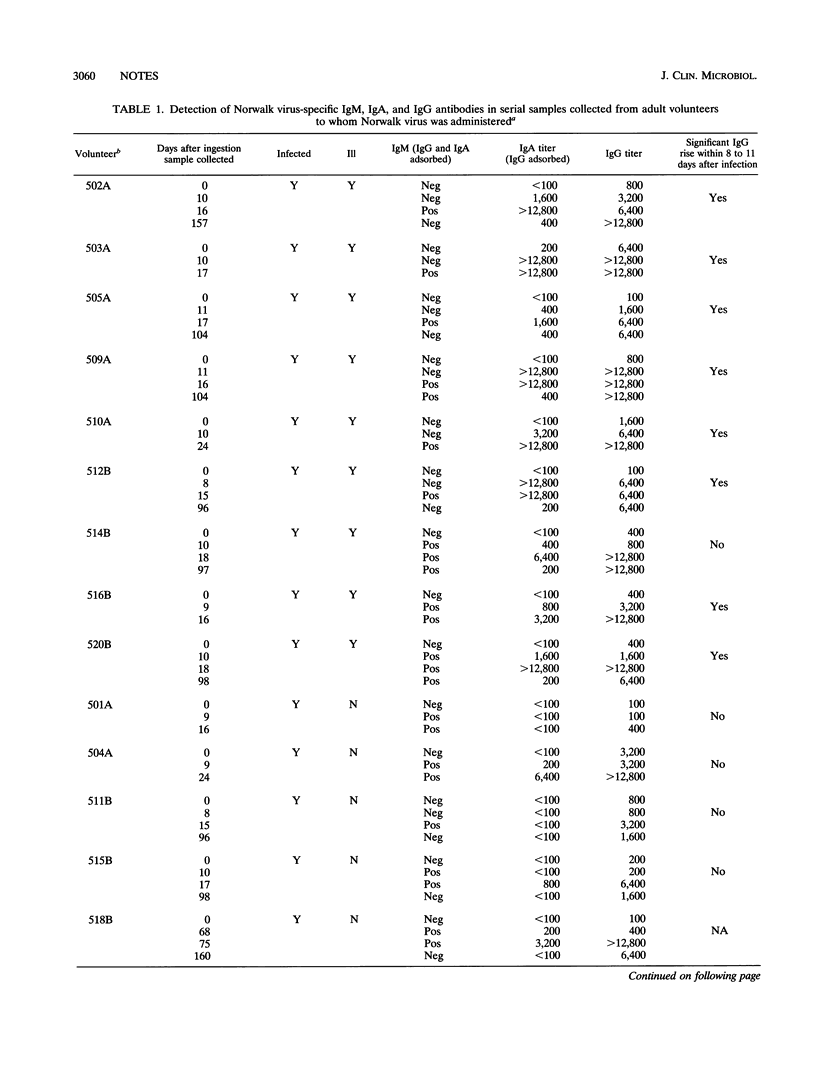
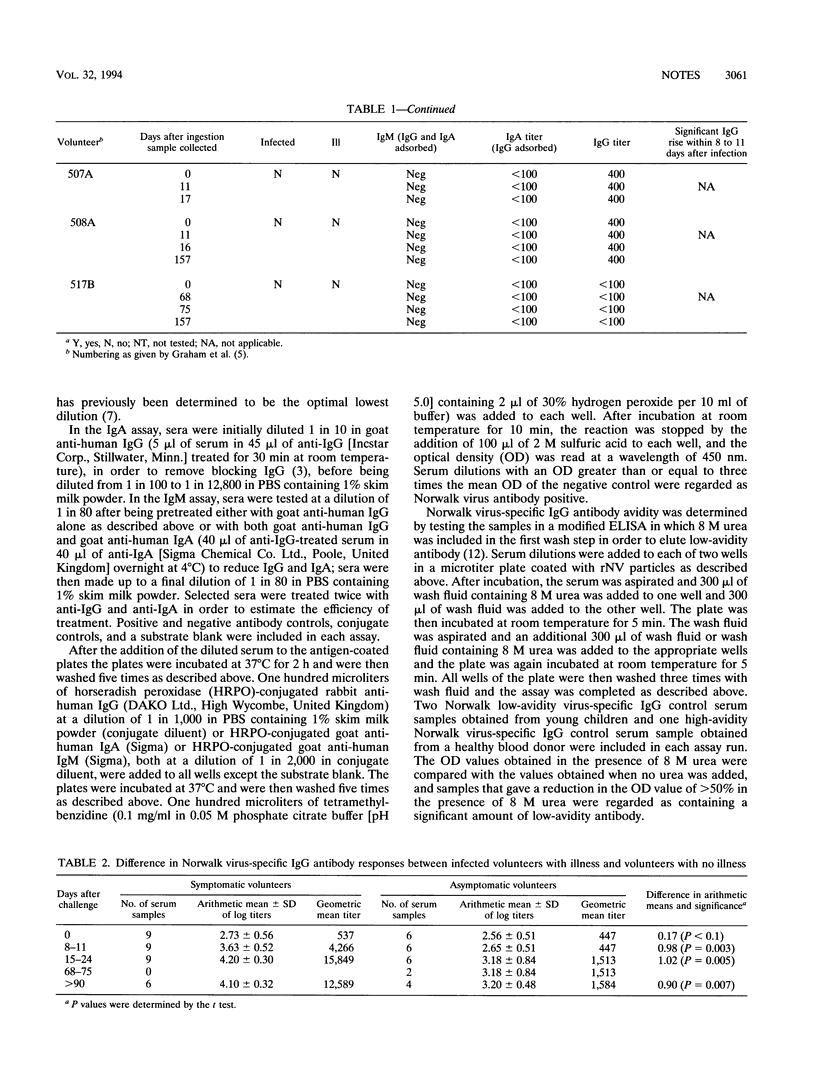
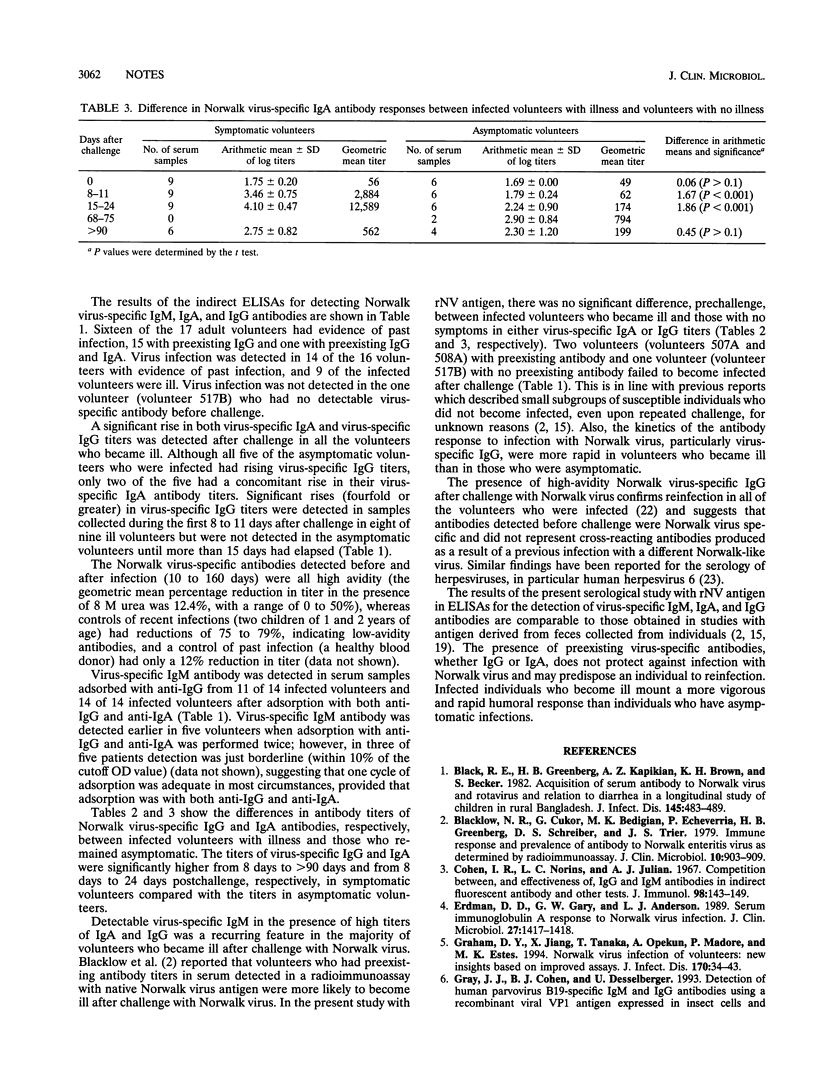
Pre- and postexposure sera collected from 17 adult volunteers challenged with Norwalk virus as described previously (D. Y. Graham, X. Jiang, T. Tanaka, A. Opekun, P. Madore, and M. K. Estes, J. Infect. Dis. 170:34-43, 1994) were examined for Norwalk virus-specific immunoglobulin M (IgM), IgA, and IgG by indirect enzyme-linked immunosorbent assays with recombinant Norwalk virus antigen bound to the solid phase. Sixteen of the 17 volunteers had evidence of past infection, all presenting with preexisting IgG antibody of high avidity; only one volunteer had no evidence of previous infection. Virus infection was detected in 14 of the 16 volunteers with evidence of past infection, and 9 of the infected volunteers had symptomatic illness. A significant rise in both virus-specific IgA and IgG titers was detected after challenge in all of the volunteers who became ill. Five of the asymptomatic volunteers who were infected had rising titers of virus-specific IgG, but only two of the five had a concomitant rise in their virus-specific IgA antibody titers. Antibody rises were detectable in eight of nine ill volunteers 8 to 11 days after challenge but in the asymptomatic volunteers only after more than 15 days had elapsed. Virus-specific IgM was detected after challenge in all 14 infected volunteers. Between symptomatic and asymptomatic volunteers there were no significant differences in titers of virus-specific IgG and IgA in serum before challenge; however, there were significantly higher titers in symptomatic volunteers between 8 and > 90 days after challenge for virus-specific IgG and 8 and 24 days after challenge for virus-specific IgA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black R. E., Greenberg H. B., Kapikian A. Z., Brown K. H., Becker S. Acquisition of serum antibody to Norwalk Virus and rotavirus and relation to diarrhea in a longitudinal study of young children in rural Bangladesh. J Infect Dis. 1982 Apr;145(4):483–489. doi: 10.1093/infdis/145.4.483. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Cukor G., Bedigian M. K., Echeverria P., Greenberg H. B., Schreiber D. S., Trier J. S. Immune response and prevalence of antibody to Norwalk enteritis virus as determined by radioimmunoassay. J Clin Microbiol. 1979 Dec;10(6):903–909. doi: 10.1128/jcm.10.6.903-909.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R., Norins L. C., Julian A. J. Competition between, and effectiveness of, IgG and IgM antibodies in indirect fluorescent antibody and other tests. J Immunol. 1967 Jan;98(1):143–149. [PubMed] [Google Scholar]
- Erdman D. D., Gary G. W., Anderson L. J. Serum immunoglobulin A response to Norwalk virus infection. J Clin Microbiol. 1989 Jun;27(6):1417–1418. doi: 10.1128/jcm.27.6.1417-1418.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Jiang X., Tanaka T., Opekun A. R., Madore H. P., Estes M. K. Norwalk virus infection of volunteers: new insights based on improved assays. J Infect Dis. 1994 Jul;170(1):34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- Gray J. J., Cohen B. J., Desselberger U. Detection of human parvovirus B19-specific IgM and IgG antibodies using a recombinant viral VP1 antigen expressed in insect cells and estimation of time of infection by testing for antibody avidity. J Virol Methods. 1993 Sep;44(1):11–23. doi: 10.1016/0166-0934(93)90003-a. [DOI] [PubMed] [Google Scholar]
- Gray J. J., Jiang X., Morgan-Capner P., Desselberger U., Estes M. K. Prevalence of antibodies to Norwalk virus in England: detection by enzyme-linked immunosorbent assay using baculovirus-expressed Norwalk virus capsid antigen. J Clin Microbiol. 1993 Apr;31(4):1022–1025. doi: 10.1128/jcm.31.4.1022-1025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Lew J. F., Jiang X., Kapikian A. Z., Estes M. K. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J Clin Microbiol. 1993 Aug;31(8):2185–2191. doi: 10.1128/jcm.31.8.2185-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., Yolken R. H., Gangarosa E., Gary W., Wyatt R. G., Konno T., Suzuki H., Chanock R. M., Kapikian A. Z. Role of Norwalk virus in outbreaks of nonbacterial gastroenteritis. J Infect Dis. 1979 May;139(5):564–568. doi: 10.1093/infdis/139.5.564. [DOI] [PubMed] [Google Scholar]
- Guigno D., Coupland B., Smith E. G., Farrell I. D., Desselberger U., Caul E. O. Primary humoral antibody response to Coxiella burnetii, the causative agent of Q fever. J Clin Microbiol. 1992 Aug;30(8):1958–1967. doi: 10.1128/jcm.30.8.1958-1967.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg C. W., Osterholm M. T. Outbreaks of food-borne and waterborne viral gastroenteritis. Clin Microbiol Rev. 1993 Jul;6(3):199–210. doi: 10.1128/cmr.6.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Hasegawa A., Matsuno S., Katow S. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol. 1984 Sep;20(3):525–529. doi: 10.1128/jcm.20.3.525-529.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang M., Graham D. Y., Estes M. K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992 Nov;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. C., Mathewson J. J., DuPont H. L., Greenberg H. B. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis. 1990 Jan;161(1):18–21. doi: 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]
- Kaplan J. E., Gary G. W., Baron R. C., Singh N., Schonberger L. B., Feldman R., Greenberg H. B. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann Intern Med. 1982 Jun;96(6 Pt 1):756–761. doi: 10.7326/0003-4819-96-6-756. [DOI] [PubMed] [Google Scholar]
- Matsui S. M., Kim J. P., Greenberg H. B., Su W., Sun Q., Johnson P. C., DuPont H. L., Oshiro L. S., Reyes G. R. The isolation and characterization of a Norwalk virus-specific cDNA. J Clin Invest. 1991 Apr;87(4):1456–1461. doi: 10.1172/JCI115152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrino T. A., Schreiber D. S., Trier J. S., Kapikian A. Z., Blacklow N. R. Clinical immunity in acute gastroenteritis caused by Norwalk agent. N Engl J Med. 1977 Jul 14;297(2):86–89. doi: 10.1056/NEJM197707142970204. [DOI] [PubMed] [Google Scholar]
- Ryder R. W., Singh N., Reeves W. C., Kapikian A. Z., Greenberg H. B., Sack R. B. Evidence of immunity induced by naturally acquired rotavirus and Norwalk virus infection on two remote Panamanian islands. J Infect Dis. 1985 Jan;151(1):99–105. doi: 10.1093/infdis/151.1.99. [DOI] [PubMed] [Google Scholar]
- Ward K. N., Gray J. J., Joslin M. E., Sheldon M. J. Avidity of IgG antibodies to human herpesvirus-6 distinguishes primary from recurrent infection in organ transplant recipients and excludes cross-reactivity with other herpesviruses. J Med Virol. 1993 Jan;39(1):44–49. doi: 10.1002/jmv.1890390109. [DOI] [PubMed] [Google Scholar]
- Xi J. N., Graham D. Y., Wang K. N., Estes M. K. Norwalk virus genome cloning and characterization. Science. 1990 Dec 14;250(4987):1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]


