Abstract
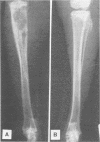
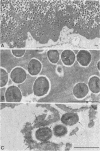
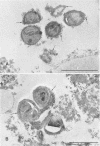
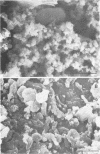
A surgical procedure allowed the placement of a silicone rubber catheter in the marrow cavity of the tibia of a rabbit and also allowed the introduction of a sclerosing agent (sodium morrhuate) and cells of Staphylococcus aureus. Osteomyelitis developed in 60% of the animals so treated, and the infecting microorganism was recovered from the infected tibias of the animals that developed this disease. All blood cultures taken 24 h after the infection were negative for S. aureus. Radiological findings consisted of osteolytic changes, the occurrence of sequestration and periosteal reactions, and sclerosis in the infected bones. Sections of bone prepared for histological examination confirmed the diagnosis of osteomyelitis. Transmission and scanning electron microscopy of samples of bone marrow, bone chips, and the catheters taken from the infected tibiae revealed gram-positive cocci embedded in a very extensive matrix of ruthenium red-staining glycocalyx adhering to the bone and the implanted catheter. It is proposed that this extensive glycocalyx served a protective function for the bacteria and was important in bacterial adherence and thus played an important role in bacterial persistence and the development of osteomyelitis in these rabbits.
Full text
PDF
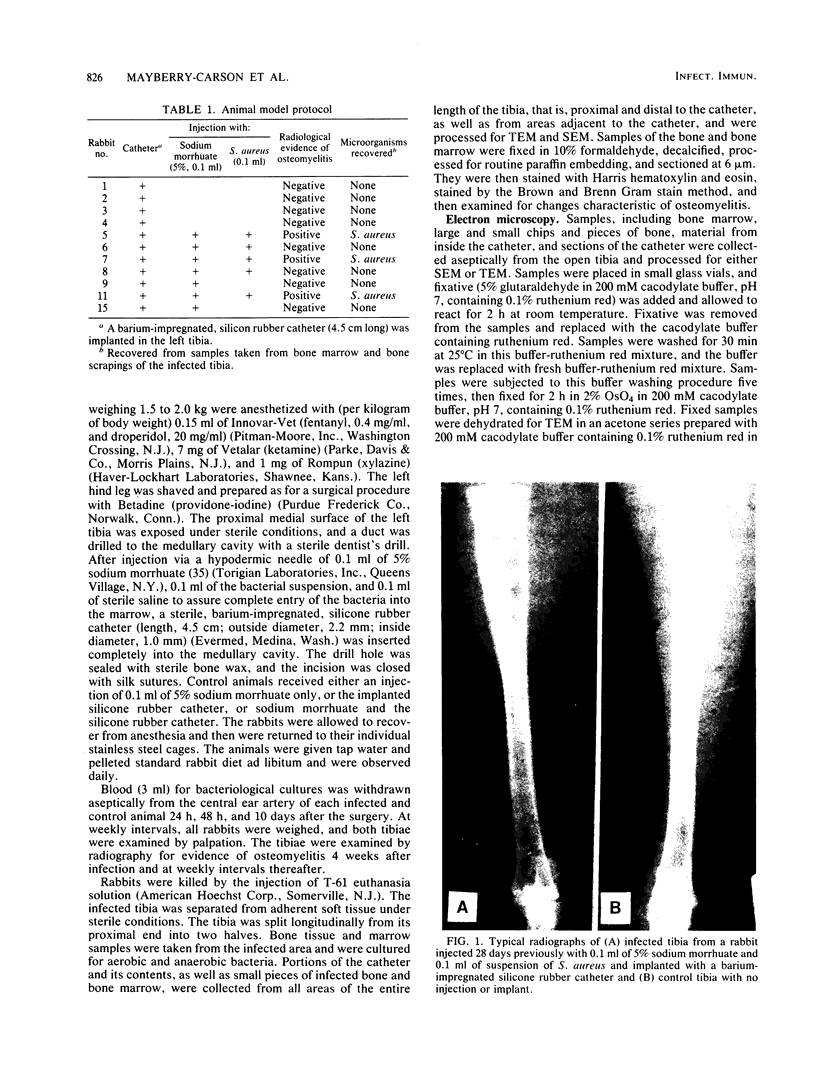
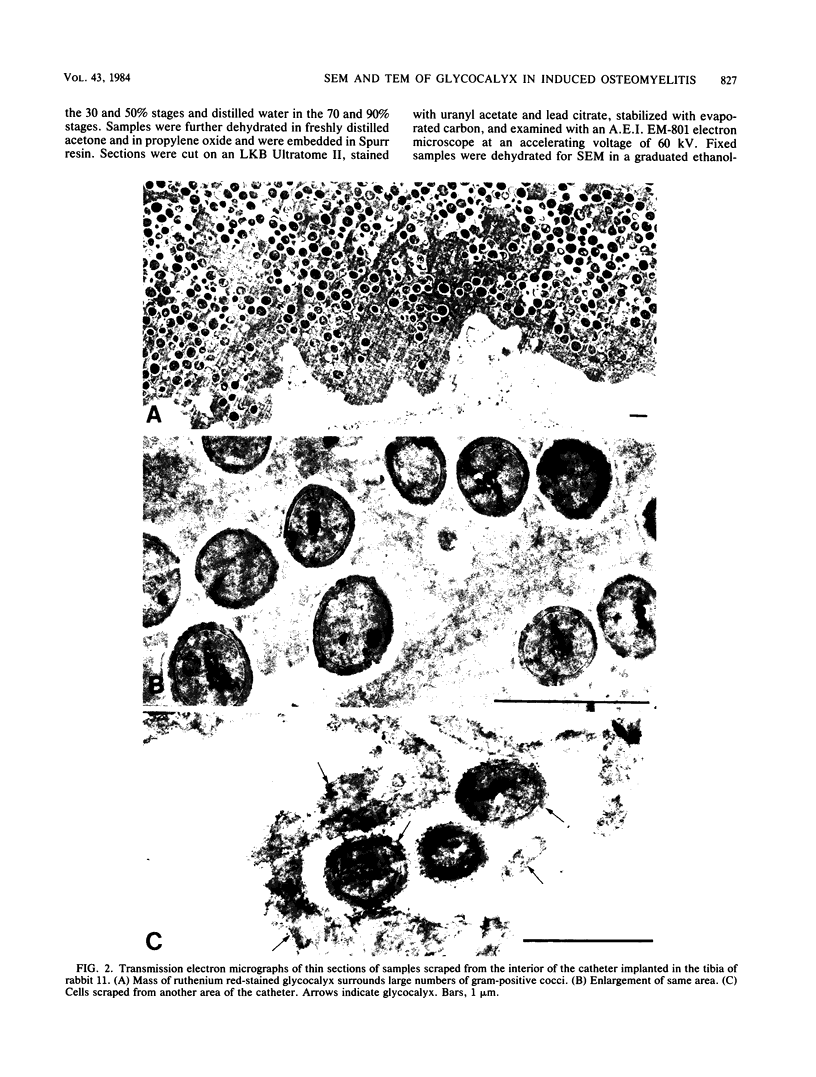

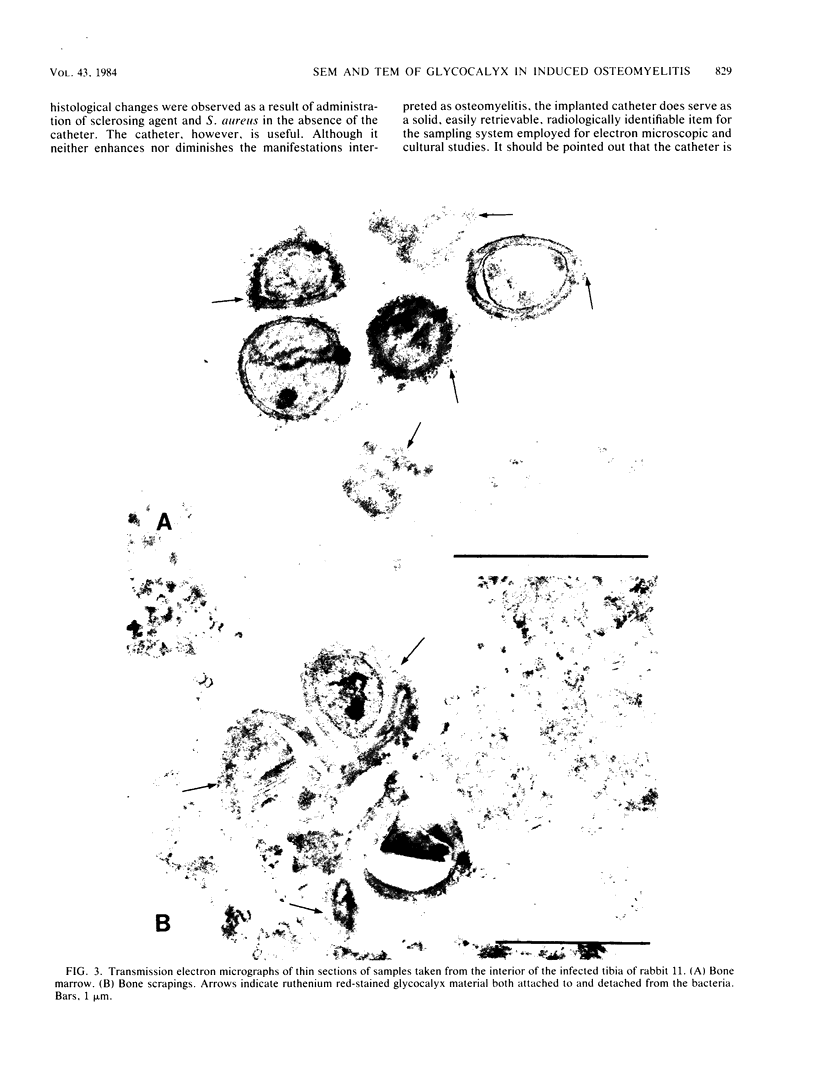
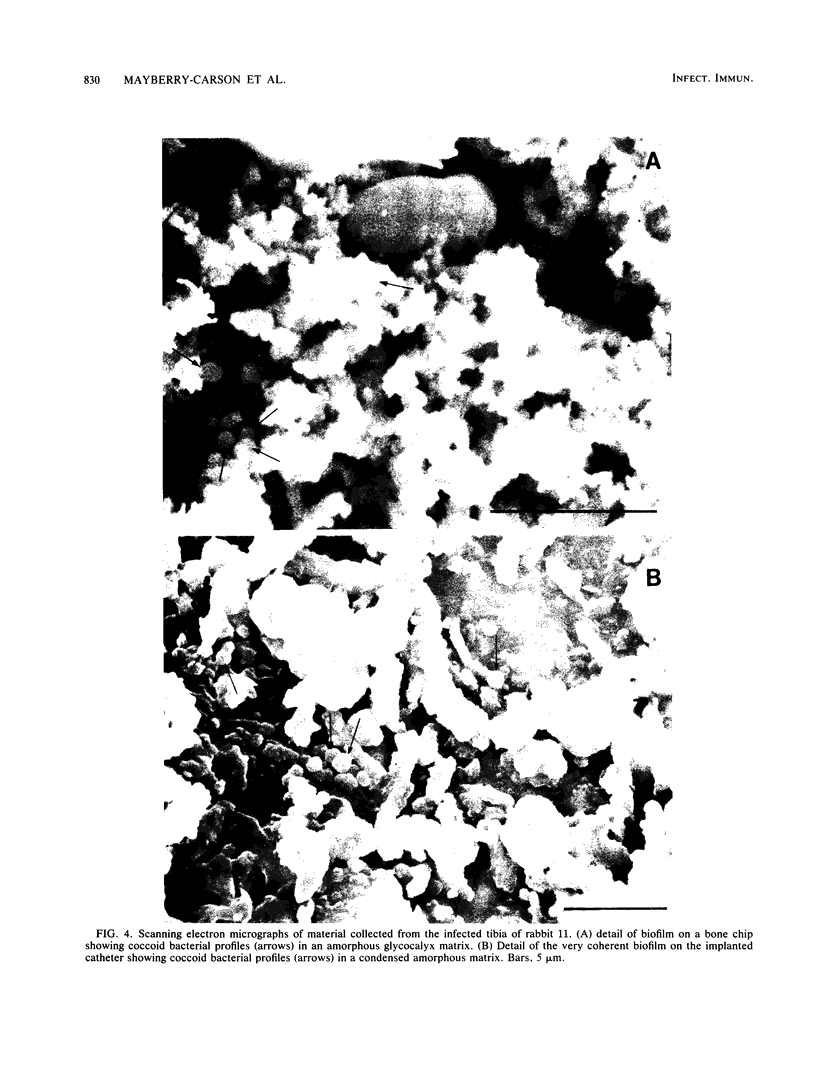
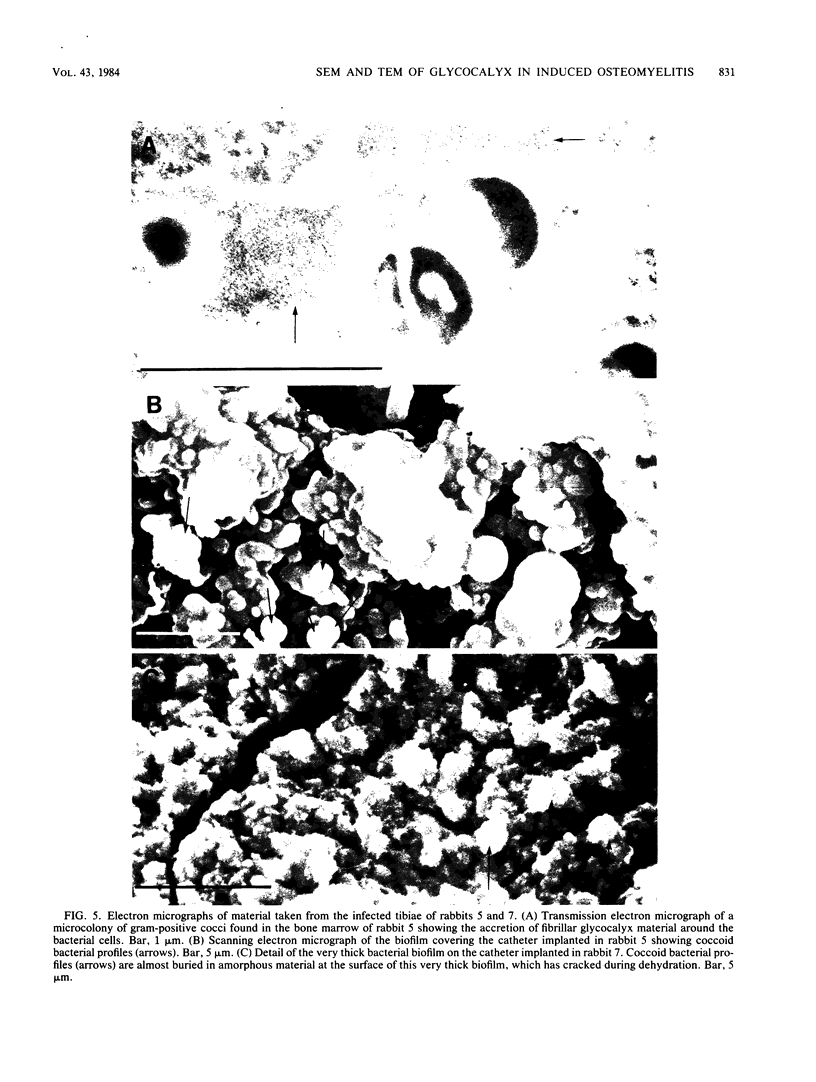


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S., Mitchell M. Immunologic investigations of mucoid strains of Pseudomonas aeruginosa: comparison of susceptibility to opsonic antibody in mucoid and nonmucoid strains. J Infect Dis. 1980 Feb;141(2):238–247. doi: 10.1093/infdis/141.2.238. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Doyle R. J., Morgenstern M. Organization of teichoic acid in the cell wall of Bacillus subtilis. J Bacteriol. 1975 Feb;121(2):726–734. doi: 10.1128/jb.121.2.726-734.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputy G. G., Costerton J. W. Morphological examination of the glycocalyces of Staphylococcus aureus strains Wiley and Smith. Infect Immun. 1982 May;36(2):759–767. doi: 10.1128/iai.36.2.759-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R., Acres S. D., Costerton J. W. Use of specific antibody to demonstrate glycocalyx, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrum-fed calves. Infect Immun. 1982 Sep;37(3):1170–1180. doi: 10.1128/iai.37.3.1170-1180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983 Apr;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- Costerton J. W. The role of electron microscopy in the elucidation of bacterial structure and function. Annu Rev Microbiol. 1979;33:459–479. doi: 10.1146/annurev.mi.33.100179.002331. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek M., Pryjma K., Bartkowski S., Heczko P. B. Anti-staphylococcal gamma hemolysin antibodies in rabbits with staphylococcal osteomyelitis. Med Microbiol Immunol. 1977 May 18;163(1):61–65. doi: 10.1007/BF02126710. [DOI] [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Mackie E. B., Brown K. N., Lam J., Costerton J. W. Morphological stabilization of capsules of group B streptococci, types Ia, Ib, II, and III, with specific antibody. J Bacteriol. 1979 May;138(2):609–617. doi: 10.1128/jb.138.2.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. A scanning and transmission electron microscopic study of the surfaces of intrauterine contraceptive devices. Am J Obstet Gynecol. 1983 Jun 15;146(4):384–394. doi: 10.1016/0002-9378(83)90818-9. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Scanning electron microscopic study of uropathogen adherence to a plastic surface. Appl Environ Microbiol. 1983 Mar;45(3):1018–1024. doi: 10.1128/aem.45.3.1018-1024.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Harding G. K., Ronald A. R., Dikkema J., Lam J., Hoban S., Costerton J. W. Influence of mucoidy on antibody coating of Pseudomonas aeruginosa. J Infect Dis. 1979 Mar;139(3):357–361. doi: 10.1093/infdis/139.3.357. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- Norden C. W. Experimental osteomyelitis. I. A description of the model. J Infect Dis. 1970 Nov;122(5):410–418. doi: 10.1093/infdis/122.5.410. [DOI] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Wilkinson B. J., Schmeling D., Michael A. F., Quie P. G. Dichotomy between opsonization and serum complement activation by encapsulated staphylococci. Infect Immun. 1978 Jun;20(3):770–775. doi: 10.1128/iai.20.3.770-775.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzmann S., Boring J. R. Antiphagocytic Effect of Slime from a Mucoid Strain of Pseudomonas aeruginosa. Infect Immun. 1971 Jun;3(6):762–767. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. Ultrastructure of the capsule of Klebsiella pneumoniae and slime of Enterobacter aerogenes revealed by freeze etching. Arch Mikrobiol. 1973 Nov 19;93(4):277–286. doi: 10.1007/BF00427925. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K. Factors affecting complement activation by Staphylococcus aureus cell walls, their components, and mutants altered in teichoic acid. Infect Immun. 1981 Apr;32(1):216–224. doi: 10.1128/iai.32.1.216-224.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Peterson P. K., Quie P. G. Cryptic peptidoglycan and the antiphagocytic effect of the Staphylococcus aureus capsule: model for the antiphagocytic effect of bacterial cell surface polymers. Infect Immun. 1979 Feb;23(2):502–508. doi: 10.1128/iai.23.2.502-508.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Sisson S. P., Kim Y., Peterson P. K. Localization of the third component of complement on the cell wall of encapsulated Staphylococcus aureus M: implications for the mechanism of resistance to phagocytosis. Infect Immun. 1979 Dec;26(3):1159–1163. doi: 10.1128/iai.26.3.1159-1163.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]