Abstract
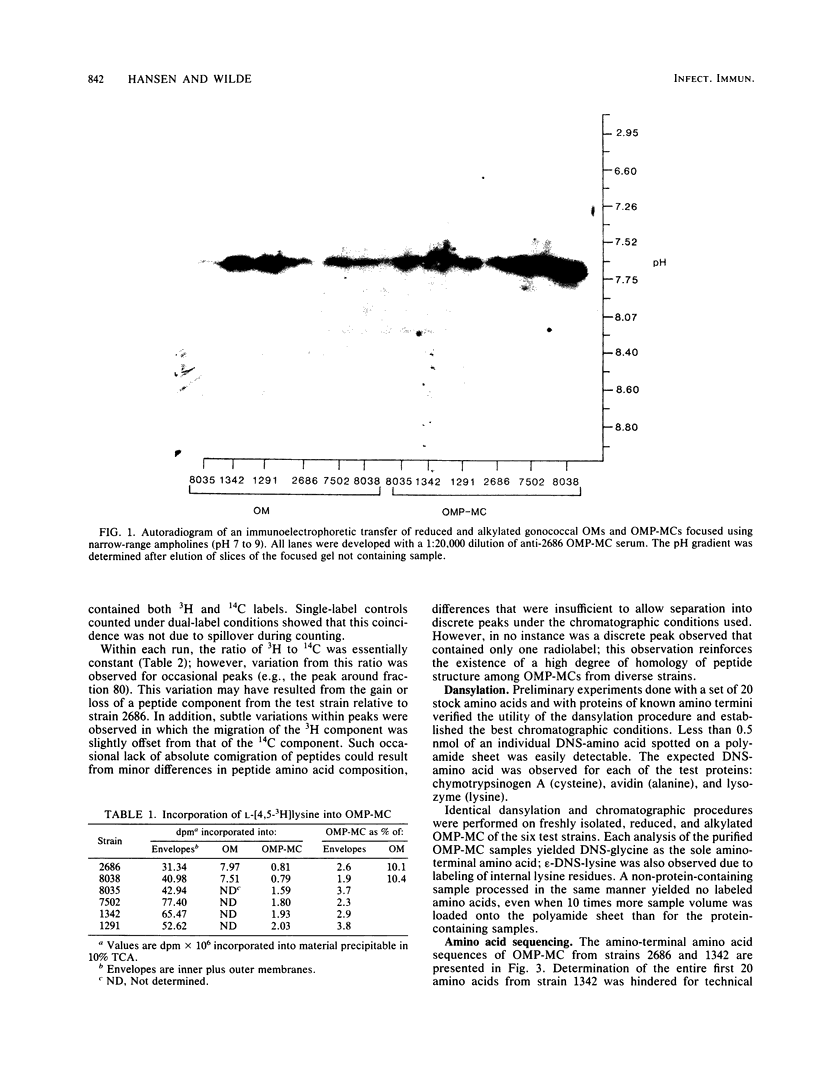
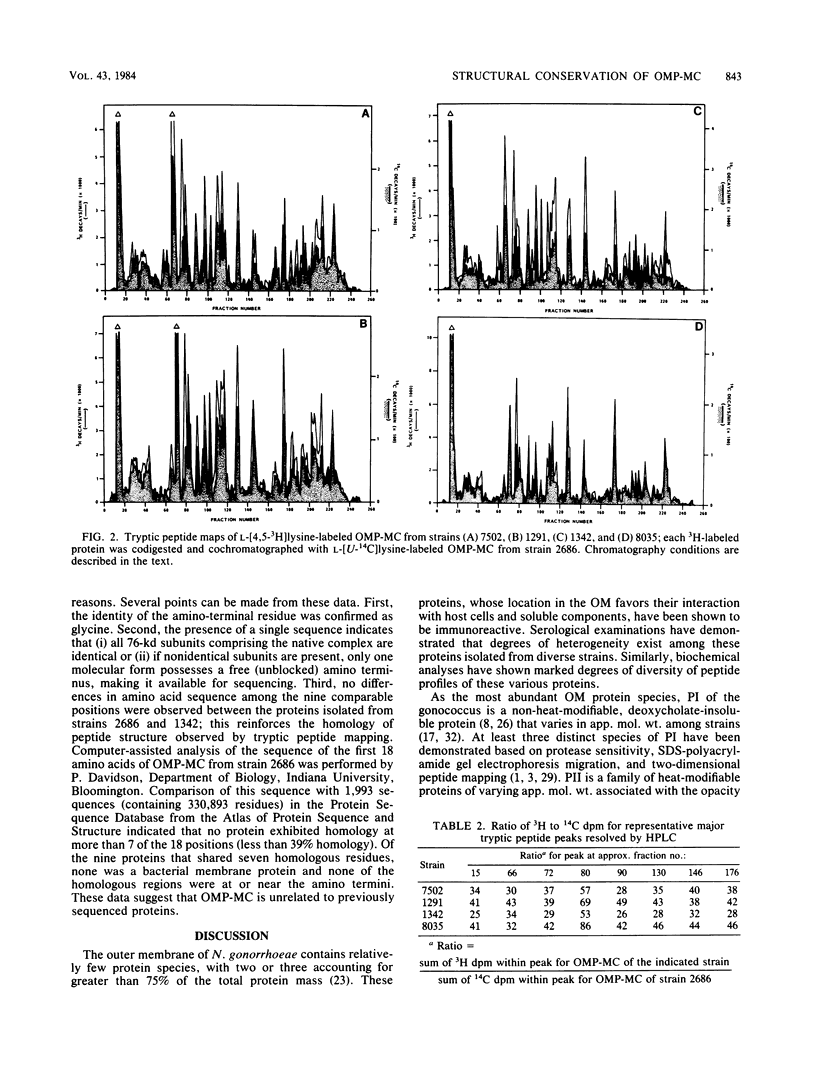
The structural conservation of an outer membrane protein of Neisseria gonorrhoeae called OMP-MC (outer membrane protein-macromolecular complex) was investigated by determining the isoelectric point and amino-terminal amino acid sequence of the protein and by using high-performance liquid chromatography for comparative tryptic peptide mapping. The 76,000-dalton subunits generated by reduction and alkylation of the native 800,000-dalton complex from six test strains focused in ultrathin gels as bands of restricted heterogeneity at an approximate pI of 7.6. Dansyl chloride labeling indicated that all strains shared glycine as the amino-terminal amino acid. Sequence analysis of OMP-MC from two strains revealed no amino acid differences within the first 11 residues. Dual-label peptide maps revealed an extremely high degree of conservation of peptide structure. The results indicate that (i) OMP-MCs isolated from various strains of N. gonorrhoeae share structural homology and (ii) the 800,000-dalton complex is a homopolymer composed of 10 to 12 apparently identical 76,000-dalton subunits.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Gotschlich E. C. Gonococcal membrane proteins: speculation on their role in pathogenesis. Prog Allergy. 1983;33:298–313. [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the major outer membrane protein of Neisseria gonorrhoeae. Infect Immun. 1982 Apr;36(1):277–283. doi: 10.1128/iai.36.1.277-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C., Swanson J. Effects of proteolytic enzymes on the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1981 Jul;33(1):212–222. doi: 10.1128/iai.33.1.212-222.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Görg A., Postel W., Westermeier R. Ultrathin-layer isoelectric focusing in polyacrylamide gels on cellophane. Anal Biochem. 1978 Aug 15;89(1):60–70. doi: 10.1016/0003-2697(78)90726-1. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. Structural comparison of Neisseria gonorrhoeae outer membrane proteins. J Bacteriol. 1981 Feb;145(2):736–742. doi: 10.1128/jb.145.2.736-742.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Surface peptide mapping of protein I and protein III of four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):632–641. doi: 10.1128/iai.37.2.632-641.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Sawyer W. D., Haak R. A. Cross-linking analysis of the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):785–791. doi: 10.1128/iai.28.3.785-791.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Wilde C. E., 3rd, Sawyer W. D., Haak R. A. High-molecular-weight antigenic protein complex in the outer membrane of Neisseria gonorrhoeae. Infect Immun. 1980 Feb;27(2):475–482. doi: 10.1128/iai.27.2.475-482.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Swanson J. 125I-labeled peptide mapping of some heat-modifiable proteins of the gonococcal outer membrane. Infect Immun. 1980 Apr;28(1):54–64. doi: 10.1128/iai.28.1.54-64.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XVIII. 125I-labeled peptide mapping of the major protein of the gonococcal cell wall outer membrane. Infect Immun. 1979 Mar;23(3):799–810. doi: 10.1128/iai.23.3.799-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhorst C., Wittmann-Liebold B., Möller W. 50-S ribosomal proteins. Peptide studies on two acidic proteins, A 1 and A 2 , isolated from 50-S ribosomes of Escherichia coli. Eur J Biochem. 1972 Jan 31;25(1):13–19. doi: 10.1111/j.1432-1033.1972.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Wong T. P., Johnston K. H. One-dimensional peptide mapping of the major outer membrane protein of Neisseria gonorrhoeae. Infect Immun. 1981 Dec;34(3):739–745. doi: 10.1128/iai.34.3.739-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. P., Shockley R. K., Johnston K. H. WSJM, a simple chemically defined medium for growth of Neisseria gonorrhoeae. J Clin Microbiol. 1980 Apr;11(4):363–369. doi: 10.1128/jcm.11.4.363-369.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]