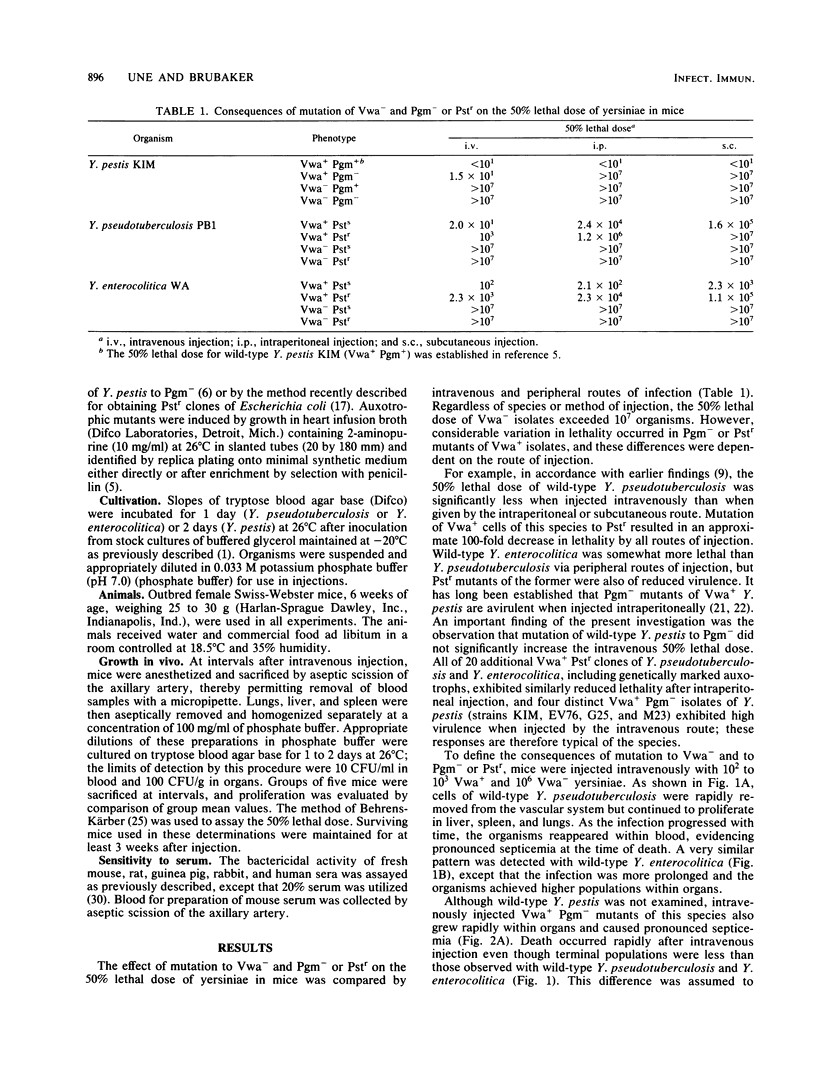
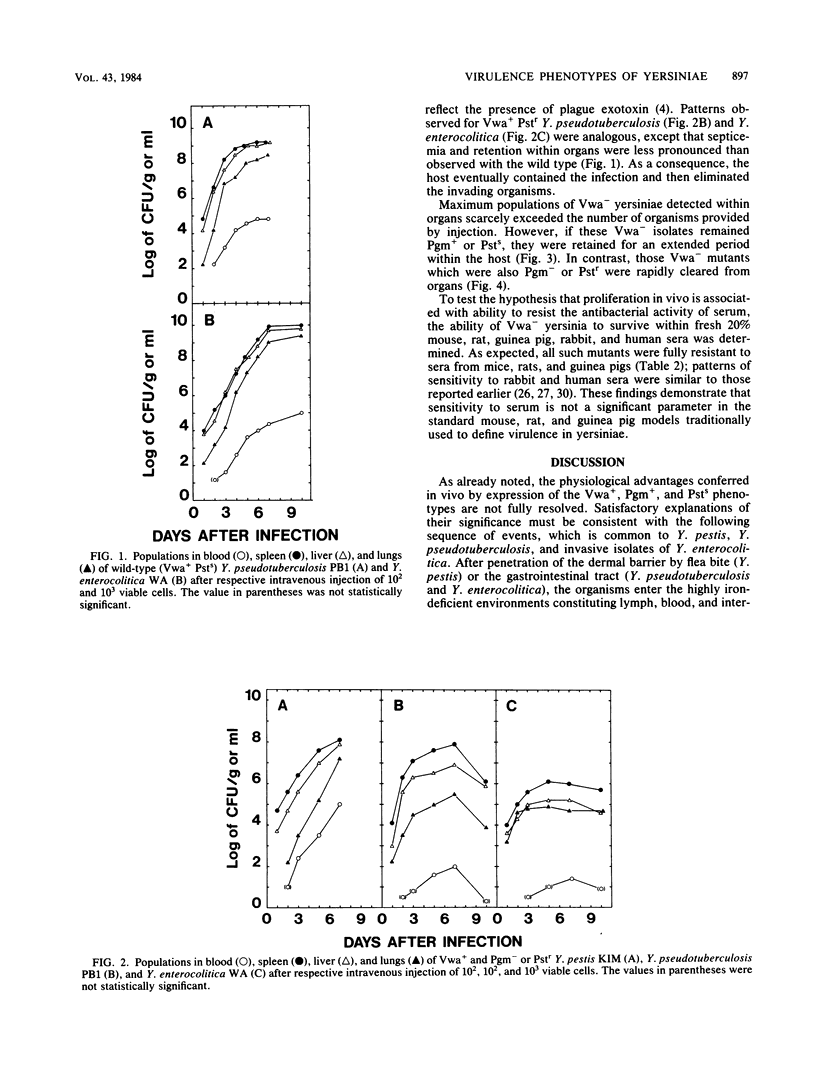
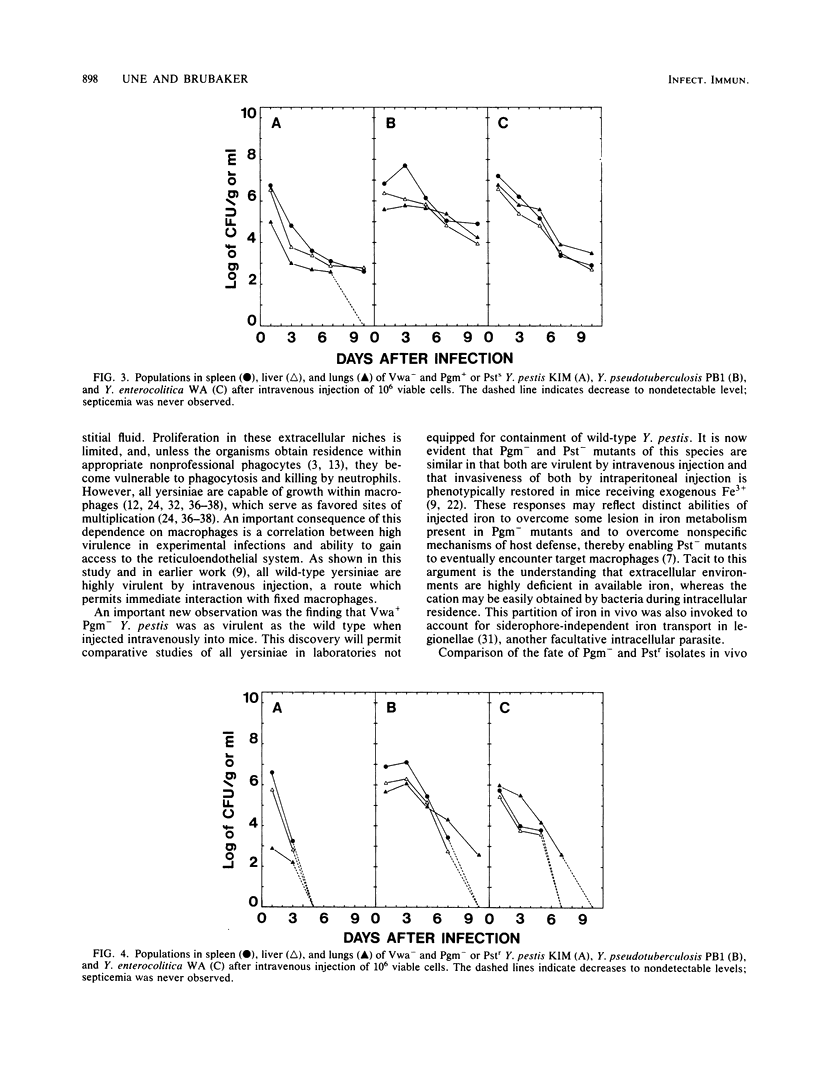
Abstract
The abilities of Yersinia pestis to undergo restriction in Ca2+-deficient medium with concomitant production of V and W antigens (Vwa+) and to absorb exogenous pigments (Pgm+) are established virulence factors. Mutation of Y. pestis to Pgm- is known to promote resistance to pesticin (Pstr) and reduced lethality by peripheral routes of injection. Vwa+ Pgm- isolates of Y. pestis were shown in this study to retain virulence in mice when injected intravenously. Although Pgm- in appearance, wild-type cells of Yersinia pseudotuberculosis and Yersinia enterocolitica may also be sensitive to pesticin. Pstr mutants of Vwa+ strains of these species were similarly of reduced virulence, especially by peripheral routes of injection. The consequences of mutation to Vwa- and Pgm- or Pstr on growth and persistence in vivo were determined. After intravenous injection, Vwa+ yersiniae of all species exhibited sustained growth in mouse spleen, liver, and lung and accumulated in blood. Septicemia was not observed after similar injection of Vwa- mutants which were unable to maintain comparable rates of net increase in tissues. Mutation to Pgm- or Pstr did not influence proliferation but resulted in enhanced clearance from organs. It is known that reticuloendothelial cells serve as favored sites of replication for all wild-type yersiniae. Our results are consistent with the hypothesis that the Vwa+ phenotype favors growth within macrophages and that the Pgm+ and pesticin-sensitive phenotypes permit long-term, probably extracellular, retention within organs. Virulence in standard animal models (mice, rats, and guinea pigs) was not correlated with resistance to the bactericidal action of serum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-GURION R., HERTMAN I. Bacteriocin-like material produced by Pasteurella pestis. J Gen Microbiol. 1958 Oct;19(2):289–297. doi: 10.1099/00221287-19-2-289. [DOI] [PubMed] [Google Scholar]
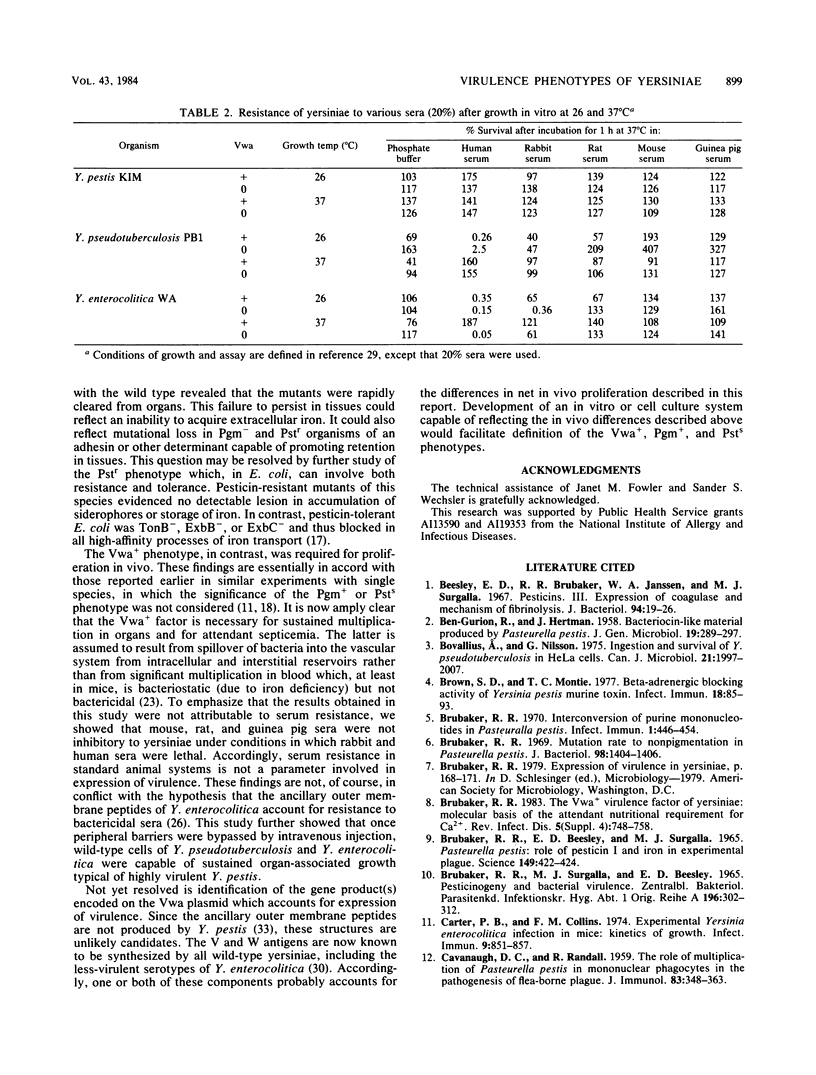
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- Beesley E. D., Brubaker R. R., Janssen W. A., Surgalla M. J. Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J Bacteriol. 1967 Jul;94(1):19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovallius A., Nilsson G. Ingestion and survival of Y. pseudotuberculosis in HeLa cells. Can J Microbiol. 1975 Dec;21(12):1997–2007. doi: 10.1139/m75-287. [DOI] [PubMed] [Google Scholar]
- Brown S. D., Montie T. C. Beta-adrenergic blocking activity of Yersinia pestis murine toxin. Infect Immun. 1977 Oct;18(1):85–93. doi: 10.1128/iai.18.1.85-93.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Beesley E. D., Surgalla M. J. Pasteurella pestis: Role of Pesticin I and Iron in Experimental Plague. Science. 1965 Jul 23;149(3682):422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Interconversion of Purine Mononucleotides in Pasteurella pestis. Infect Immun. 1970 May;1(5):446–454. doi: 10.1128/iai.1.5.446-454.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. Mutation rate to nonpigmentation in Pasteurella pestis. J Bacteriol. 1969 Jun;98(3):1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVANAUGH D. C., RANDALL R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–363. [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect Immun. 1974 May;9(5):851–857. doi: 10.1128/iai.9.5.851-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J. A., Schiemann D. A. HeLa cell infection by Yersinia enterocolitica: evidence for lack of intracellular multiplication and development of a new procedure for quantitative expression of infectivity. Infect Immun. 1981 Apr;32(1):48–55. doi: 10.1128/iai.32.1.48-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgat M., Ben-Gurion R. Mode of action of pesticin. J Bacteriol. 1969 May;98(2):359–367. doi: 10.1128/jb.98.2.359-367.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUI G. M., LAWTON W. D., JANSSEN W. A., SURGALLA M. J. Response of guinea pig lungs to in vivo and in vitro cultures of Pasteurella pestis. J Infect Dis. 1957 Jan-Feb;100(1):103–107. doi: 10.1093/infdis/100.1.103. [DOI] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Mode of action of pesticin: N-acetylglucosaminidase activity. J Bacteriol. 1979 Aug;139(2):495–501. doi: 10.1128/jb.139.2.495-501.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Fowler J. M., Brubaker R. R. Mutations to tolerance and resistance to pesticin and colicins in Escherichia coli phi. J Bacteriol. 1981 May;146(2):506–511. doi: 10.1128/jb.146.2.506-511.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. C., Brubaker R. R. Characterization of pesticin. Separation of antibacterial activities. J Biol Chem. 1974 Aug 10;249(15):4749–4753. [PubMed] [Google Scholar]
- JACKSON S., MORRIS B. C. Enhancement of growth of Pasteurella pestis and other bacteria in serum by the addition of iron. Br J Exp Pathol. 1961 Aug;42:363–368. [PMC free article] [PubMed] [Google Scholar]
- Janssen W. A., Surgalla M. J. Plague bacillus: survival within host phagocytes. Science. 1969 Feb 28;163(3870):950–952. doi: 10.1126/science.163.3870.950. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983 Sep;41(3):921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., DeStephano L. Serum resistance associated with virulence in Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):605–611. doi: 10.1128/iai.35.2.605-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977 Oct;18(1):94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Accumulation of iron by yersiniae. J Bacteriol. 1979 Mar;137(3):1290–1298. doi: 10.1128/jb.137.3.1290-1298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Schooley R., Dolin R., Ennis F. A., Gross P., Gwaltney J. M. Serologic responses and systemic reactions in adults after vaccination with monovalent A/USSR/77 and trivalent A/USSR/77, A/Texas/77, B/Hong Kong/72 influenza vaccines. Rev Infect Dis. 1983 Jul-Aug;5(4):748–757. doi: 10.1093/clinids/5.4.748. [DOI] [PubMed] [Google Scholar]
- Reeves M. W., Pine L., Neilands J. B., Balows A. Absence of siderophore activity in Legionella species grown in iron-deficient media. J Bacteriol. 1983 Apr;154(1):324–329. doi: 10.1128/jb.154.1.324-329.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M., Harkness T. K. Intracellular Pasteurella pseudotuberculosis: Multiplication in Cultured Spleen and Kidney Cells. Infect Immun. 1970 Nov;2(5):631–639. doi: 10.1128/iai.2.5.631-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Localization in Yersinia pestis of peptides associated with virulence. Infect Immun. 1982 Apr;36(1):129–135. doi: 10.1128/iai.36.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgalla M. J., Beesley E. D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969 Nov;18(5):834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. I. Experimental infection in rabbits. Microbiol Immunol. 1977;21(7):341–363. [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. II. Interaction with cultured cells in vitro. Microbiol Immunol. 1977;21(7):365–377. doi: 10.1111/j.1348-0421.1977.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. III. Comparative studies between Y. enterocolitica and Y. pseudotuberculosis. Microbiol Immunol. 1977;21(9):505–516. doi: 10.1111/j.1348-0421.1977.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]


