Abstract
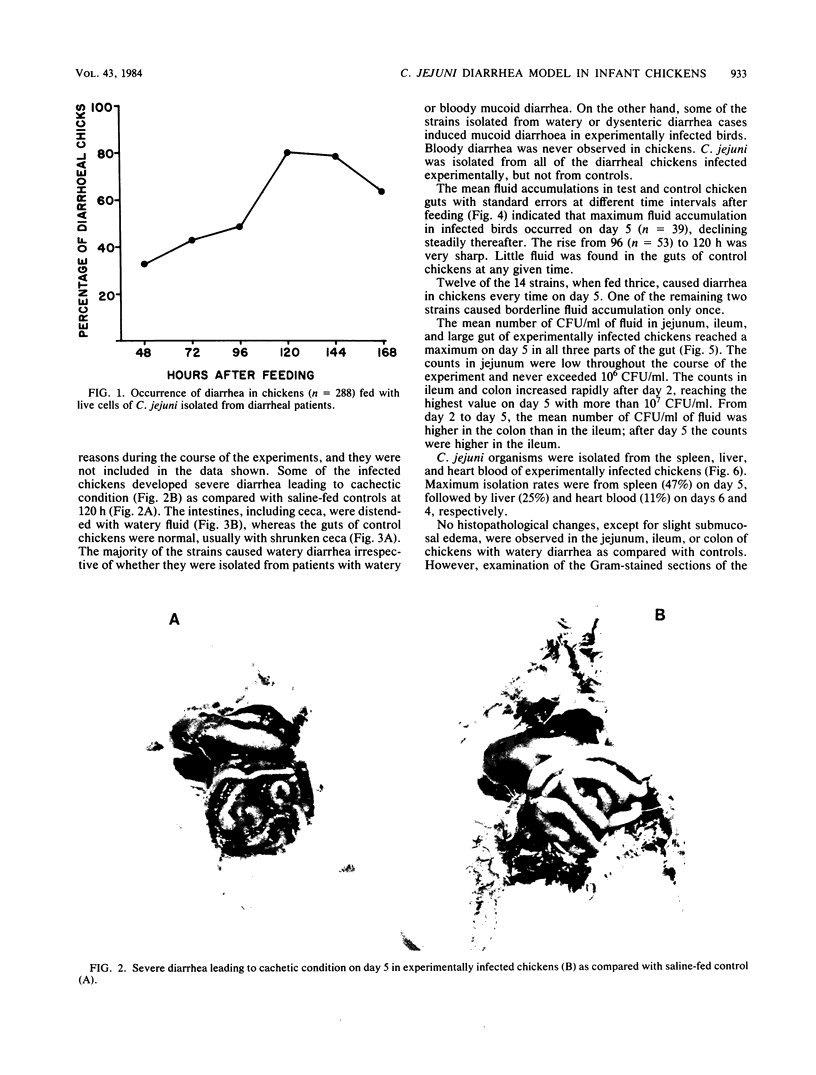
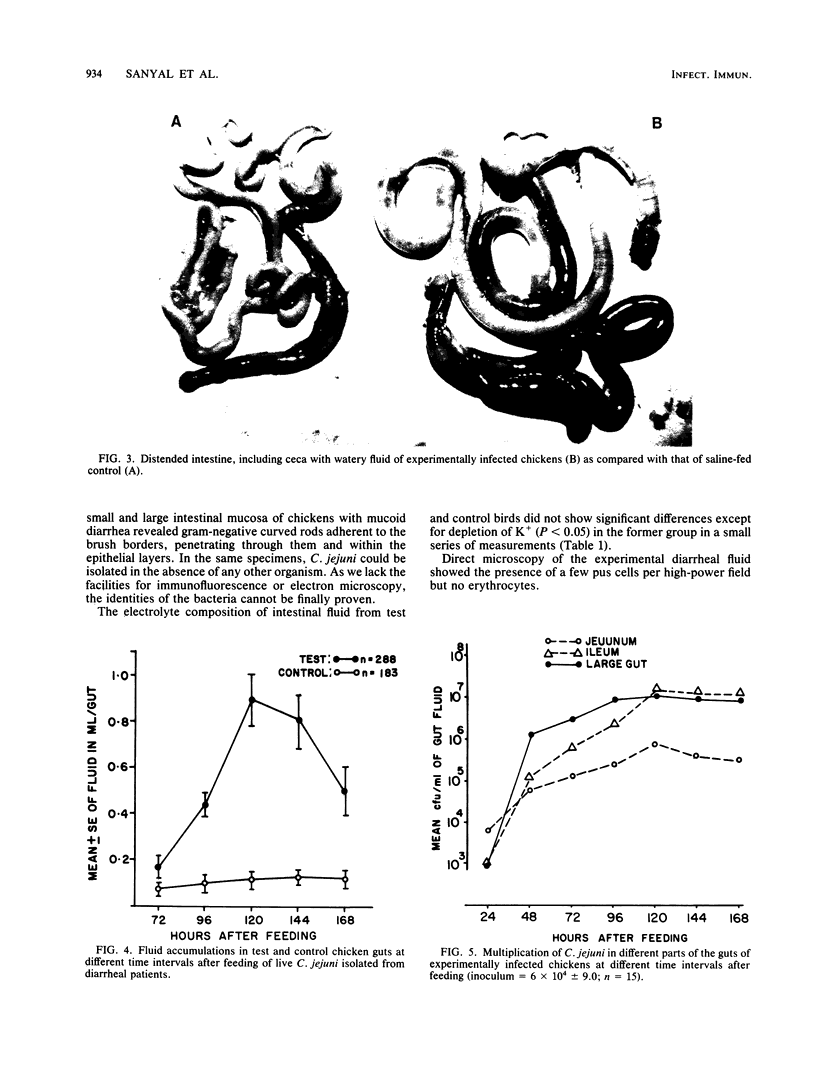
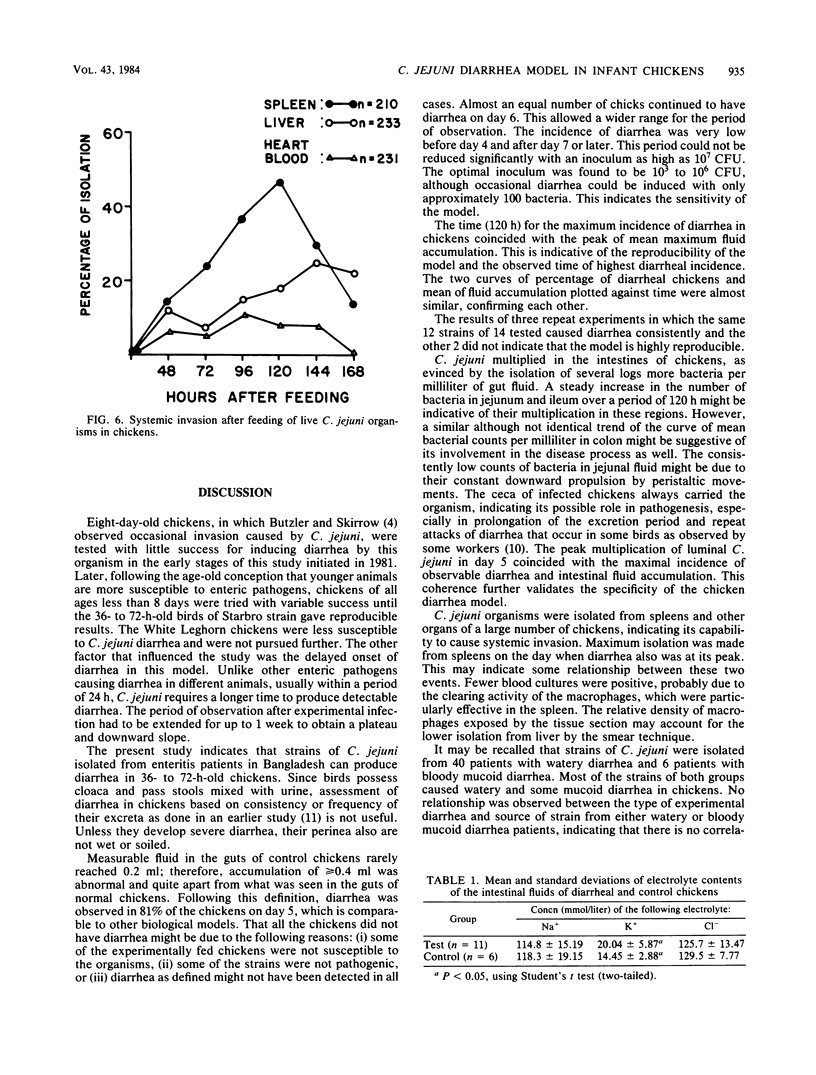
To study the pathogenic mechanisms of Campylobacter jejuni infection, 36- to 72-h-old chickens were fed 10(3) to 10(6) live cells, using strains isolated from 40 patients with watery diarrhea and 6 with bloody mucoid diarrhea from whom no other known enteropathogen was detected. Chickens of Starbro strain were more likely to develop C. jejuni-induced diarrhea than were White Leghorn chickens. Diarrhea was defined on the basis of amounts of gut fluid in 288 chicks fed with live C. jejuni versus 183 saline-fed control as an accumulation greater than or equal to 0.4 ml of fluid in the guts (excluding ceca) of chickens. Twenty-five percent of the chickens developed diarrhea on day 2, 49% on day 4, and 81% on day 5. The intestines, including ceca, were distended with watery fluid. The majority of the strains, irrespective of whether they were isolated from watery or bloody mucoid enteritis patients, caused watery diarrhea in chickens, and a few caused mucoid diarrhea. No correlation was observed between the source of a strain and the outcome in the experimental model. Bloody diarrhea was never observed in chickens. The peak incidence of diarrhea on day 5 coincided with the mean of maximum fluid accumulation. The organisms multiplied by 3 to 4 logs in all parts of the intestine, with a steady increase in number until day 5. Systemic invasion occurred frequently: C. jejuni could be recovered from the spleen in 47% of the chickens on day 5, in 25% from the liver on day 6, and in 11% from heart blood on day 4. Histopathological examination of gut tissue of the chickens having watery diarrhea did not reveal any abnormality except slight submucosal edema. However, in chickens with mucoid diarrhea, the organisms were found to adhere to brush borders and penetrate into the epithelial cells with formation of a breach in continuity of the brush border lining. The electrolyte composition of the intestinal fluid from chickens infected with C. jejuni and from saline-fed controls did not show significant differences, except for depletion of K+ in the test group. The results obtained in this highly reproducible chicken diarrhea model indicate that (i) most chickens develop nonexudative watery diarrhea 2 to 5 days after oral feeding of 10(3) to 10(6) live cells of C. jejuni; (ii) the organism multiples in all parts of a chicken intestine, (iii) systemic invasion is common, and (iv) local invasion is sometimes observed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Mashat R. R., Taylor D. J. Production of diarrhoea and dysentery in experimental calves by feeding pure cultures of Campylobacter fetus subspecies jejuni. Vet Rec. 1980 Nov 15;107(20):459–464. doi: 10.1136/vr.107.20.459. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Berkowitz I. D., LaForce F. M., Cravens J., Reller L. B., Wang W. L. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med. 1979 Aug;91(2):179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Glass R. I., Huq M. I., Stoll B., Kibriya G. M., Alim A. R. Isolation of Campylobacter fetus subsp. jejuni from Bangladeshi children. J Clin Microbiol. 1980 Dec;12(6):744–747. doi: 10.1128/jcm.12.6.744-747.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Firehammer B. D., Myers L. L. Campylobacter fetus subsp jejuni: its possible significance in enteric disease of calves and lambs. Am J Vet Res. 1981 Jun;42(6):918–922. [PubMed] [Google Scholar]
- Manninen K. I., Prescott J. F., Dohoo I. R. Pathogenicity of Campylobacter jejuni isolates from animals and humans. Infect Immun. 1982 Oct;38(1):46–52. doi: 10.1128/iai.38.1.46-52.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J. F., Barker I. K., Manninen K. I., Miniats O. P. Campylobacter jejuni colitis in gnotobiotic dogs. Can J Comp Med. 1981 Oct;45(4):377–383. [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Palacios G. M., Escamilla E., Torres N. Experimental Campylobacter diarrhea in chickens. Infect Immun. 1981 Oct;34(1):250–255. doi: 10.1128/iai.34.1.250-255.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi A. R., Wachsmuth K., Huq M. I., Mahbub M., Agbonlahor D. E. An attempt to detect Yersinia enterocolitica infection in Dacca, Bangladesh. Trop Geogr Med. 1982 Jun;34(2):151–154. [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]