Abstract
Growth cone responses to guidance cues provide the basis for neuronal pathfinding. Although many cues have been identified, less is known about how signals are translated into the cytoskeletal rearrangements that steer directional changes during pathfinding. Here we show that the response of dorsal root ganglion (DRG) neurons to Semaphorin 3A gradients can be divided into two steps: growth cone collapse and retraction. Collapse is inhibited by overexpression of myosin IIA or growth on high substrate-bound laminin-1. Inhibition of collapse also prevents retractions; however collapse can occur without retraction. Inhibition of myosin II activity with blebbistatin or by using neurons from myosin IIB knockouts inhibits retraction. Collapse is associated with movement of myosin IIA from the growth cone to the neurite. Myosin IIB redistributes from a broad distribution to the rear of the growth cone and neck of the connecting neurite. High substrate-bound laminin-1 prevents or reverses these changes. This suggests a model for the Sema 3A response that involves loss of growth cone myosin IIA to facilitate actin meshwork instability and collapse, followed by myosin IIB concentration at the rear of the cone and neck region where it associates with actin bundles to drive retraction.
INTRODUCTION
Correct responses to extracellular signaling molecules during development are essential for the formation of the mammalian central and peripheral nervous systems (Sobeih and Corfas, 2002; Hagg, 2005). Growth cones navigate through the tissue environment in vivo by integrating growth-promoting signals with guidance cue signals at choice points and then responding through two opposing processes: extension and retraction (Buettner, 1994; Dontchev and Letourneau, 2003). This steers the growing axon to the correct target location. Cues are classified as either attractive or repulsive depending on their ability to invoke responses. In vitro attractants generally stimulate growth and turning toward the source of the cue, whereas repulsive cues induce turning away from the cue (Lohof et al., 1992; Fan and Raper, 1995). Repulsive cues can also cause growth cone collapse and temporary cessation of outgrowth (Luo et al., 1993; Wahl et al., 2000). It is unclear to what degree full or partial collapse or even retractions are associated with growth cone steering in vivo (Knobel et al., 1999; Mason and Erskine, 2000; Kalil et al., 2000). The complex in vivo environment is likely to result in a variety of behaviors that reflect the integration of signals from multiple cues within a short time period. Some of these interactions occur at the receptor level (Stein and Tessier-Lavigne, 2001; Halloran and Wolman, 2006), but others may converge on common growth cone effector mechanisms. To understand growth cone behavior responsible for pathfinding in vivo, it is important to fully dissect the responses to individual cues in vitro.
Sema 3A is secreted by cells in the developing spinal cord and repels dorsal root ganglion (DRG) neurons that are sensitive to nerve growth factor (NGF; Quach et al., 2004). The Sema 3A–dependent repulsive response is associated with growth cone collapse and is known to involve the Rho family of small GTPases that act as molecular switches (Jin and Strittmatter, 1997; Kuhn et al., 1999; Turner et al., 2004) and their downstream effectors, the actin cytoskeleton and focal contacts (Fan et al., 1993; Fritsche et al., 1999; Woo and Gomez, 2006). However the pathways that are involved and their relationship to growth cone responses has not been fully characterized. For example, cofilin phosphorylation is necessary, but not sufficient for growth cone collapse induced by Sema 3A (Aizawa et al., 2001).
Recently myosin II has been shown to be a key component for growth cone motility. It contributes to retrograde flow and actin filament organization and advance (Bridgman et al., 2001; Medieros et al., 2006). In addition it is important for turning in response to borders of substrate-bound laminin-1 (Turney and Bridgman, 2005) and for neurite retraction including that induced by Sema 3A (Gallo et al., 2002; Wylie and Chantler, 2003; Gallo, 2006), indicating that it may be of general importance for growth cone steering. Several studies have shown axon retraction can be prevented by inhibiting upstream regulators of myosin motors (Ahmad et al., 2000) or by reducing the amount of active myosin II in neuroblastoma cells (Wylie and Chantler, 2003). Inhibition of Rho kinase, a myosin II regulatory protein, can prevent retraction in response to guidance factors (Wahl et al., 2000; Gallo, 2006). In COS-7 cells coexpressing Plexin-A1 and NP-1, Sema 3A collapse was not dependent on Rho or Rho kinase (Turner et al., 2004). However, COS-7 cells express little or no myosin IIA (Buxton et al., 2003), suggesting that myosin IIA may be particularly important for the Sema 3A response in neurons. DRG neurons express multiple forms of myosin II, including myosin IIA, which has been shown to be involved in neurite retraction of neuroblastoma cells (Wylie and Chantler, 2003). However, it remains unclear if both collapse and retraction are normally part of the response to Sema 3A and if so, to what degree these different phases of response depend on myosin II isoforms. Sema 3A also contributes to the inhibitory environment for growth at sites of spinal cord injury (Niclou et al., 2006; Kaneko et al., 2006). Understanding the mechanisms for the repulsive response to Sema 3A will be important for overcoming barriers to growth after injury. The major goals of this study were to more fully characterize the in vitro response to Sema 3A, to determine if growth-promoting factors can modulate the response, and then to test the hypothesis that the various myosin II isoforms play different roles in the response.
MATERIALS AND METHODS
Cell Culture
DRG neurons from embryonic day (E) 13.5–14 mice were used for all experiments. Peripheral nerve culture methods and the means of identifying myosin IIB knockout (KO) mouse embryos have previously been described by Bridgman et al. (2001). DRG neurons from wild-type or heterozygous mice were used as controls. Explants or cells from the two different populations were plated on separate coverslips coated with poly-l-ornithine (PLO; 0.1 mg/ml) and laminin-1 (9.6 or 32 μg/ml) and then grown overnight at 37°C in medium containing 50 ng/ml NGF (mouse 2.5s, Harlan, Indianapolis, IN; Bridgman et al., 2001). For the collapse assay of fixed cells, 40 min before application of Sema 3A the cultures were switched to low-NGF (5 ng/ml) medium. For most bath application experiments DRG neurons were grown in a culture dish with a glass bottom that was separated into two chambers using a Sylgard seal. The two sides were coated with different concentrations of laminin-1. For imaging experiments using micropipette application, explant cultures were mounted in an open imaging chamber containing either a HEPES/carbonate-buffered mixture (25 mM each) and low-NGF (5 ng/ml) medium or normal carbonate-buffered medium with the same low concentration of NGF. Cultured DRG neurons grow at normal rates in an open chamber using the HEPES buffer system provided the medium is covered with mineral oil to slow pH changes. For time-lapse observations in normal carbonate-buffered medium a microscope-based CO2 incubation system was used.
Application of Sema 3A and Time-Lapse Recording
For bath application Sema 3A was dissolved in low-NFG medium (preequilibrated with CO2 to the correct pH) and then carefully added to the culture. Controls received only new medium. For the collapse assay the cells were fixed at 30 min with 4% paraformaldehyde. For imaging experiments of bath-applied Sema 3A the same procedure was used except that imaging was started before Sema 3A addition, paused, and then started again immediately after application. Imaging was done on an inverted Zeiss LSM 510 Meta confocal microscope (Thornwood, NY) with a transmitted light detector. A 633-nm laser line was used for illumination, and images were taken at 1-min intervals using the multitime macro. An Olympus 20× phase objective (0.7 NA) was used for the imaging (Melville, NY). For local gradient application Sema 3A (7.5 μg/ml in PBS) was applied through a micropipette with a 2-μm tip diameter using continuous pressure pulses (2 Hz, 20-ms pulse, 1–2.5 psi). The gradient produced by the pulses was monitored by including FITC-dextran in the pipette. We confirmed that the FITC-dextran accurately modeled the Sema 3A gradient by comparing Cy3-conjugated Sema 3A with the FITC dextran. The pipette tip was set at a standard 50 μm (in plane distance) from the growth cone at the beginning of the recording. A grid pattern on a video monitor was used to maintain a relatively constant distance between the pipette tip and growth cone. Recordings were standardized for 40 min at 1-min intervals using a Zeiss 40× oil objective (1.0 NA) on a standard Zeiss inverted microscope. Illumination was with a mercury arc lamp attenuated with long-pass and neutral density filters to 10% transmittance. A computer controlled shutter coupled to image capture by a CCD camera kept light exposure to a minimum. For controls, only fluorescent dextran (in PBS) was applied from the micropipette tip. A layer of mineral oil was used to slow pH changes and inhibit evaporation. The pH change with mineral oil overlay was <0.04 pH units/h. Multiple recordings from a single dish were stopped at 2.5–3 h.
Immunofluorescence
For immunofluorescence, cells were prepared as previously described (Brown and Bridgman, 2003). Images were taken on an Olympus confocal or an inverted microscope equipped with a Cooke Sensicam (The Cooke Corporation, Romulus, MI). For ratio imaging and analysis, fixed cells were permeabilized with Triton X-100 and then incubated for 1 h with activated Cy3 (CyDye; Amersham Pharmacia, Piscataway, NJ) to label total protein (Kolega, 2006). After rinsing, cells were treated using standard methods for antibody labeling (Brown and Bridgman, 2003).
Quantitative Analysis
For the collapse assay using fixed cells, collapse was defined and scored as previously described as a loss of lamellipodia and most filopodia (Luo et al., 1993). Fixed cultures were imaged using phase microscopy on the confocal microscope and then scored for collapse. ANOVA and t test's were used to determine significant changes. In time-lapse recordings retraction was defined a priori as a rearward movement of the neurite tip of 5 μm or greater. A 2 × 2 contingency table analysis with Fisher's exact test was used to compare retraction frequencies. For comparison of different outgrowth rates or change in growth areas, an unpaired t test was used using SE for all error bars shown. To determine changes in growth cone area the area of the growth cone was measured before application of Sema 3A and from images taken just before retraction. In the cases where retraction did not occur, the second measurement was taken from an image at the frame nearest to the average time for onset of retraction (14.6 min). The % change in area was then calculated from these measurements using the formula (Areastart − Areaend/Areastart) × 100. Collapse was defined as a decrease in area greater than 50%.
For ratio imaging and analysis Cy3 dye images of growth cones (or subregions) were individually traced by hand using the ROI tool in IPLab (Scanalytics, Billerica, MA). If no discernable growth cone was present, the last 10 μm of the neurite was used for analysis. The ROI boundary was then transferred to the complementary immunofluorescence image. The mean intensity values per pixel within the complementary ROIs were measured and expressed as a ratio. Image exposures were taken at times to keep the intensity values within the linear range of the camera or detector. Analysis of camera sensitivity indicated that the slope of the intensity curve differed by 10% at the different wavelengths used. However both controls and Sema 3A-treated ratios should be equally affected, allowing accurate comparisons of fluorescence intensity. For most of the analysis we did not separate collapsed from noncollapsed growth cones because a preliminary assessment indicated that segregating the growth cones into such classes did not appear to substantially change the results. However in a few cases where segregating the growth cones into collapsed and noncollapsed would potentially give more information, the two classes were analyzed separately.
Cell Transfection
Cells were transfected by two different methods. The first method used biolistics ∼5 h after plating (Bridgman et al., 2003). The second method used a nucleofector device (Amaxa, Gaithersburg, MD) before plating. The latter gave higher rates of transfection for low-density cultures (program G-013), but otherwise the relative levels of expression (based on fluorescence intensity) were indistinguishable. Some live cultures were imaged before and after bath application of Sema 3A, but most quantitation of collapse was done on fixed cultures.
Reagents
Stock solutions of blebbistatin (100 mM; Calbiochem, La Jolla, CA), active (−) and inactive (+) forms, were prepared in DMSO and stored at −70°C. For treatment of cultures the stock was diluted in warm culture medium, immediately mixed, and then filtered through a 0.2-μm filter. The active (−) form was used at a standard concentration of 10 μM except where indicated. The inactive (+) form was used at either 10 or 100 μM. Latrunculin A was from Invitrogen (Carlsbad, CA). Sema 3A was purchased from R&D Systems (Minneapolis, MN). Stock solutions were stored at −70°C for up to 3 mo. Positive controls (Sema 3A–induced retraction) were run before all experiments. All other reagents were purchased from Sigma (St. Louis, MO).
RESULTS
Bath-applied Sema 3A Induces Frequent Retractions above a Threshold Concentration
Previous work has shown that DRG neurons respond to high concentrations of bath-applied Sema 3A by collapse and retraction (Gallo, 2006). Retraction, but not collapse, was shown to be myosin II dependent. However, the relationship between these two different phases remains unclear. To understand these processes more fully and for comparison purposes we first studied the responses of DRG neurons to different concentrations of bath-applied Sema 3A using time-lapse imaging.
Time-lapse observations of control DRG explants growing in low-NGF medium (5 ng/ml) and on low laminin-1 (coated with 9.6 μg/ml) revealed concentration-dependent responses to bath-applied Sema 3A. Applications of relatively high concentrations of Sema 3A (800 ng/ml) produced collapse or retraction of most neurites visible in the field. For instance in one experiment from a recording of 23 neurons, 39% retracted and another 39% collapsed but did not retract. Lower concentrations (600 ng/ml) produced a similar response. For example, in one 30-min recording some neurites retracted (33%) and others collapsed and stopped advance (40%), whereas some continued to show motility and advance (27%). However, if the concentration of Sema 3A was reduced further (500 ng/ml), the mix of responses remained, but the proportions change: in one 30-min recording 7% of neurites retracted, 51% collapsed and stopped advance, and 42% continued to advance. This indicates that for bath application of Sema 3A, concentrations in the range of 600–800 ng/ml are more likely to induce collapse and retraction, whereas concentrations of 500 ng/ml or lower tend to produce only collapse. At 500 ng/ml Sema 3A retraction sometimes occurs in neurons that are adjacent to those undergoing advance (Figure 1, A–D). In recordings from control cultures spontaneous retractions were sometimes seen, but at a very low rate (three retractions observed in 76 neurons recorded for 2.5 h). Therefore both collapse and retraction are normal components of the DRG neuronal response to bath-applied Sema 3A, but high concentrations are required for frequent retraction. When using bath application it is often difficult to determine if collapse precedes retraction, because retraction is usually abrupt.
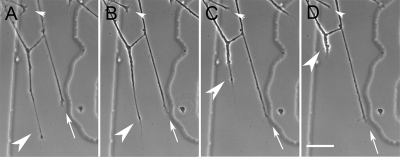
Figure 1.
Characterization of DRG growth cone responses to bath-applied Sema 3A. (A–D) Time-lapse images showing the three responses observed during bath application of Sema 3A. A growth cone collapses (small white arrowhead) while another retracts (large white arrowhead). A third growth cone extends normally (arrow). Images are at 2-min intervals. Scale bar, 20 μm.
Gradient Application of Sema 3A Produces a Distinct Biphasic Response: Collapse Followed by Retraction
Bath application of Sema 3A has several drawbacks. Because the entire neuron is exposed to the Sema 3A, the response may involve both the entire neurite and the growth cone. Bath application also results in desensitization, and neurons recover from the application over time, particularly when using lower Sema 3A concentrations. In addition, we noticed in time-lapse observations that the nonneuronal cells in explant cultures sometimes pull on neurites, and this pulling may potentially increase upon application of Sema 3A, influencing the retraction response. To more fully investigate the changes in growth cone morphology and the roles of myosin II in the response to Sema 3A, we switched from bath application to micropipette application (Lohof et al., 1992). This was to ensure that the response involves only the growth cone and distal portion of the neurite. Pulsed application from a micropipette allows the formation of gradients that may more closely resemble the interaction of growth cones with diffusible guidance cues. The tip of the micropipette is positioned so that it is roughly aligned with the neurite and is 50 μm from the growth cone leading edge. Pulsed application of Sema 3A to DRG neurons grown on low laminin-1 resulted in a consistent biphasic response; growth cones collapsed and then retracted (Figure 2, A–C). Collapse of peripheral extensions (filopodia and lamellipodia) always preceded retraction of the growth cone central domain. The collapse phase was associated with a significant and substantial decrease in growth cone area (reduction in area = 76.9%, n = 10, p = 0.0001; Supplemental Figure S1) before retraction. The decrease in area results in the elimination of lamellipodia and most filopodia (Figure 2B), consistent with previous studies documenting collapse (Luo et al., 1993). The frequency of retraction (90%) with micropipette application was consistently higher than with the highest concentration used in bath application of Sema 3A (800 ng/ml).
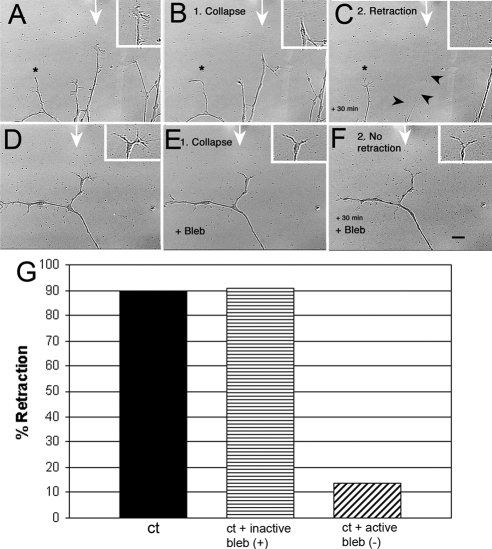
Figure 2.
DRG neurons for control mice show myosin II–dependent retraction in response to a Sema 3A gradient. Cultures were preincubated with either 100 μm inactive blebbistatin (+) or 10 μm active blebbistatin (−) for 30 min. A gradient of Sema 3A (7.5 μg/ml in the pipette) was applied for 30 min during time-lapse imaging (1-min intervals) via micropipette. The time-lapse movie was used to score for extension, collapse, retraction, and turning. (A–C) A sequence showing typical DRG neurite responses to a Sema 3A gradient when cells are grown on low laminin-1. The micropipette tip was just outside the field at the point indicated by the white arrow. A growth cone in line with the Sema 3A gradient first collapses (15 min) (B) and then retracts (30 min; C). Arrowheads, residual retracted neurite. An adjacent neurite (*) further from the gradient does not retract. (D–F) Blebbistatin (Bleb) treatment does not prevent collapse (E), but does prevent retraction (F). Scale bar, 10 μm. (G) Inactive blebbistatin (+) had no effect on retraction compared with untreated controls, but active blebbistatin (−) caused a significant drop in the number of cells retracting (p = 0.0001; Ct, n = 28; Bleb (−), n = 37; Bleb inactive, n = 11) indicating that myosin II activity is necessary for retraction in response to Sema 3A gradients.
Retraction Induced by a Sema 3A Gradient is Myosin II Dependent
Retraction in response to bath-applied Sema 3A is myosin II dependent (Gallo, 2006). To better understand the isoform dependent role of myosin II in the Sema 3A response to a gradient, we first inhibited all isoforms of myosin II with blebbistatin (Straight et al., 2003), a specific inhibitor for the force-producing state of myosin II (Allingham et al., 2005), which can has been shown to inhibit endothelial cell contraction (Goeckeler et al., 2008). Cultured DRG neurons were preincubated (1–3 h) with medium containing blebbistatin. Untreated neurons or DRG neurons treated with an inactive form of blebbistatin collapsed and then retracted in response to application of Sema 3A (Figure 2, Supplemental Figure S1), whereas those pretreated with the active form of blebbistatin collapsed but rarely retracted (Figure 2, D–F). The differences in retraction were statistically significant (Figure 2G). The active form of blebbistatin only prevents retraction, and although collapse meets our minimum criterion (Supplemental Figure S1), it was less dramatic (52.4% decrease in area, n = 10) but still significant (p = 0.0072). This is probably because growth cones of blebbistatin-treated neurons are smaller before Sema 3A application, resulting in less change in area. These results indicate that blebbistatin inhibits retraction, but not collapse, in response to Sema 3A gradients. This is consistent with previous results obtained using bath application of Sema 3A (Gallo, 2006).
Blebbistatin treatment has persistent effects on retraction. Pretreatment with blebbistatin, followed by Sema 3A application in medium without blebbistatin, produces a similar initial response (collapse followed by greatly a reduced incidence of retraction). The low retraction rate remains the same until ∼90 min after removal of the blebbistatin. The cells then recover from the blebbistatin treatment, and the retraction rate returns to that observed in the absence of blebbistatin (90% retracted, n = 12).
Increasing the concentration of the active form of blebbistatin used for treatment from 10 to 100 μM does not significantly change the frequency of Sema 3A–dependent retraction (data not shown), but does significantly retard the rate of outgrowth of neurites during the 40-min recording period by ∼40% (decreased average rate by 20 μm/h, n = 17, 10 respectively; t test, p = 0.038; Supplemental Figure S2).
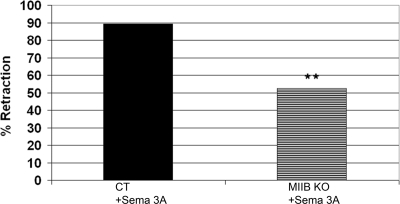
Myosin IIB Activity Contributes to Retraction
Previous work has shown that growth cones from myosin IIB KO mice have defects in motility, are smaller in area, have reduced filopodia-mediated traction force, and do not consistently turn at laminin-1 borders (Bridgman et al., 2001; Turney and Bridgman, 2005). It has been suggested that myosin IIB is mainly responsible for outgrowth, whereas myosin IIA is responsible for adhesion and retraction (Wylie et al., 1998; Wylie and Chantler, 2003). To test whether this segregation of function could be applied to Sema 3A–induced retraction, we tested DRG neurons from myosin IIB KO mice using the micropipette application. Sema 3A–treated DRG neurons derived from KO embryos showed significantly reduced retraction rates (Figure 3). Collapse of growth cones, though less pronounced because of the smaller area, still occurs even in the absence of retraction (decrease in average area = 51.6%, n = 9, p = 0.0005). This indicates that myosin IIB contributes to the Sema 3A–induced retraction phase. Because the absence of myosin IIB does not fully eliminate retraction, whereas treatment with the general myosin II inhibitor blebbistatin nearly does, other myosin II isoforms (myosin IIA and/or myosin IIC) are likely to also contribute to the retraction response, indicating partial overlap in function.
Figure 3.
Retraction in response to a Sema 3A gradient is partially myosin IIB–dependent. DRG neurons from control type (ct) or myosin IIB knockout (KO) where subjected to a Sema 3A gradient during time-lapse imaging and scored for extension, collapse, or retraction. Myosin IIB knockout neurites showed a significant (36.9%) reduction in retraction frequency (p = 0.0075; Ct, n = 28; KO, n = 25).
Relationship between Collapse and Retraction in Sema 3A Gradients
The consistent biphasic reaction of growth cones to a Sema 3A gradient application (collapse followed by retraction) is different from that typically observed with bath application. Bath application usually results in collapse, but retraction is rarer except at relatively high concentrations of Sema 3A (Gallo, 2006). In addition, when retraction occurs in response to bath-applied Sema 3A, the response is relatively rapid and without a distinct biphasic response. To test if this difference is due to the interaction of a polarized growth cone structure with a Sema 3A gradient, we apply the gradient at different orientations. In all the previous trials the microelectrode was roughly aligned parallel to the neurite, and the pipette tip was 50 μm from the leading edge of the growth cone. Positioning the pipette perpendicular to this orientation at an equal distance from the side of the growth cone causes the same collapse and retraction response (92% collapsed and then retracted, n = 12, Table 1A). Keeping the same orientation, but moving the electrode toward the cell body so that only the neurite is exposed to the gradient eliminates the collapse and retraction response (6.3% retraction, n = 16, Table 1A). Positioning the microelectrode 180° from the original position so that the tip is the same 50-μm distance from the growth cone, but the gradient encounters the connecting neurite and rear of the cone first, also causes retraction. In this orientation retraction occurs significantly faster (onset occurs at 5.2 ± 1.1 vs. 14.6 ± 5.3 min, ±SD, n = 10 for each; p = 0.0001, Table 1B). Although collapse begins, the change in growth cone area is less, suggesting that collapse is incomplete upon the onset of retraction (decrease in growth cone area = 24 vs. 76.8%, n = 10 each, Table 1C). When the microelectrode tip is positioned so that only the neck of the growth cone and connecting neurite is exposed to Sema 3A, a swelling of that area is observed, and this enlarged mass moves back along the process as application continues. The growth cone does not collapse or retract; however, no significant advance was seen. Taken together these results suggest that the machinery required for retraction may partially reside at the rear of the cone and connecting neurite, but relocalization of the machinery may be required to enhance the retraction response.
Table 1.
Effect of micropipette orientation on the growth cone responses
| A. Retraction vs. orientation | ||
|---|---|---|
| % Retraction | n | |
| Ct | 89.3 | 28 |
| Puffing behind growth cone | 89.5 | 19 |
| Puffing on cell body | 6.3 | 16 |
| B. Retraction speed with regard to Sema 3A gradient orientation | ||||||
|---|---|---|---|---|---|---|
| Time of retraction onset (min) | Time of retraction finish (min) | SD start | SD finish | n | p | |
| Ct puffing in front | 14.6 | 26.9 | 5.3 | 7.5 | 10 | |
| Puffing behind growth cone | 5.2 | 13.8 | 1.1 | 2.4 | 10 | 0.0001 |
| C. Growth cone collapse | |||
|---|---|---|---|
| % Area lost | n | p | |
| Ct | 76.8 | 10 | |
| Puffing behind growth cone | 24 | 10 | 0.0001 |
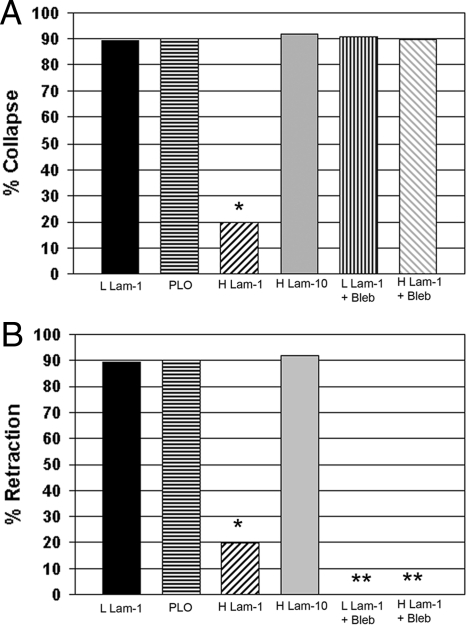
Increasing the Concentration of Substrate-bound laminin-1 Inhibits Collapse and Retraction in Response to Gradients of Sema 3A
The responsiveness of guidance cues can be influenced by the growth substrate (Hopker et al., 1999; Nguyen-Ba-Charvet et al., 2001). To test the dependence of micropipette applied Sema 3A on substrate concentration or composition, we compared responses of neurons growing on substrates coated with two concentrations of laminin-1. Neurons grown on substrates coated with the two concentrations of laminin-1 exhibited identical rates of outgrowth and had the same growth cone areas (Supplemental Figures S1 and S2). However, growth cones on high laminin-1 rarely collapsed during Sema 3A treatment (Figure 4A; change in growth cone area was not significant, n = 9, p = 0.38; Supplemental Figure S2), but outgrowth sometimes ceased during the time of recording. Furthermore, the frequency of retraction in response to Sema 3A application was significantly reduced (Figure 4B). This indicates that both collapse and retraction can be inhibited by increasing the amount of substrate-bound laminin-1 when exposed to a set gradient of Sema 3A. However, if the concentration of Sema 3A in the micropipette was increased from 7.5 to 50 μM, then three of four growth cones tested collapsed and then retracted. This indicates that the inhibition of collapse and retraction can be overridden by increasing the level of Sema 3A exposure. To evaluate whether this protective quality of laminin-1 was the result of general substrate adhesiveness or the product of more specific interactions, DRGs were grown on substrates coated with a high concentration of laminin-10 or PLO. Although laminin-10 produced the same outgrowth rate and growth cone area as laminin-1 (Supplemental Figures S1 and S2) and may have similar adhesive properties, it does not bind the same integrins (Ferletta and Ekblom, 1999). Poly-l-ornithine is a synthetic polymer very similar to poly-l-lysine that reduces outgrowth rates (Lochter et al., 1995; Turney and Bridgman, 2005), but is similar in adhesive strength to laminin-1 when the concentration is appropriately adjusted (Zheng et al., 1994). Both of these alternative substrates failed to prevent collapse and retraction in response to micropipette application of Sema 3A (Figure 4), suggesting that laminin-1 has specific protective affects on growth cones when bound to the substrate above a certain concentration.
Figure 4.
Increased amounts of substrate-bound laminin-1 can reduce the frequency of collapse and retraction in response to a Sema 3A gradient. Inhibiting myosin II with Bleb only prevents retraction. DRG cultures (normal control-type cells) on coverslips coated with low laminin-1 (9.6 μg/ml), poly-l-ornithine (PLO-0.1 mg/ml), high laminin-1 (32 μg/ml), or high laminin-10 (32 μg/ml) were subjected to a Sema 3A gradient and then scored for collapse (A) and retraction (B). (A) Only high laminin-1 prevented collapse (*p = 0.0001, n = 25 for each condition). Addition of active Bleb to DRG neurons growing on either low laminin-1 or high laminin-1 before exposure to the Sema 3A gradient resulted in collapse. Therefore Bleb treatment eliminated the protective effect of high laminin-1. (B) The same cells were also scored for retraction. High laminin-1 significantly inhibited retraction at the same rate as collapse (*p = 0.0001, n = 25). Active Bleb inhibited retraction for cells growing on both low and high laminin-1. *No retraction was observed in the Bleb-treated cells.
To determine if the amount of laminin-1 bound to the substrate is significantly greater on coverslips coated with the higher concentrations in solution, we bound increasing concentrations of fluorescently conjugated laminin-1 (mixed with unconjugated at the same concentration 1:4) to poly-l-lysine– or PLO-coated coverslips for different times. Two- or 24-h incubation produced different binding curves (Supplemental Figure S3), but in both cases 32 μg/ml laminin-1 produced significantly brighter substrates than 10 μg/ml. For 24-h incubations the difference was about fourfold. The increased brightness also correlated with a more even coating. We also compared the ratio of immunofluorescence intensity on coverslips coated for the shorter time (2 h) with the two different concentrations in separate areas. The higher concentration has a twofold greater intensity (Supplemental Data Table S1). From this data we conclude that increasing the concentration of laminin-1 applied to the substrate from 10 to 32 μg/ml increases the amount that binds to the substrate (per μm2). This probably increases the amount of laminin-1 contacted by the growth cone at any one time and potentially leads to greater sustained integrin activation (Lemons and Condic, 2006). This may provide the basis for the difference in response to Sema 3A, although other mechanisms such as increased adhesion may also contribute.
The Ability of Laminin-1 to Prevent Growth Cone Collapse in Response to Sema 3A Depends on Myosin II Activity
High concentrations of substrate-bound laminin-1 can reduce growth cone collapse in response to micropipette Sema 3A. This effect seems to be independent of adhesive strength or ability to stimulate outgrowth suggesting that it may involve a myosin-independent stabilization of the growth cone cytoskeleton. To determine if this was the case, DRG explants were grown on high concentrations of laminin-1 and were incubated with 10 μM blebbistatin 30 min before application of Sema 3A via micropipette to the growth cone. Surprisingly, inhibiting myosin II activity prevented the high concentration of substrate-bound laminin-1 from protecting against Sema 3A–induced collapse (Figure 4A). Blebbistatin treatment did inhibit retraction (Figure 4B). Because blebbistatin is a general inhibitor of myosin II activity, it suggests that signaling through integrins may mediate a myosin II–dependent stabilization of the actin cytoskeleton when laminin-1 is highly concentrated on the substrate to inhibit collapse during Sema 3A treatment.
Collapse Occurs over a Wide Concentration Range and Is the Main Response to Low Concentrations of Bath-applied Sema 3A
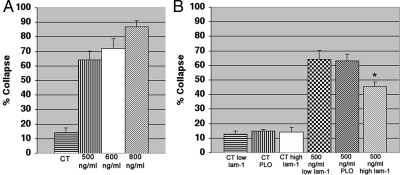
Fixed cultures have been used to score the collapse response to Sema 3A in many studies (Campbell et al., 2001) and is much more amenable to comparing changes in levels of proteins using immunostaining of cells fixed during the response (Eickholt et al., 2002; Brown et al., 2004). Therefore we used this approach for quantitative comparisons of collapse and protein levels using immunofluorescence staining. Retraction is likely to be missed in assays of fixed cultures because retracted neurons are not easily identified. Therefore we did not attempt to score for retracted neurons. The collapse frequencies of fixed cultures were consistent with the responses observed by time-lapse recording of bath-applied Sema 3A. As expected, collapse frequencies increased with higher concentrations of Sema 3A (Figure 5A). Although the concentrations differ from that indicated by the manufacturer for inducing collapse, they are consistent with results obtained using the same source for Sema 3A from a recent report (Gallo, 2006). At relatively low concentrations of Sema 3A (500 ng/ml) that rarely cause retraction, collapse occurred in greater than 60% of growth cones (Figure 5A). Thus at this concentration of Sema 3A collapse predominates, but many growth cones appeared relatively unperturbed.
Figure 5.
Bath applied Sema 3A produces mainly collapse at low concentrations and the response is affected by the amount of substrate-bound laminin-1. (A) The concentration dependence of collapse to bath-applied Sema 3A. DRG cultures were fixed at 30 min of exposure. (B) The amount of substrate-bound laminin-1 has no effect on spontaneous collapse in controls (CT). On exposure to Sema 3A (500 ng/ml) the frequency of collapse on high laminin (*) was significantly reduced compared with low laminin (p = 0.034, n = 4 explants for each condition with a minimum 40 growth cones per explant).
Collapse in Response to Bath-applied Sema 3A Is Inhibited by High Concentrations of Substrate-bound Laminin-1
Laminin-1 has been shown to affect the response of growth cones to several different guidance cues (Hopker et al., 1999; Nguyen-Ba-Charvet et al., 2001), and we observed that increasing the amount bound to the substrate protects against collapse and retraction in response to Sema 3A gradients (Figure 4). Therefore we also tested the effects of coating the substrate with the two different concentrations of laminin-1 on bath-applied Sema 3A. When 500 ng/ml Sema 3A was applied to the bath during time-lapse recordings of neurons growing on substrates coated with the higher concentration of laminin, the collapse responses were typically attenuated, suggesting a protective effect for the high concentration of laminin-1. From the more extensive analysis of fixed cultures, this protective effect was found to be only significant in response to 500 ng/ml Sema 3A (Figure 5B). Coating coverslips with the higher concentration of laminin-1 had no significant effects on responses to bath-applied Sema 3A at higher concentrations (600–800 ng/ml; Supplemental Figure S4). Both concentrations of substrate-bound laminin-1 gave the same outgrowth rates (Supplemental Figure S2).
In addition to using two different concentrations of laminin-1, we compared the frequency of collapse in response to 500 ng/ml Sema 3A between neurons grown on substrates coated with low laminin-1 with those grown on substrates coated with PLO. Although there was a large decrease in outgrowth rate on PLO compared with low laminin, there was no difference in collapse frequency (Figure 5B). This indicates that high concentrations of substrate-bound laminin-1 protect against collapse, and a decrease in growth-promoting activity alone is insufficient to induce collapse. It also indicates that collapse may not require integrin-dependent pathways, but can potentially be influenced by these pathways when activated by high concentrations of substrate-bound laminin.
DRG Growth Cones Contain All Three Isoforms (IIA, IIB, and IIC) of Nonmuscle Myosin II
The Sema 3A response involves multiple mechanisms, including the disruption of the actin cytoskeletal and focal complexes (Fan et al., 1993; Fritsche et al., 1999; Woo and Gomez, 2006). Collapse of COS-7 cells coexpressing Plexin-A1 and NP-1 does not seem to involve contractile responses (Turner et al., 2004); however, retraction of DRG neurons does involve myosin II (Gallo, 2006). Rodent peripheral nerves and their growth cones have been shown to contain all three isoforms of nonmuscle myosin II (Rochlin et al., 1995; Turney and Bridgman, 2005), unlike COS-7 cells that lack myosin IIA. This suggests that the different isoforms may contribute in varying degrees to the retraction and collapse responses to Sema 3A. To confirm that all three isoforms are present in DRG neurons, we used immunostaining (Supplemental Figure S5). All three were present and had varying distributions similar to those previously reported for superior cervical ganglion (SCG) neurons (Rochlin et al., 1995; Turney and Bridgman, 2005), although myosin IIA was consistently more peripherally located in DRG neurons compared with SCG.
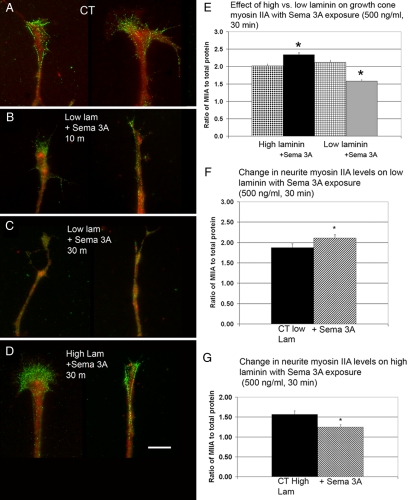
Quantitative Differences in Myosin IIA Immunostaining after Bath-applied Sema 3A Are Dependent on the Concentration of Substrate-bound Laminin-1
If increased activation of integrins by high laminin-1 stabilizes the actin cytoskeleton through a myosin II–dependent mechanism, then it may involve a specific myosin II isoform. Therefore we stained for specific myosin II isoforms in the DRG neurons growing on either high or low substrate-bound laminin-1 and after bath-applied Sema 3A (500 and 800 ng/ml; Figures 6–8). To compensate for changes in morphology, the cells were stained for total protein and the average intensity of staining was expressed as ratios. Treatment of DRG neurons growing on low laminin with low concentrations (500 ng/ml) for 10–30 min caused a progressive drop in the growth cone myosin IIA–staining ratio (Figure 6, A–C and E). In contrast DRG neurons growing on high laminin treated with low concentrations of Sema 3A (500 ng/ml) for the same amounts of time showed a small but significant increase in the growth cone myosin IIA–staining ratio (Figure 6, D and E). Thus treatments that normally induce mainly collapse induced a significant drop in growth cone myosin IIA–staining intensities suggesting that the loss of myosin IIA from the growth cone roughly coincides with collapse. The increase in myosin IIA–staining ratio caused by Sema 3A treatment on high laminin occurs under a condition where collapse rates are <50%, suggesting that the noncollapsed growth cones influenced the overall result. Therefore, we analyzed collapsed and noncollapsed growth cones separately. Only the noncollapsed growth cones showed a significant increase in the myosin IIA–staining ratio compared with controls (CT = 1.30 ± 0.07, collapsed = 1.5 ± 0.09, noncollapsed = 1.6±.07, n = 40, 23, and 20, p = 0.03).
Figure 6.
Low concentrations of bath-applied Sema 3A decrease growth cone Myosin IIA immunofluorescence staining intensity ratio. High laminin-1 produces the opposite effect. (A) Control (CT) DRG neurons grown on low laminin and then fixed and antibody labeled for myosin IIA (green) and stained for total protein (red). (B) Antibody labeling for myosin IIA and total protein staining after 10 min of Sema 3A treatment (500 ng/ml). The myosin IIA immunofluorescence staining appears more diffuse. Cells were growing on low laminin. (C) Antibody labeling for myosin IIA and staining for total protein after 30 min of Sema 3A treatment. Growth cones have partially collapsed and the myosin IIA staining is decreased. Cells were growing on low laminin-1. (D) Cells labeled for myosin IIA and total protein after 30 min exposure to bath-applied Sema 3A (500 ng/ml). Cells shown were growing on high laminin-1 and did not collapse. Myosin IIA labeling appears similar to control. Scale bar, 5 μm. (E) For quantitative comparisons the antibody staining fluorescence intensity in the growth cone was expressed as a ratio to fluorescence labeling intensity of total protein using activated Cy3 dye. Cells exposed to Sema 3A (500 ng/ml) for 30 min growing on high laminin-1 showed a significant increased in myosin IIA immunofluorescence staining (*p = 0.0001). In contrast cells exposed to Sema 3A (500 ng/ml) for 30 min growing on low laminin-1 showed significantly decreased staining for myosin IIA (*p = 0.0001). For each condition N = 40 growth cones. (F) Myosin IIA staining redistributes within the neurite after Sema 3A exposure. The same cells used for E were analyzed for changes in myosin II staining intensity in the neurite after Sema 3A (500 ng/ml) for 30 min. The ratio of myosin IIA to total protein in the neurite, defined as the segment starting 50 μm behind the growth cone. In cases where growth cones were collapsed this measurement was taken starting 10 μm from the tip of the process. The addition of Sema 3A significantly increased the staining ratio for myosin IIA (*p = 0.044, n = 25). (G) The same analysis was done for cells growing on high laminin. Myosin IIA staining ratio in the neurite showed a significant decrease (*p = 0.0037, n = 40). Thus the changes are the opposite of those shown in E, suggesting movement of myosin IIA between the growth cone and neurite.
Figure 7.
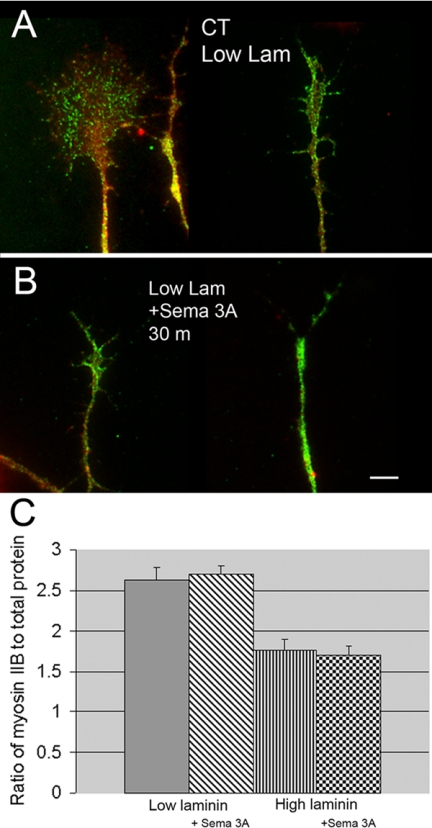
Low concentrations (500 ng/ml) of bath-applied Sema 3A do not change growth cone myosin IIB immunofluorescence staining intensity ratios. (A) Growth cones for control type DRG neurons growing on low laminin-1 fixed and antibody labeled for myosin IIB (green) and stained for total protein (red). (B) Antibody labeling for myosin IIB and staining for total protein after 30 min of Sema 3A treatment (500 ng/ml). Growth cones have partially collapsed but the myosin IIB staining intensity appears unchanged. Cells were growing on low laminin. The staining results on high laminin were the same except that collapse was inhibited. (C) For quantitative comparisons the antibody staining fluorescence intensity in the growth cone was expressed as a ratio to fluorescence labeling intensity of total protein using activated Cy3 dye. Cells exposed to Sema 3A (500 ng/ml) for 30 min growing on either low or high laminin-1 showed no significant change in the myosin IIB immunofluorescence staining ratios (low laminin p = 0.7, n = 60, high laminin p = 0.6, n = 25). Scale bar, 6 μm.
Figure 8.
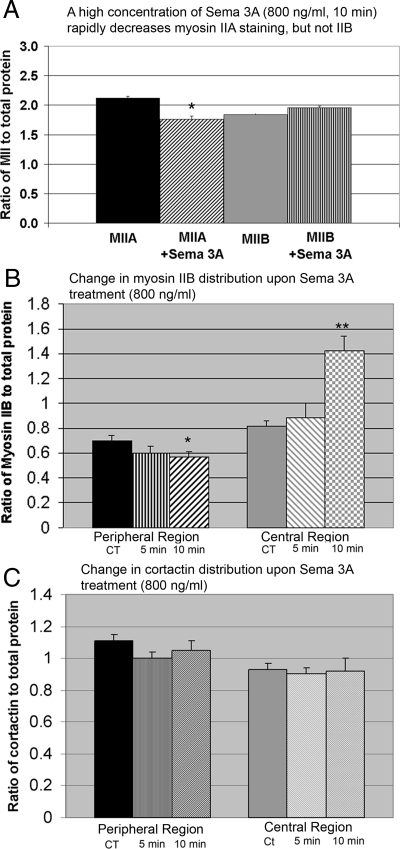
High concentrations of bath-applied Sema 3A induce changes in total growth cone myosin IIA–staining intensity ratio, but not myosin IIB. (A) Exposure of DRG neurons to bath-applied Sema 3A at a high concentration (800 ng/ml) for 10 min induced a significant decrease in myosin IIA–staining ratio. Under the same conditions, the myosin IIB staining ratio did not change. Most growth cones collapse and many neurites retract under these conditions. For fully collapsed neurites the analysis was performed on the last 10 μm. Retracted neurites were not included in the analysis. (B) High concentrations (800 ng/ml) of bath-applied Sema 3A induce redistributions of myosin IIB in the growth cone at short times (5 and 10 min). The myosin IIB staining ratio was analyzed in peripheral and central regions of growth cones. Only noncollapsed growth cones were selected for analysis at the different time points. For comparison the immunofluorescence staining intensity of a specific actin-binding protein (cortactin) was analyzed in the same growth cones (C). Peripheral myosin IIB showed a significant decrease at 10 min (*p = 0.0001, n = 18) and a corresponding significant increase in the central region (*p = 0.03, n = 18). The magnitude of the increase in the central region was greater than the decrease in peripheral region. However the area of the peripheral region is much larger than the central region. (C) In the same growth cones the cortactin staining ratio did not significantly change.
The changes in staining intensity could result from loss or addition of myosin IIA bipolar filaments within the growth cone (Bridgman, 2002). Myosin IIA has been shown to redistribute in other cell types, and this movement is inhibited by filament assembly (Kolega, 2006). Thus myosin IIA filament disassembly could lead to movement between the growth cone and the neurite. Therefore we determined the ratio in staining intensity of myosin IIA with and without Sema 3A application in the neurite, 50 μm behind the neck of the growth cone in the same cells. For cells growing on low laminin-1, the staining ratio significantly increases upon Sema 3A application (Figure 6F). In contrast, the cells growing on high laminin-1 showed a decrease in neurite myosin IIA staining in response to Sema 3A (Figure 6G). These data strongly suggest that the change in the levels of myosin IIA staining observed in response to Sema 3A treatment is mainly due to myosin IIA movement between the growth cone and the neurite.
Under the same conditions and treatments there was no detectable change in the myosin IIB staining ratio in growth cones (Figure 7). This suggests that growth cone myosin IIB levels on average are unaffected by increased substrate-bound laminin-1 or low concentrations of Sema 3A.
To determine if higher concentrations of Sema 3A that normally induce collapse and retraction might alter the distribution of myosin IIB, we analyzed cells treated for short times with high concentrations of Sema 3A. Treatment with high concentrations of Sema 3A (800 ng/ml) of cells grown on low laminin produced a significant drop in the myosin IIA–staining ratio in cells that had not retracted. The decrease in staining was similar to that observed with low concentrations of Sema 3A except that the effect was detectable in a shorter time (Figure 8). There was no significant change in the overall growth cone myosin IIB staining under the same conditions (Figure 8). However, we noticed that myosin IIB seemed to be brighter in the central region of partially or noncollapsed growth cones after Sema 3A application. Therefore we analyzed growth cones for regional differences in myosin IIB immunostaining induced by Sema 3A treatment at different times (5 and 10 min). Under the treatment conditions many growth cones had collapsed, but a minority population of growth cones was still present (collapse frequency: 72 ± 5% at 5 min and 81 ± 4% at 10 min). Only growth cones that had not collapsed were selected for analysis to determine if myosin IIB redistributes at early time points before collapse. For this analysis we used the total protein (Cy3) staining intensity to define the central and peripheral regions of growth cones because their shapes are highly variable. We compared the myosin IIB staining to immunostaining for cortactin in the same growth cones. Cortactin is an actin-binding protein that associates preferentially with actin meshworks in growth cones (Cosen-Binker and Kapus, 2006). Peripheral myosin IIB staining progressively decreased upon Sema 3A treatment, and the difference was significant at 10 min (Figure 8B). Staining for myosin IIB significantly increased in the central region at the same time point, but the increase was of greater magnitude. In contrast cortactin staining did not significantly change in the same growth cones (Figure 8C). Normally cortactin staining decreases with longer treatments of Sema 3A (500 ng/ml for 30 min) that induce collapse (data not shown). This indicates that in the absence of collapse, myosin IIB partially redistributes from the periphery to the central region of the cone upon Sema 3A treatment at early time points. The change is before that observed for actin-binding proteins that redistribute with collapse. Further redistribution may lead to collapse and/or retraction in some cells at a later time because the collapse rate in response to bath-applied Sema 3A at this concentration (800 ng/ml) increased to 87% at 30 min (Supplemental Figure S4). This suggests that rearward movement and concentration of myosin IIB at the rear of the cone and neck may occur before collapse and contribute to retraction. This conclusion should be taken with caution because in collapse assays using fixed cells retraction is not scored and so we cannot correlate the change in myosin IIB distribution directly to retraction. However in gradients of Sema 3A collapse appears to be required for retraction. If the noncollapsed cells that were analyzed were caught as they were about to retract, then the myosin IIB in the rear of the cone and neck is in position to drive retraction, but has not gone to completion yet. Rearward movement of green fluorescent protein (GFP)-myosin IIB in live growth cones does correlate closely with collapse induced by lower concentrations of Sema 3A (Supplemental Figure S6) that only rarely induce retraction, indicating that the redistribution may also begin to occur in cells that only collapse.
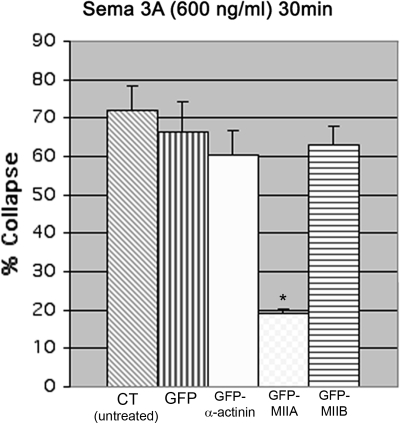
Overexpression of Myosin IIA, But Not IIB Can Prevent Collapse in Response to Sema 3A
Bath-applied Sema 3A produces a decrease in MIIA staining in growth cones on low laminin-1. This change is prevented by high laminin-1 and is associated with inhibition of collapse. Therefore we tested whether overexpression of myosin IIA could prevent collapse of cells on low laminin-1. Cells were transfected with GFP-MIIA or for comparison with either GFP-MIIB or the actin-bundling protein GFP-α-actinin. Transfection with GFP alone was also used as a control. Expression of GFP, GFP-α-actinin or GFP-MIIB did not prevent collapse or retraction in response to high concentrations of bath-applied Sema 3A. The expressed GFP-myosin IIB did show redistribution during treatment that correlated with collapse (Supplemental Figure S6). In contrast overexpression of GFP-MIIA significantly reduced the number of growth cones that collapsed in response to Sema 3A (Figure 9). Overexpression of the IIA heavy chain is likely to increase the number of myosin IIA bipolar filaments in growth cones (Bridgman, 2002). If collapse requires that the number of myosin IIA bipolar filaments decrease below a threshold, then adding more filaments may prevent Sema 3A treatment from reducing the number sufficiently. The myosin IIA filaments may in turn stabilize actin filaments preventing collapse.
Figure 9.
Over expression of GFP-myosin IIA, but not GFP-myosin IIB blocks collapse in response to relatively high bath-applied Sema 3A (600 ng/ml, 30 min). DRG neurons were transfected with GFP, GFP-α-actinin, GFP-MIIA, and GFP-MIIB. Cultures were fixed and scored for collapse or imaged by time lapse and fixed before scoring for collapse. Only cells expressing GFP-MIIA showed a significant reduction in collapse frequency (*p ≤ 0.001, in order n = 80, 45, 36, 32, and 49) compared with GFP-expressing cells. An additional experiment gave similar results.
Contribution of Actin to Collapse and Retraction Induced by Gradients of Sema 3A
Myosin II requires actin filaments to produce force in cells. To test the contribution of actin in collapse and retraction, we also applied a gradient of latrunculin A via micropipette to growth cones. Latrunculin A sequesters actin monomers, causing net depolymerization (Coué et al., 1987). If Sema 3A causes collapse by depolymerizing peripheral actin, then application of latrunculin A may mimic collapse and perhaps induce retraction. Latrunculin A applied by micropipette (25 μM) causes a significant reduction in growth cone area, and the change is of sufficient magnitude to meet our criterion for collapse (reduction in average area = 63.8%, n = 9, p = 0.005, Supplemental Figure S2). But, unlike Sema 3A–induced collapse, latrunculin A causes a generalized thinning of any peripheral remnants and sometimes increases the volume of the central domain (giving it a swollen appearance). Retraction is not observed. Therefore depolymerization of actin by a latrunculin A gradient mimics collapse but does so poorly. In contrast bath application of 2 μM latrunculin A causes peripheral thinning and central swelling, but does not induce collapse because a reduction in growth cone area is not observed (n = 10, p = 0.49, Supplemental Figure S1B).
Bath application of latrunculin A may not cause collapse because it affects f-actin in all parts of the growth cone and neurite simultaneously. For instance, it is likely to prevent the localized formation of actin bundles. If this is correct and retraction requires myosin II–driven contractions that required actin bundles within the growth cone rear/central domain and/or connecting neurite (Gallo, 2006), then bath application of latrunculin A before Sema 3A application by micropipette should prevent retraction. Therefore we applied Sema 3A via micropipette to growth cones in cultures that had received prior bath treatment with latrunculin-A, for 20–30 min. Retraction is not observed (retraction = 0%, n = 10, p = 0.0001). Taken together these results suggest that the biphasic response of growth cones to an oriented Sema 3A gradient requires both a polarized structure and a sequential set of changes mediated through signal transduction pathways that include, but are not limited to, changes in the polymerization state of peripheral f-actin followed by localized actin bundle formation and concentration of myosin II that leads to actomyosin driven contractions in the growth cone rear/central domain and connecting neurite (Figure 10).
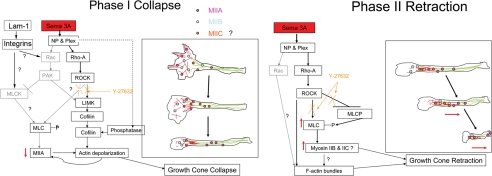
Figure 10.
A model for the molecular interaction involved in the two phases of the Sema 3A response. Phase 1 is collapse and involves loss of myosin IIA and actin filaments from the growth cone. The loss may be simultaneous or sequential and is likely to lead to a transient decrease in tension on the neurite. Activation of integrins by high substrate-bound laminin-1 can modify the response, but the pathways remain unclear. ROCK appears to be partially involved in controlling collapse (Gallo, 2006), but other pathways may also contribute (indicated in gray). Phase II is retraction and involves a redistribution of myosin IIB (and possibly IIC), as well as an increase in bundled actin at the rear of the growth cone or neck region followed by actomyosin drive contractions to increase tension. If the contractions are sufficiently compressed in time and tension overcomes the adhesive interactions with the substrate, then the neurite fully retracts. ROCK appears to also control this phase, although its interactions are complex and need further work to obtain a complete picture. Other pathways may contribute to this phase.
DISCUSSION
Characterization of the Response of DRG Neurons to Sema 3A
Application of Sema 3A gradients generates a reproducible biphasic response. The first phase is growth cone collapse (>50% reduction in area resulting in elimination of lamellipodia and most filopodia), and the second phase is neurite retraction (net rearward movement of the remaining central domain; Figure 10). The two phases can be separated by their different requirements and sensitivities to Sema 3A application that correlated with changes in the different myosin II isoforms. Bath application of a relatively low concentration of Sema 3A produces widespread collapse of DRG growth cones, but only rarely retraction. Thus it seems to reproduce only the first phase observed using gradient application. If the concentration of bath-applied Sema 3A is increased then the response shifts from collapse to rapid collapse and retraction. The difference in the ability of gradients versus bath application to clearly show the two phases during a single application of Sema 3A can be explained by the more localized effects and orientation of the Sema 3A gradient relative to the different functional domains of the growth cone. When the leading edge of the cone is exposed to the gradient first, collapse occurs first and then retraction occurs after a delay. When the rear of the growth cone is exposed to the gradient first, then retraction occurs quickly, similar to retraction observed with bath application of high Sema 3A concentrations. This indicates that retraction requires spatially restricted actomyosin contractions as was suggested in a previous report (Gallo, 2006). Thus the rear of the cone and a short segment of the connecting neurite are the main sites for myosin II–dependent contractions that lead to retraction. Normally myosin II–dependent contractions that contribute to the traction force required for growth cone advance are likely to be isometric (no structural shortening). However, Sema 3A treatment may decrease growth cone adhesion and coordinate myosin II–dependent contractions to overcome the residual adherence to the substratum, leading to detachment and the initiation of retraction.
Semaphorin, first identified as collapsin (Luo et al., 1993), has been studied in cultures mostly by bath application followed by fixation (Campbell et al., 2001; Behar et al., 1999). Until recently the retraction phase was not clearly identified (Gallo, 2006). Although collapse can occur without retraction, retraction does not occur unless collapse is initiated first. However, complete collapse is not necessary for retraction to begin. Thus retraction appears to be dependent on initiation of collapse and the cytoskeletal and adhesive changes that result. Retraction represents the most extreme response of DRG growth cones to Sema 3A. Whether or not biphasic responses occur in vivo remains to be determined. However, because diffusible guidance factors are likely to presented as gradients (Schmitt et al., 2006; Kennedy et al., 2006), the response of growth cones in vivo may depend upon their orientation relative to the gradient as well as the steepness of the gradient (Kennedy et al., 2006).
Cytoskeletal Dependence of Sema 3A-induced Collapse
Collapse is known to involve loss of actin filaments (Fan et al., 1993), but the molecular mechanisms leading to this loss have not been fully identified (Aizawa et al., 2001). Retraction involves both myosin II activity and the redistribution of actin (Gallo, 2006). Collapse has generally been thought to be myosin II independent. We now show that both phases can be influenced by myosin II activity, and the isoform specificity and degree of dependency differs. The changes in myosin IIA that we observe during the collapse phase could be the consequence of actin filament loss. Increases in substrate-bound laminin-1, as well as overexpression of myosin IIA, prevent collapse and are all treatments that are likely to either stabilize growth cone actin filaments or prevent their depolymerization. The protective effects of laminin-1 on collapse is sensitive to blebbistatin treatment. Therefore it seems likely that myosin II (mainly IIA) bipolar filaments have the capacity to stabilize the actin cytoskeleton to prevent collapse through its cross-linking function. This is consistent with recent work in nonneuronal cells (Choi et al., 2008). Pathways activated by Sema 3A may act simultaneously or in close sequence on myosin IIA and actin to cause loss of the peripheral actomyosin network (Figure 10). Further work will be needed to distinguish between these possibilities. Collapse can be altered by circumstances that potentially cause up-regulation of myosin IIA activity. For instance, exposure of DRG neurons to higher concentrations of laminin and NGF could potentially increase myosin IIA filament levels contributing to a synergistic protective effect of these factors to dampen the Sema 3A response.
Cytoskeletal Dependence of Sema 3A-induced Retraction
In contrast to myosin IIA, the amount of growth cone myosin IIB does not change upon application of Sema 3A, and overexpression does not prevent collapse. A high concentration of bath-applied Sema 3A does cause myosin IIB to redistribute concentrating it at the rear of the cone and neck. Taken together these results suggests a model for growth cone repulsion that involves a specific sequence, collapse followed by retraction. Collapse is destabilization and loss of peripheral actin rich lamellipodia and filopodia that contain and can be stabilized by myosin IIA. These regions are also known to be associated with focal contacts in neuroblastoma cells (Wylie and Chantler, 2001), and focal complexes in growth cones from Xenopus spinal neurons can be destabilized by Sema 3A treatment potentially leading to decreased adhesion (Woo and Gomez, 2006). Collapse also leads to redistribution and reorganization of actin to bundles within the neurite (Gallo, 2006) close or overlapping with regions where myosin IIB concentrates. Myosin IIA moves further into the connecting neurite. These initial changes may reduce tension on the neurite. In endothelial cells myosin II contributes to both basal tone and production of force during thrombin induced contraction (Goeckeler et al., 2008), suggesting that nonmuscle myosin II may play similar roles in neurons. Therefore it seems reasonable to propose that localized myosin IIB–driven contractions then provide the main driving force for retraction in the second phase (Figure 10), although myosin IIA and perhaps myosin IIC can also contribute. This supports the general idea that neurons contain different myosin II isoforms to perform distinct functions, but often those functions can overlap.
Growth-promoting Signals Dampen the Response to Sema 3A
Previous studies have shown that high levels of NGF in the medium can dampen the response of DRG growth cones to Sema 3A (Dontchev and Letourneau, 2002). Under conditions where the NGF concentration is low, a high concentration of substrate-bound laminin-1 also inhibits collapse irrespective of the method used to apply Sema 3A. This suggests that the level of integrin activation by laminin-1 may also modulate the degree of the Sema 3A response. Integrin membrane levels of growth cones are sensitive to the amount of substrate-bound laminin, increasing with decreasing density of laminin on the substrate (Condic and Letourneau, 1997). However, the proportion of activated integrins goes up with higher laminin densities (Lemons and Condic, 2006). We used coating densities that are relatively high, differ by 2–4-fold, and are equal in their ability to stimulate growth. Thus any decrease in integrin density in the membrane is likely to be small compared with the ability of the greater density of laminin to give higher integrin activation levels. Semaphorins may regulate integrins (Nakamoto et al., 2004), and integrin activation may also influence the Rho family of small GTPases (Edwards et al., 1999; DeMali et al., 2003). Thus cross-talk between the two pathways may be bidirectional. Previous studies of the Sema 3A response did not investigate the effects of different levels of substrate-bound laminin (Luo 1993; Campbell et al., 2001). Responses to netrin (Hopker et al., 1999) and Slit-2 (Stevens and Jacobs, 2002) are also influenced by integrin signaling. Integrins may modulate responses by altering actin polymerization or by controlling myosin light chain (MLC) phosphorylation (Chan et al., 2007). Both factors may also work together to dampen responses. This seems likely because both laminin-1 and NGF are growth-promoting factors that will oppose the effects of repulsive guidance cues such as Sema 3A. If other guidance factors are able to modulate myosin activity by altering MLC phosphorylation, then this may be a point of convergence in their control of growth responses. Even though collapse can occur through mechanisms that may be independent of integrin pathways as indicated by the high rate of collapse on PLO, the influence of the concentration of laminin-1 on responses to Sema 3A demonstrates that extracellular matrix (ECM) component levels may be critical for determining the degree of response to guidance factors in vivo. The in vivo environment during development may have different levels of both NGF and laminin-1 than are used for in vitro experiments. Thus the response to Sema 3A in vivo may vary depending upon local conditions. This is especially pertinent to regeneration studies that attempt to overcome repulsive factors inhibiting regrowth of axons. Sema 3A is a contributing component to the inhibitory environment of the glial scar in spinal cord injury (Niclou et al., 2006; Kaneko et al., 2006). The combination of high concentrations of laminin-1 and NGF may help overcome this part of the inhibitory signal at injury sites.
Supplementary Material
ACKNOWLEDGMENTS
Grady Phillips provided expert technical help. The antiserum to myosin IIC and the mice expressing GFP-myosin IIB were gifts of Dr. Robert Adelstein, National Institutes of Health (NIH). This work was supported by NIH Grants NS26150 (P.C.B), Neuroscience Blueprint Core Grant NS057105 to Washington University, and the Bakewell Family Foundation Grants; and NIH Grants HL-45788 and P20-RR16440 (R.B.W.).
Abbreviations used:
- MII
myosin II
- Sema 3A
Semaphorin 3A
- Bleb
blebbistatin
- lam-1
Laminin-1
- MLC
myosin light chain
- PLO
poly-l-ornithine
- ROCK
Rho kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-01-0065) on December 24, 2008.
REFERENCES
- Ahmad F. J., Hughey J., Wittmann T., Hyman A., Greaser M., Baas P. W. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat. Cell Biol. 2000;5:276–280. doi: 10.1038/35010544. [DOI] [PubMed] [Google Scholar]
- Allingham J. S., Smith R., Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- Aizawa H., Wakatsuki S., Isii A., Moriyama K., Sasaki Y., Ohashi K., Sekine-Aizawa Y., Sehara-Fujisawa A., Mizuno K., Goshima Y., Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Behar O., Mizuno K., Badminton M., Woolf C. J. Semaphorin 3A growth cone collapse requires a sequence homologous to tarantula hanatoxin. Proc. Natl. Acad. Sci. USA. 1999;96:13501–13505. doi: 10.1073/pnas.96.23.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman P. C., Dave S., Asnes C. F., Tullio A. N., Adelstein R. S. Myosin IIB is required for growth cone motility. J. Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman P. C. Growth cones contain myosin II bipolar filament arrays Cell Motil. Cytoskelet. 2002;52:91–96. doi: 10.1002/cm.10038. [DOI] [PubMed] [Google Scholar]
- Bridgman P. C., Brown M. E., Balan I. Biolistic Transfection. Methods in Cell Biol. 2003;71:353–368. doi: 10.1016/s0091-679x(03)01017-3. [DOI] [PubMed] [Google Scholar]
- Brown M. E., Bridgman P. C. Retrograde flow rate is increased in growth cones from myosin IIB knockout mice. J. Cell Sci. 2003;116:1087–1094. doi: 10.1242/jcs.00335. [DOI] [PubMed] [Google Scholar]
- Brown M., Jacobs T., Eickholt B., Ferrari G., Teo M., Monfries C.Qi.R.Z., Leung T., Lim L., Hall C. lpha2-chimaerin, cyclin-dependent Kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse. J. Neurosci. 2004;24:8994–9004. doi: 10.1523/JNEUROSCI.3184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner H. M. Nerve growth dynamics. Quantitative models for nerve development and regeneration. Ann. NY Acad. Sci. 1994;745:210–221. [PubMed] [Google Scholar]
- Buxton D. B., Golomb E., Adelstein R. S. Induction of nonmuscle myosin heavy chain II-C by butyrate in RAW 264.7 mouse macrophages. J. Biol. Chem. 2003;278:15449–15455. doi: 10.1074/jbc.M210145200. [DOI] [PubMed] [Google Scholar]
- Campbell D. S., Regan A. G., Lopez J. S., Tannahill D., Harris W. A., Holt C. E. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J. Neurosci. 2001;21:8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. T., Cortesio C. L., Huttenlocher A. Integrins in cell migration. Methods Enzymol. 2007;426:47–67. doi: 10.1016/S0076-6879(07)26003-3. [DOI] [PubMed] [Google Scholar]
- Choi C. K., Vicente-Manzanares M., Zareno J., Whitmore L. A., Mogilner A., Horwitz A. R. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condic M. L., Letourneau P. C. Ligand-induced changes in integrin expression regulate neuronal adhesion and neurite outgrowth. Nature. 1997;389:852–856. doi: 10.1038/39878. [DOI] [PubMed] [Google Scholar]
- Cosen-Binker L. I., Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology. 2006;21:352–361. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- Coué M., Brenner S. L., Spector I., Korn E. D. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- DeMali K. A., Wennerberg K., Burridge K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Dontchev V. D., Letourneau P. C. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J. Neurosci. 2002;22:6659–6669. doi: 10.1523/JNEUROSCI.22-15-06659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontchev V. D., Letourneau P. C. Growth cones integrate signaling from multiple guidance cues. J. Histochem. Cytochem. 2003;51:435–444. doi: 10.1177/002215540305100405. [DOI] [PubMed] [Google Scholar]
- Edwards D. C., Sanders L. C., Bokoch G. M., Gill G. N. Activation of Lim-kinase by Pak1 couples Rac/Cdc42 GTPase signaling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Eickholt B. J., Walsh F. S., Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J. Cell Biol. 2002;157:211–217. doi: 10.1083/jcb.200201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Mansfield S. G., Redmond T., Gordon-Weeks P. R., Raper J. A. The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J. Cell Biol. 1993;121:867–878. doi: 10.1083/jcb.121.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Raper J. A. Localized collapsing cues can steer growth cones without inducing their full collapse. Neuron. 1995;14:263–274. doi: 10.1016/0896-6273(95)90284-8. [DOI] [PubMed] [Google Scholar]
- Ferletta M., Ekblom P. Identification of laminin-10/11 as a strong cell adhesive complex for a normal and a malignant human epithelial cell line. J. Cell Sci. 1999;12:1–10. doi: 10.1242/jcs.112.1.1. [DOI] [PubMed] [Google Scholar]
- Fritsche J., Reber B. F., Schindelholz B., Bandtlow C. E. Differential cytoskeletal changes during growth cone collapse in response to hSema III and thrombin. Mol. Cell Neurosci. 1999;14:398–418. doi: 10.1006/mcne.1999.0777. [DOI] [PubMed] [Google Scholar]
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J. Cell Sci. 2006;119:3413–3423. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G., Yee H. F., Jr., Letourneau P. C. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J. Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeckeler Z. M., et al. “Nonmuscle myosin II is responsible for maintaining endothelial cell basal tone and stress fiber integrity.”. Am. J. Physiol. Cell Physiol. 2008;295(4):C994–C1006. doi: 10.1152/ajpcell.00318.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;11:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Halloran M. C., Wolman M. A. Repulsion or adhesion: receptors make the call. Curr. Opin. Cell Biol. 2006;18:533–540. doi: 10.1016/j.ceb.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Hopker V. H., Shewan D., Tessier-Lavigne M., Poo M., Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Jin Z., Strittmatter S. M. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil K., Szebenyi G., Dent E. W. Common mechanisms underlying growth cone guidance and axon branching. J. Neurobiol. 2000;44:145–158. [PubMed] [Google Scholar]
- Kaneko S., et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat. Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Kennedy T. E., Wang H., Marshall W., Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: A gradient of netrin protein in the developing spinal cord. J. Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobel K. M., Jorgensen E. M., Bastinai M. J. Growth cones stall and collapse during axon outgrowth in Caenorhabditis elegans. Development. 1999;126:4489–4498. doi: 10.1242/dev.126.20.4489. [DOI] [PubMed] [Google Scholar]
- Kolega J. The role of myosin II motor activity in distributing myosin asymmetrically and coupling protrusive activity to cell translocation. Mol. Biol. Cell. 2006;17:4435–4445. doi: 10.1091/mbc.E06-05-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn T. B., Brown M. D., Wilcox C. L., Raper J. A., Bamburg J. R. Myelin and Collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of Rac1. J. Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons M. L., Condic M. L. Combined integrin activation and intracellular cAMP cause Rho GTPase dependent growth cone collapse on laminin-1. Exp. Neurol. 2006;202:324–335. doi: 10.1016/j.expneurol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Lochter A., Taylor J., Braunewell K. H., Holm J., Schachner M. Control of neuronal morphology in vitro: interplay between adhesive substrate forces and molecular instruction. J. Neurosci. Res. 1995;42:145–158. doi: 10.1002/jnr.490420202. [DOI] [PubMed] [Google Scholar]
- Lohof A. M., Quillan M., Dan Y., Poo M. -M. Asymmetrical modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Raible D., Raper J. A. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75(2):217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Mason C., Erskine L. Growth cone form, behavior, and interactions in Vivo: Retinal axon pathfinding as a model. J. Neurobiol. 2000;44:260–270. doi: 10.1002/1097-4695(200008)44:2<260::aid-neu14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Medieros N. A., Burnette D. T., Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Nakamoto T., Kain K. H., Ginsberg M. H. Neurobiology: New connections between integrins and axon guidance. Curr. Biol. 2004;14:R121–R123. [PubMed] [Google Scholar]
- Niclou S. P., Erich M.E.E., Verhaagen J. Chemorepellent axon guidance molecules in spinal cord injury. J. Neurotrauma. 2006;23:409–421. doi: 10.1089/neu.2006.23.409. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet K., Brose K., Marillat V., Sotelo C., Tessier-Lavigne M., Chedotal A. Sensory axon response to substrate-bound slit2 is modulated by laminin-1 and cyclic GMP. Mol. Cell Neurosci. 2001;17:1048–1058. doi: 10.1006/mcne.2001.0994. [DOI] [PubMed] [Google Scholar]
- Quach T. T., Duchemin A. M., Rogemond V., Aguera M., Honnorat J., Belin M. F., Kolattukudy P. E. Involvement of collapsin response mediator proteins in the neurite extension induced by neurotrophins in dorsal root ganglion neurons. Mol. Cell Neurosci. 2004;25:433–443. doi: 10.1016/j.mcn.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Rochlin M. W., Itoh K., Adelstein R. S., Bridgman P. C. Localization of myosin IIA and B isoforms in cultured neurons. J. Cell Sci. 1995;108:3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Schmitt A. M., Shi J., Wolf A. M., Lu C. -C., King L. A., Zou Y. Wnt-Ryk signaling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Sobeih M. M., Corfas G. Extracellular factors that regulate neuronal migration in the central nervous system. Int. J. Dev. Neurosci. 2002;20:349–357. doi: 10.1016/s0736-5748(02)00040-0. [DOI] [PubMed] [Google Scholar]
- Stein E., Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;279:105–107. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Stevens A., Jacobs J. R. Integrins regulate responsiveness to slit repellent signals. J. Neurosci. 2002;22(11):4448–55. doi: 10.1523/JNEUROSCI.22-11-04448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Turner L. J., Nicholls S., Hall A. The activity of the plexin-A1 receptor is regulated by Rac. J. Biol. Chem. 2004;279:33199–33205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- Turney S. G., Bridgman P. C. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat. Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- Wahl S., Barth H., Ciossek T., Aktories K., Mueller B. K. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J. Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S., Gomez T. M. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J. Neurobiol. 2006;26:1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie S. R., Wu P. J., Patel H., Chantler P. D. A conventional myosin motor drives neurite outgrowth. Proc. Natl. Acad. Sci. USA. 1998;95:12967–12972. doi: 10.1073/pnas.95.22.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie S. R., Chantler P. D. Separate but linked functions of conventional myosins modulate adhesion and neurite outgrowth. Nat. Cell Biol. 2001;1:88–92. doi: 10.1038/35050613. [DOI] [PubMed] [Google Scholar]
- Wylie S. R., Chantler P. D. Myosin IIA drives neurite retraction. Mol. Biol. Cell. 2003;11:4654–4666. doi: 10.1091/mbc.E03-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Buxbaum R. E., Heidemann S. R. Measurements of growth cone adhesion to culture surfaces by micromanipulation. J. Cell Biol. 1994;127:2049–2060. doi: 10.1083/jcb.127.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.