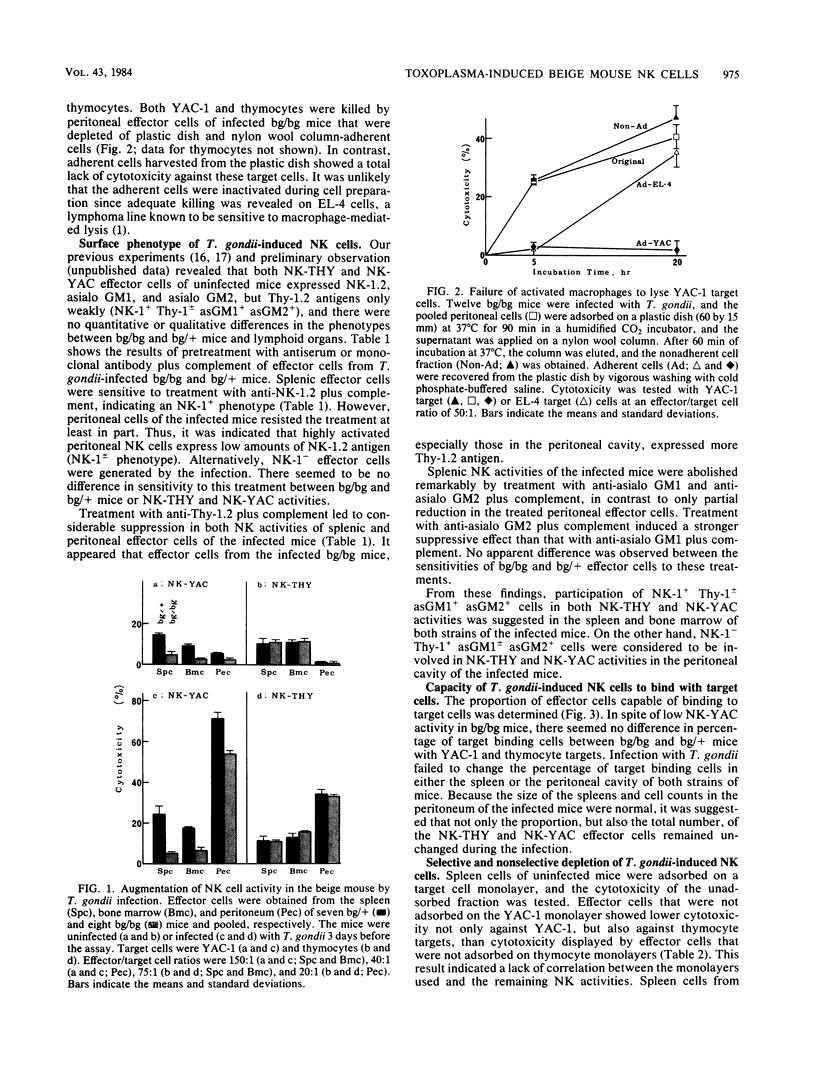
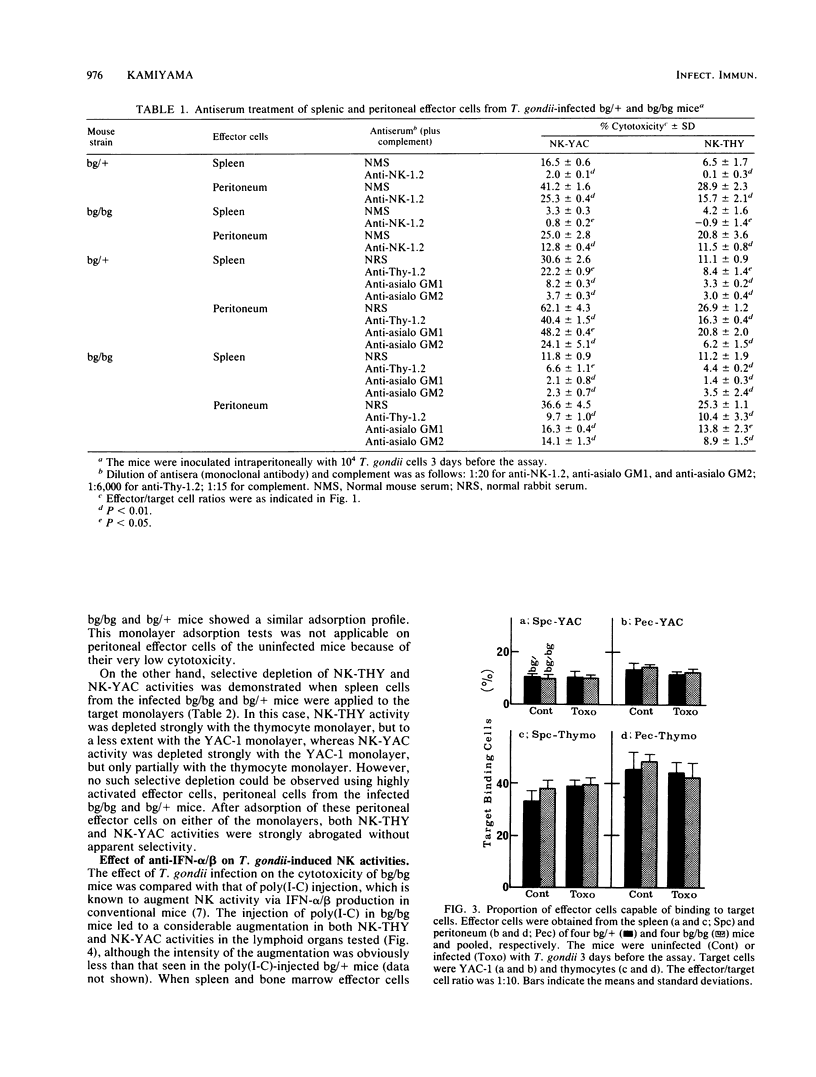
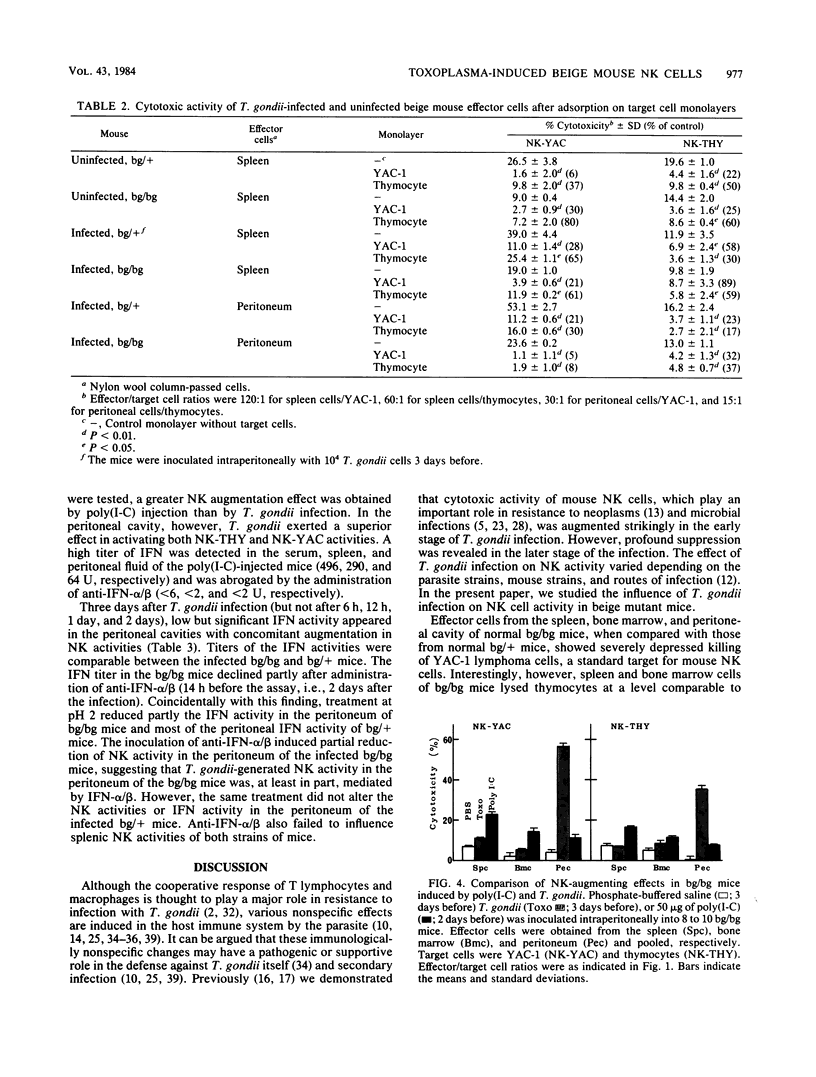
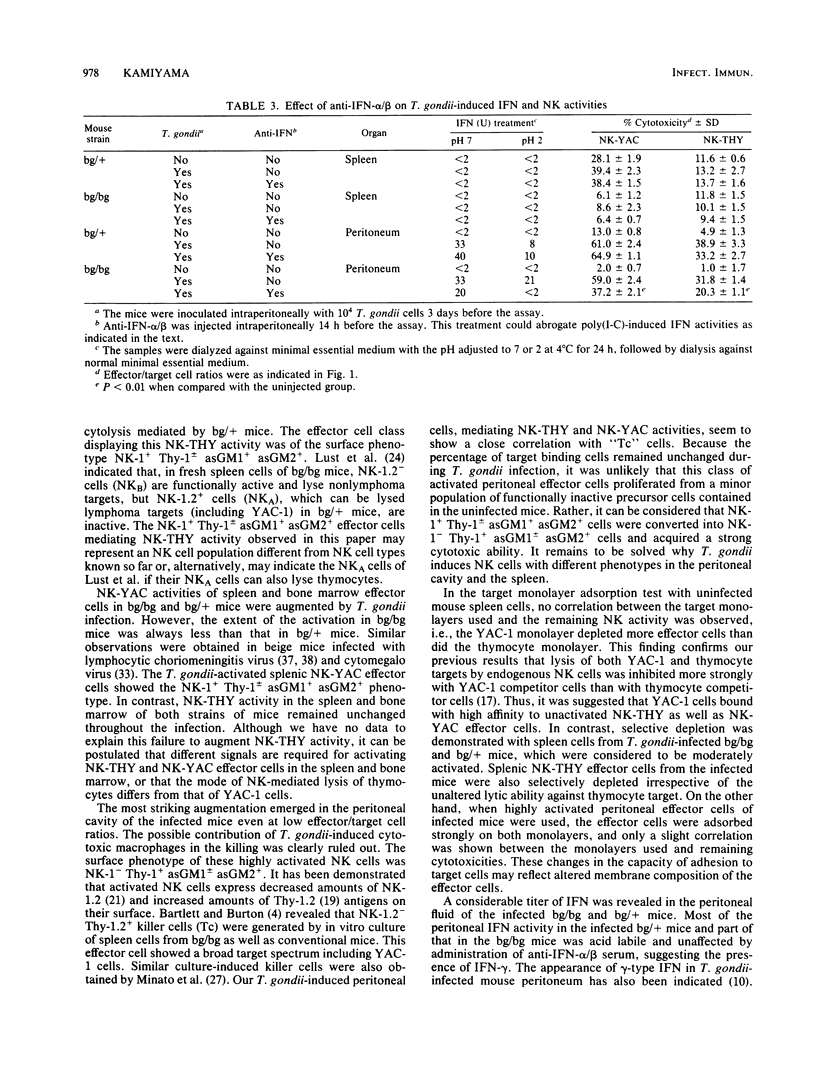
Abstract
Homozygous (bg/bg) and heterozygous (bg/+) beige mice were infected with Toxoplasma gondii, and splenic and peritoneal natural killer (NK) cell activities were assayed against YAC-1 lymphoma (NK-YAC) and thymocyte (NK-THY) targets. Although uninfected bg/bg mice were devoid of NK-YAC activity when compared with bg/+ mice, NK-THY activity was at a completely normal level. Both effector cells showed NK-1.2+ Thy-1.2 +/- asialo GM1+ asialo GM2+ phenotype. T. gondii infection induced a marked augmentation in splenic NK-YAC activity of bg/+ mice, whereas a slight increase was shown in the bg/bg mouse spleen cells. On the other hand, the infection did not change the splenic NK-THY activity of either strain of mice. An increased expression of Thy-1.2 antigen was shown on both NK-THY and NK-YAC effector cells from the infected mouse spleen. The T. gondii-induced augmentation was dramatic in the peritoneal cavity of the both mice. The activated peritoneal NK cells were of the NK-1.2- Thy-1.2+ asialo GM1 +/- asialo GM2+ phenotype and were considered to be generated from functionally inactive peritoneal cells. Splenic effector cells obtained from the infected mice were selectively depleted with target cell monolayer, whereas peritoneal cells from the infected mice were strongly absorbed by the target monolayers without selectivity. A weak but significant interferon (IFN) titer was detected in the peritoneum, but not in the spleen, of the infected mice. Most of the IFN titer was acid labile. Treatment with anti-IFN-alpha/beta resulted in partial decline of both NK and IFN activities of bg/bg mice, but not bg/+ mice. Thus, involvement of both IFN-alpha/beta and IFN-gamma in the generation of peritoneal NK cells and IFN-independent augmentation of splenic NK cells in toxoplasmosis were suggested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagawa K. S., Tokunaga T. Appearance of a cell surface antigen associated with the activation of peritoneal macrophages in mice. Microbiol Immunol. 1982;26(9):831–842. doi: 10.1111/j.1348-0421.1982.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Anderson S. E., Bautista S., Remington J. S. Induction of resistance to Toxoplasma gondii in human macrophages by soluble lymphocyte products. J Immunol. 1976 Aug;117(2):381–387. [PubMed] [Google Scholar]
- Argov S., Cochran A. J., Kärre K., Klein G. O., Klein G. Incidence and type of tumors induced in C57BL bg/bg mice and +/bg littermates by oral administration of DMBA. Int J Cancer. 1981 Dec;28(6):739–746. doi: 10.1002/ijc.2910280613. [DOI] [PubMed] [Google Scholar]
- Bartlett S. P., Burton R. C. Studies on natural killer (NK) cells. III. The effects of in vitro culture on spontaneous cytotoxicity of murine spleen cells. J Immunol. 1982 Mar;128(3):1070–1075. [PubMed] [Google Scholar]
- Biron C. A., Welsh R. M. Activation and role of natural killer cells in virus infections. Med Microbiol Immunol. 1982;170(3):155–172. doi: 10.1007/BF02298196. [DOI] [PubMed] [Google Scholar]
- Burton R. C., Winn H. J. Studies on natural killer (NK) cells. I. NK cell specific antibodies in CE anti-CBA serum. J Immunol. 1981 May;126(5):1985–1989. [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Elin R. J., Edelin J. B., Wolff S. M. Infection and immunoglobulin concentrations in Chediak-Higashi mice. Infect Immun. 1974 Jul;10(1):88–91. doi: 10.1128/iai.10.1.88-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugui E. M., Allison A. C. Differences in susceptibility of various mouse strains to haemoprotozoan infections: possible correlation with natural killer activity. Parasite Immunol. 1980 Winter;2(4):277–292. doi: 10.1111/j.1365-3024.1980.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Freshman M. M., Merigan T. C., Remington J. S., Brownlee I. E. In vitro and in vivo antiviral action of an interferon-like substance induced by Toxoplasma gondii. Proc Soc Exp Biol Med. 1966 Dec;123(3):862–866. doi: 10.3181/00379727-123-31625. [DOI] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E., Cerrone M. C., Burton R. C. Increased natural killer cell activity in experimental American trypanosomiasis. J Immunol. 1981 Sep;127(3):1126–1130. [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Natural killer cells induced by acute and chronic toxoplasma infection. Cell Immunol. 1982 May 15;69(2):330–346. doi: 10.1016/0008-8749(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Kamiyama T., Hagiwara T. Augmented followed by suppressed levels of natural cell-mediated cytotoxicity in mice infected with Toxoplasma gondii. Infect Immun. 1982 May;36(2):628–636. doi: 10.1128/iai.36.2.628-636.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama T., Tatsumi M. Effect of Toxoplasma infection on the sensitivity of mouse thymocytes to natural killer cells. Infect Immun. 1983 Nov;42(2):789–795. doi: 10.1128/iai.42.2.789-795.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Eriksson E., Hallenbeck L. A., Welsh R. M. A comparative analysis of the cell surface properties of activated vs endogenous mouse natural killer cells. J Immunol. 1980 Oct;125(4):1551–1557. [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P. Leishmaniasis in beige mice. Infect Immun. 1982 Dec;38(3):1208–1216. doi: 10.1128/iai.38.3.1208-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Barnes M. C., Bennett M., Burton R. C. Differential lysis of endogenous and activated natural killer cells by anti-NK-1.2 antibody and complement. J Immunol. 1982 Mar;128(3):1482–1484. [PubMed] [Google Scholar]
- Kärre K., Klein G. O., Kiessling R., Klein G., Roder J. C. In vitro NK-activity and in vivo resistance to leukemia: studies of beige, beige//nude and wild-type hosts on C57BL background. Int J Cancer. 1980 Dec 15;26(6):789–797. doi: 10.1002/ijc.2910260613. [DOI] [PubMed] [Google Scholar]
- Lane P. W., Murphy E. D. Susceptibility to spontaneous pneumonitis in an inbred strain of beige and satin mice. Genetics. 1972 Nov;72(3):451–460. doi: 10.1093/genetics/72.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C., Kirkpatrick D., Fitzgerald P. A., Ching C. Y., Pahwa R. N., Good R. A., Smithwick E. M. Studies of the cell lineage of the effector cells that spontaneously lyse HSV-1 infected fibroblasts (NK(HSV-1)). J Immunol. 1982 Aug;129(2):824–828. [PubMed] [Google Scholar]
- Lust J. A., Kumar V., Burton R. C., Bartlett S. P., Bennett M. Heterogeneity of natural killer cells in the mouse. J Exp Med. 1981 Aug 1;154(2):306–317. doi: 10.1084/jem.154.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Strickland G. T., Warren K. S. Toxoplasmosis and the host-parasite relationship in murine schistosomiasis mansoni. J Infect Dis. 1977 Mar;135(3):408–413. doi: 10.1093/infdis/135.3.408. [DOI] [PubMed] [Google Scholar]
- Mahoney K. H., Morse S. S., Morahan P. S. Macrophage functions in beige (Chédiak-Higashi syndrome) mice. Cancer Res. 1980 Nov;40(11):3934–3939. [PubMed] [Google Scholar]
- Minato N., Reid L., Bloom B. R. On the heterogeneity of murine natural killer cells. J Exp Med. 1981 Sep 1;154(3):750–762. doi: 10.1084/jem.154.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Salimonu L. S., Williams A. I., Akinwolere O. A., Shabo R., Alm G. V., Wigzell H. Positive correlation between degree of parasitemia, interferon titers, and natural killer cell activity in Plasmodium falciparum-infected children. J Immunol. 1981 Dec;127(6):2296–2300. [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Pelster B., Suzuki N., Piekarski G., Brandis H. Immunity to Toxoplasma gondii induced in vitro in non-immune mouse macrophages with specifically immune lymphocytes. J Immunol. 1975 Oct;115(4):1151–1158. [PubMed] [Google Scholar]
- Shellam G. R., Allan J. E., Papadimitriou J. M., Bancroft G. J. Increased susceptibility to cytomegalovirus infection in beige mutant mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5104–5108. doi: 10.1073/pnas.78.8.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata T., Shimizu K. Production and properties of immune interferon from spleen cell cultures of Toxoplasma-infected mice. Microbiol Immunol. 1980;24(11):1109–1120. doi: 10.1111/j.1348-0421.1980.tb02915.x. [DOI] [PubMed] [Google Scholar]
- Strickland G. T., Ahmed A., Sell K. W. Blastogenic response of Toxoplasma-infected mouse spleen cells to T- and B-cell mitogens. Clin Exp Immunol. 1975 Oct;22(1):167–176. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Kobayashi A. Suppression of unprimed T and B cells in antibody responses by irradiation-resistant and plastic-adherent suppressor cells in Toxoplasma gondii-infected mice. Infect Immun. 1983 Apr;40(1):1–7. doi: 10.1128/iai.40.1.1-7.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Kiessling R. W. Natural killer cell response to lymphocytic choriomeningitis virus in beige mice. Scand J Immunol. 1980;11(4):363–367. doi: 10.1111/j.1365-3083.1980.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Sawyer S., Remington J. S. Role of activated macrophages in resistance of mice to infection with Trypanosoma cruzi. J Infect Dis. 1976 Dec;134(6):610–623. doi: 10.1093/infdis/134.6.610. [DOI] [PubMed] [Google Scholar]


