Abstract
Objective
The goal of this study is to determine whether C-reactive protein (CRP) gene variants affect baseline and training-induced changes in plasma CRP levels.
Methods and Results
Sixty-three sedentary men and women aged 50 to 75 years old underwent baseline testing (VOmax, body composition, CRP levels). They repeated these tests after 24 weeks of exercise training while on a low-fat diet. The CRP +219G/A variant significantly associated with CRP levels before and after training after accounting for the effects of demographic and biological variables. CRP −732A/G genotype was significantly related on a univariate basis to CRP levels after training. The CRP +29T/A variant did not affect CRP levels before or after training. In regression analyses, the +219 and −732 variants each had significant effects on CRP levels before and after training. Subjects homozygous for the common A/G −732/+219 haplotype exhibited the highest CRP levels, and having the rare allele at either site was associated with significantly lower CRP levels. CRP levels decreased significantly with training (−0.38±0.18 mg/L; P=0.03). However, none of the CRP variants was associated with the training-induced CRP changes.
Conclusion
CRP +219G/A and −732A/G genotypes and haplotypes and exercise training appear to modulate CRP levels. However, training-induced CRP reductions appear to be independent of genotype at these loci.
Keywords: C-reactive protein, genetics, exercise training
Atherosclerosis, which is the main mechanism underlying most cardiovascular (CV) diseases, is now considered to be, at least partly, an inflammatory disorder. C-reactive protein (CRP) has been proposed as a marker of inflammation,1 with lower CRP levels associated with reduced CV disease risk2 and even slight CRP elevations associated with increased CV events.1 Cross-sectional studies have found that CRP levels are lowest in those with the highest levels of habitual physical activity;3 however, very few studies have assessed the impact of an exercise training intervention on CRP levels.4
Recent evidence indicates that common polymorphisms at the CRP gene locus affect CRP levels.5,6 Four single nucleotide polymorphisms (SNPs) have been identified in the CRP gene: −732 A/G, an adenine/guanine transition in the promoter region of the CRP gene;7 +29 T/A, a thymine/adenine transversion in intron 1, 29-bp downstream (3′) of exon 1; +1059 G/C, a silent guanine/cytosine transversion in the exon 2 coding region;8 and +219 G/A, a guanine/adenine transition in the 3′ flanking sequence of the CRP gene, 219-bp downstream (3′) of exon 2. Because genetic variations affect the responses of other CV disease risk factors to exercise training,9,10 it is possible that these CRP gene variants may interact with exercise training to differentially affect plasma CRP levels. Thus, we hypothesized that CRP levels at baseline and after 6 months of exercise training will differ among CRP genotype groups, CRP levels will decrease as a result of exercise training, and these training-induced CRP decreases will be dependent on CRP genotype. These hypotheses were also assessed based on CRP gene haplotypes.
Methods
Sedentary white and nonwhite men and women aged 50 to 75 years were screened via telephone to ascertain their interest, suitability, and ability to participate in an exercise training intervention. The Institutional Review Board at the University of Maryland College Park and Howard University approved the study. Written informed consent was obtained during the participants’ first laboratory visit. Eligible volunteers were sedentary, nondiabetic, normotensive, or hypertensive with blood pressure (BP) controlled with medication (systolic BP <160, diastolic BP <90 mmHg), nonsmokers, body mass index <37 kg/m2, no ongoing regular aerobic exercise, and no previous history of CV disease. All women were postmenopausal and maintained the same hormone replacement therapy (HRT), either using or not using HRT, throughout the study.
On their first laboratory visit, medical histories were reviewed to ensure subjects met the study inclusion criteria. Body mass index <37 was ascertained by measuring height and weight. Participants had blood chemistry and fasting plasma glucose levels determined and underwent a 2-hour 75-gram oral glucose tolerance test. Those with fasting glucose >126 mg/dL or 2-hour glucose >200 mg/dL were excluded. Participants had to have ≥1 National Cholesterol Education Program lipid abnormality because this study was a part of a larger trial assessing the genetics of training-induced plasma lipoprotein-lipid changes. Maximal treadmill exercise tests with BP and electrocardiogram monitoring were performed to screen for CV disease. Participants whose exercise test was terminated for CV disease signs and symptoms or showed other evidence of CV disease were excluded.
Participants then completed 6 weeks of instruction on the principles of an American Heart Association Step 1 diet (<30% calories from fat; ≈55% from carbohydrates; ≈15% from protein; cholesterol intake <300 mg/d).11 Participants completed a 7-day food record and adhered to the diet for >3 weeks before baseline testing. Participants maintained this diet for the duration of the study. A registered dietitian analyzed all food records (Computrition Inc Software). Participants completed food records during the exercise training intervention to ensure adherence to the diet.
Participants then completed baseline testing consisting of body composition, Vomax, and CRP level assessments. Body composition was assessed by dual-energy X-ray absorptiometry (DPX-L; Lunar Corp). Vomax was measured using a graded treadmill protocol.12 On the day of blood sampling to determine CRP levels, participants must have had no alcohol for 24 hours, no exercise for 24 to 36 hours, and no infections in the preceding week. CRP was measured using an enzyme-linked immunosorbent assay system (minimum detectable CRP level 0.35 ng/mL, interassay and intra-assay coefficient of variation 3 to 7 and 2% to 4%, respectively) (Alpha Diagnostic International). Processed samples were stored at −80°C until assayed. Each subject's before and after training samples were analyzed in the same assay to eliminate the effect of interassay variation.
Genomic DNA was extracted from peripheral lymphocytes using standard methods.13 Restriction fragment length polymorphism analysis14 was used to genotype the +1059 G/C SNP using Bsp1286 I. Fluorescence polarization15 was used to genotype the remaining 3 polymorphisms (−732 A/G, +29 T/A, and +219 G/A). Haplotypes for the −732 and +219 loci were generated using PHASE v2.16 Unequivocal haplotypes were assigned to all subjects with the exception of double heterozygotes. Subjects were grouped for analysis as indicated in Table 1.
TABLE 1.
Definition of CRP Haplotype Groups for the −732 and +219 Polymorphisms
| Group | Haplotypes | Definition |
|---|---|---|
| Common homozygotes | A/G,A/G | Subjects homozygous for the common allele at both polymorphisms |
| 1 SNP heterozygotes | A/G,g/G and A/G,A/a | Subjects heterozygous for the rare allele at only 1 polymorphism |
| 1 SNP rare homozygotes | g/G,g/G and A/a,A/a | Subjects homozygous for the rare allele at only 1 polymorphism |
| Double heterozygotes* | A/G,g/a and/or A/a,G,g | Subjects heterozygous for the rare allele at both polymorphisms |
Haplotype represented as −732/+219, −732/+219.
Rare allele designated with lowercase font.
No subjects possessed the rare g/a,g/a haplotype combination.
Actual haplotype indeterminate.
Participants underwent 3 supervised exercise training sessions per week for 6 months. Initial sessions consisted of 20 minutes of 50% Vo2max exercise and progressed until 40 minutes of 70% Vo2max exercise were completed during each session.12 Exercise consisted of treadmill walking/jogging, stair-stepping, and cycle and rowing ergometry. Participants added a lower intensity unsupervised 45- to 60-minute walk on the weekend after 12 weeks of training.
After exercise training, body composition, Vomax, and CRP assessments were completed as before training. Participants’ food records were examined and dietary compliance determined. Samples for plasma CRP measurements were drawn 24 to 36 hours after the subject's training session.
Statistical analyses were performed using the SAS statistical software system.17 According to the American Heart Association criteria for CRP levels,18 5 persons with CRP levels ≥10 mg/L were excluded from our analyses. CRP levels were not normally distributed and were square-root transformed for analyses. Because of the low frequency of the C allele at the +1059 locus (Table 2), it was not included in subsequent analyses. A χ2 test determined that the genotype distribution at the remaining 3 SNPs did not differ from Hardy–Weinberg expectations (Table 2). Rare allele homozygotes for each SNP were combined with heterozygotes as carriers of the rare allele. Each carrier group was then compared with the noncarrier group for all statistical analyses. For each of the 3 SNPs, bivariate ANOVA was performed using the general linear models. For the bivariate models, complete data were available on 61 subjects (27 men, 34 women) for CRP +219G/A genotype, 63 subjects (28 men, 35 women) for CRP −732A/G genotype, and 62 subjects (28 men, 34 women) for +29T/A genotype. Next, each model was adjusted for demographic variables (age, gender, and ethnicity). The final model included adjustment for demographic and biological variables (body weight, percent total body fat). Similar models were constructed for CRP levels after training and for changes in CRP levels with training. The 3 CRP variants included in the analyses showed no evidence of significant linkage disequilibrium between any of the pairs (D′=0.34 to 0.46). Consequently, the 3 SNPs were examined together as predictor variables of CRP levels at baseline, after training, and the changes in CRP levels with training, with adjustments made for demographic and biological variables. Haplotype comparisons were made using preplanned contrasts within an ANOVA framework while covarying for age, gender, and ethnicity. Statistical significance was accepted at P≤0.05.
TABLE 2.
CRP Variant Allele and Genotype Frequencies
| SNP | Allele Frequency | Genotype Frequency | |||
|---|---|---|---|---|---|
| +219G/A (n=61) |
G=0.70 | A=0.30 | GG=0.48 (n=29) |
GA=0.44 (n=27) |
AA=0.08 (n=5) |
| −732A/G (n=63) |
A=0.74 | G=0.26 | AA=0.56 (n=35) |
AG=0.37 (n=23) |
GG=0.08 (n=5) |
| +29T/A (n=62) |
T=0.69 | A=0.31 | TT=0.52 (n=32) |
TA=0.36 (n=22) |
AA=0.13 (n=8) |
| +1059G/C (n=46) |
G=0.97 | C=0.03 | GG=0.94 (n=43) |
GC=0.07 (n=3) |
CC=0 |
n is the sample size.
Results
CRP +219G/A Genotype
In the total sample, and within both CRP +219G/A genotype groups, there were approximately the same number of men and women and a similar number of women using HRT and not using HRT (Table 3). Furthermore, there was a comparable distribution of whites and nonwhites in both CRP +219G/A genotype groups.
TABLE 3.
Demographic and Physical Characteristics of the Total Study Sample and by CRP +219G/A Genotype
| CRP +219G/A Genotype |
|||
|---|---|---|---|
| Characteristics | Total (n=61) | GG Homozygotes (n=29) | A Allele Carriers (n=32) |
| Age | 58.6±0.8 | 59.5±1.2 | 57.8±1.0 |
| Gender men/women | 27/34 | 13/16 | 14/18 |
| Women using HRT yes/no | 12/22 | 7/9 | 5/13 |
| Ethnicity whites/nonwhites | 44/17 | 20/9 | 24/8 |
| Body weight, kg baseline | 79.9±1.8 | 81.8±2.5 | 78.2±2.6 |
| After training | 78.4±1.7* | 80.0±2.5* | 77.1±2.4* |
| Total body fat, kg baseline | 34.7±1.3 | 36.0±2.0 | 33.4±1.7 |
| After training | 33.5±1.3* | 34.4±2.1* | 32.7±1.7† |
| VO2max, L/min baseline | 2.02±0.07 | 2.04±0.09 | 1.99±0.09 |
| After training | 2.31±0.08* | 2.35±0.12* | 2.27±0.11* |
Values are means±SE.
Training effect within group significant at P<0.05.
Training effect between group significant at P<0.05.
Baseline Vomax, body weight, and percent body fat did not differ between CRP +219G/A genotype groups. However, baseline CRP levels were significantly different among CRP +219G/A genotype groups (P=0.02) (Table 4) with the GG homozygotes having ≈70% higher baseline CRP levels than A allele carriers. These genotype-dependent baseline CRP differences were similar between men and women (P=0.10), between whites and nonwhites (P=0.79), and between women using and not using HRT (P=0.27). CRP +219G/A genotype explained 9% of the variation in baseline CRP levels. A multivariate model adding demographic covariates accounted for 16% of the variation in baseline CRP levels, with CRP +219G/A genotype still being associated significantly with baseline CRP levels (P=0.03). Further adjustments for biological variables increased the variation accounted for by the model to 40% and the CRP +219G/A genotype association with baseline CRP levels remained significant (P=0.04), still accounting for ≈9% of the inter-individual variance in baseline CRP levels.
TABLE 4.
Baseline, After Training, and Change With Training CRP Levels by CRP +219G/A Genotype
| CRP +219G/A Genotype |
||||
|---|---|---|---|---|
| Total (n=60) | GG (n=29) | A Allele Carriers (n=31) | P | |
| Baseline CRP levels | 2.64±0.3 | 3.37±0.5 | 1.97±0.4 | 0.02 |
| After training CRP levels | 2.26±0.3 | 2.84±0.4 | 1.73±0.4 | 0.02 |
| Change with training CRP levels | −0.38±0.2* | −0.54±0.3* | −0.25±0.2 | 0.41 |
Values are means±SE expressed in units of mg/L.
P are for the differences between genotype groups.
Significant change within group with exercise training at P<0.05.
After exercise training, CRP levels remained significantly higher in the +219 GG homozygotes compared with A allele carriers (P=0.02) (Table 4). CRP +219G/A genotype again accounted for 9% of the variation in CRP levels after training. Adjustment for demographic variables increased the variation accounted for by the model to 16% with the association between CRP +219G/A genotype and final CRP levels still being significant (P=0.04). Additional adjustment for biological variables increased the variation accounted for to 33%, although the CRP +219G/A genotype main effect only tended toward significance (P=0.08).
Training-induced body weight, percent body fat, and Vo2max changes were similar in the +219 GG and A allele carrier groups (Table 3). In the total population, CRP levels were significantly reduced after 6 months of aerobic exercise training (−0.38±0.18 mg/L; P=0.03) (Table 4). However, a significant reduction in CRP levels with training was only evident in the GG genotype group (−0.54±0.26 mg/L P=0.04), but not in the A allele carrier group (−0.25±0.24 mg/L P=0.32) (Table 4), although the training-induced CRP changes did not differ significantly between these 2 genotype groups (P=0.41). Adjustment for demographic and biological variables still resulted in nonsignificant relationships between CRP +219G/A genotype and training-induced CRP changes (P=0.40 and P=0.56, respectively). Further adjustment for baseline CRP levels also had no effect on this relationship.
CRP −732A/G Genotype
Baseline Vomax, body weight, and percent body fat did not differ significantly between CRP −732A/G genotype groups (data not shown). CRP −732A/G genotype had a tendency to associate with baseline CRP levels on a univariate basis (P=0.13). The significance level remained generally similar in multivariate analyses adding the demographic and biological variables.
After exercise training, CRP levels were significantly higher in CRP −732 AA homozygotes than in G allele carriers (P=0.04). Adjustment for demographic variables increased the model contribution to explained variance to 16% whereas the −732A/G genotype effect remained significant (P=0.04). Additional adjustment for biological variables increased the model's contribution to explained variance to 33%, with the −732A/G genotype main effect tending toward significance (P=0.051) and accounting for ≈6% of the variation in CRP levels. Changes in CRP levels with training were not associated with CRP −732A/G genotype in the bivariate or adjusted models.
CRP +29T/A Genotype
The CRP +29T/A polymorphism did not affect CRP levels at baseline, after 6 months of exercise training, or changes with exercise training (data not shown). Adjustment for demographic and biological variables did not result in significant associations between CRP +29T/A genotype and baseline, after training, or change with training CRP levels.
Combined Influence of CRP +219G/A, −732A/G, and +29T/A Genotypes
Fifty-four participants had complete data for all demographic, biological, and SNP variables. Multivariate analysis of the initial model including all 3 genotypes revealed that the CRP +219G/A and the −732A/G SNP significantly and independently influenced CRP levels at baseline (P<0.01, and P=0.04, respectively) and after exercise training (P<0.01, and P<0.01, respectively). At baseline, the influence of +219G/A and −732A/G genotypes was maintained after adjusting for demographic variables (P<0.01, and P=0.05, respectively), but not after adding the biological variables. After exercise training, adding demographic variables and biological variables increased the contribution of the model to 42%, whereas the CRP +219G/A and −732A/G genotype effects remained significant and independent (P<0.01, and P=0.02, respectively) with both accounting for ≈9% of the variation in CRP levels. CRP +29T/A genotype had no influence on CRP levels at baseline or after exercise training in these models.
Based on these findings, analysis of −732/+219 haplotype was conducted, revealing a significant association between −732/+219 haplotype and CRP levels before and after exercise training (Figure 1). At baseline, subjects homozygous for the common A/G haplotype exhibited significantly higher CRP levels than subjects heterozygous for the rare allele at one or both polymorphisms. After exercise training, subjects homozygous for the common A/G haplotype exhibited significantly higher CRP levels than all other haplotype groups.
Discussion
Most major CV disease risk factors are heritable, and recently many common genetic polymorphisms that affect these heritable CV disease risk factors have been identified. Environmental factors, including endurance exercise training, clearly also have a substantial impact on many of these same CV disease risk factors. Furthermore, we and others have begun to provide evidence of the interactive effects of common genetic polymorphisms and exercise training on CV disease risk factors.9,10 Thus, in the present study we hypothesized that common CRP gene polymorphisms and CRP gene haplotypes would affect CRP levels at baseline and after training and that these polymorphisms and haplotypes would affect the change in CRP levels elicited with endurance exercise training. However, although we found that CRP gene polymorphisms and haplotypes clearly had a substantial effect on CRP levels at baseline and after exercise training, they did not interact with exercise training to differentially affect CRP level responses in our middle-aged to older-aged individuals.
Elevated CRP levels have been associated with a greater risk for CV disease and CV events.19 This relationship has been proposed to be the result of CRP levels being a marker for vascular inflammation.20 It is not entirely clear if this relationship is causal, that is, the CV disease outcomes are the result of the direct effect of the release of CRP as a major acute phase protein, or merely an association, that is, the CRP levels provide an easily accessible and measurable index of the low-grade vascular inflammation proposed to underlie CV disease. Although it is important to determine which of these 2 scenarios is correct, it is widely acknowledged that CRP levels represent a very useful measure of CV disease risk and a substantial research effort in the past 5 to 10 years has been directed toward a better understanding of the implications of elevated CRP levels.
A number of previous studies have found that CRP levels are heritable,21 which indicates that common genetic polymorphisms may account for a portion of the interindividual differences in CRP levels. Zee and Ridker reported that CRP +1059 GC heterozygotes had ≈30% lower CRP levels than GG homozygotes at this locus.6 Szalai et al reported that persons with an intermediate number of dinucleotide repeats in intron 1 of the CRP gene had twice the CRP levels of those with a low or high number.5 Most recently, Brull et al found that military recruits and coronary artery bypass graft patients with the TT genotype at the CRP +1444 variant in the 3′ untranslated region had 30% to 40% higher CRP levels than C allele carriers,7 and +1444 genotype accounted for 2% to 5% of the interindividual variance in baseline CRP levels. They found that CRP −732 and +1059 genotypes were not associated with CRP levels. Regulation of CRP gene expression has been shown to occur mainly at the transcriptional level.22 Consequently, CRP gene sequence variation may affect this regulation, because CRP gene polymorphisms have the potential to result in the disruption of supramolecular complexes of gene-specific combinations of transcription factors and cis-DNA that interact with promoters of inducible genes to enhance their expression.23
In the present study, we examined the effect of 4 genetic polymorphisms at the CRP locus on CRP levels at baseline and after exercise training, and their changes with endurance exercise training. We did not have adequate power to address our hypothesis relative to CRP +1059 genotype because of the low frequency of the rare allele. When assessing the effect of each SNP independently, the CRP +219G/A variant showed the strongest and most consistent relationships with CRP levels both before and after exercise training. The A allele carriers at this locus had ≈40% lower CRP levels than GG genotype individuals. These relationships generally persisted after accounting for a number of demographic and biological variables that influence CRP levels, including age, gender, ethnicity, body weight, and percent body fat. CRP −732 genotype was significantly related on a univariate basis to CRP levels after exercise training both before and after correcting for the effect of the different demographic and biological variables. The CRP +29 variant did not significantly affect CRP levels either before or after exercise training with or without correcting for demographic and biological variables. In regression analyses that included all 3 CRP SNPs, the +219 and the −732 variants each had a significant independent effect on CRP levels before exercise training after correcting for the influence of demographic variables but not after accounting for biological variables. Both of these variants had a significant independent effect on CRP levels after exercise training both before and after correcting for the effects of the demographic and biological variables. These 2 variants accounted for ≈13% and ≈18% of the interindividual variance in CRP levels at baseline and after training, respectively.
Haplotype analysis indicated that subjects homozygous for the common A/G haplotype exhibited the highest CRP levels, and that the presence of the rare allele at either the −732 or the +219 SNP was associated with significantly lower CRP levels. These extreme haplotype groups had 3-fold to 4-fold differences in plasma CRP levels both at baseline and after exercise training. Although not statistically significant (P=0.12), our data indicate that the presence of one rare allele at both the −732 and +219 polymorphisms (double heterozygotes) may be associated with further reduced CRP levels compared with those in subjects possessing the rare allele at only 1 locus. This would be indicative of an independent, additive effect of variation at the CRP −732 and +219 SNPs, consistent with the multivariate analysis.
A number of cross-sectional studies have found that higher physical activity levels are associated with lower CRP levels.3,24-28 In general, most cross-sectional studies have reported that individuals with the highest fitness or physical activity levels have CRP levels 25% to 40% lower than those of the least fit or least active individuals.24-28 Recently Esposito et al reported a ≈30% reduction in CRP levels with a diet and physical activity program in 20- to 46-year-old obese women.29 However, this study did not assess the independent effect of exercise training on CRP levels and, to our knowledge, the independent effect of exercise training on CRP levels has not yet been addressed.
In the present study in middle-aged to older-aged at-risk men and women, CRP levels decreased ≈15% with 6 months of endurance exercise training. Although our study was not designed to provide definitive evidence of CRP changes with endurance exercise training, it makes a substantial contribution to this question because of the dietary control imposed in the study, the control of recent inflammation/infection before blood sampling, the prolonged exercise training program, the screening of subjects to exclude those with comorbidities that affect CRP levels, and the long-term stability of plasma CRP levels.30 Our training-induced reductions in plasma CRP levels were one-third to one-half (15% versus 25% to 40%) those generally found for differences between fit and unfit or active and inactive individuals. Our somewhat smaller effect may be because of the longitudinal nature of the present investigation. In addition, it is possible that CRP levels may be reduced further with more prolonged exercise training, because the fit individuals in the previous cross-sectional studies generally had been undergoing their physical activity programs for much longer than the 6-month duration of the present study.
Recently a number of studies have shown that pharmacological interventions reduce plasma CRP levels in individuals at high risk for CV disease.31 CRP levels were reduced 29% by the ingestion of 300 mg of aspirin per day for 3 weeks.32 Statin-class medications have been shown to reduce CRP levels by 13% to 17%.33-35 Thus, the 15% reduction in CRP levels observed with 6 months of endurance exercise training in our study is very similar to the effects of standard pharmacological therapies on plasma CRP levels. These data clearly indicate that endurance exercise training interventions should be included as another viable strategy to reduce CRP levels in those at risk.
Although in our study both genotype/haplotype and exercise training independently affected plasma CRP levels, no significant gene–exercise training interactive effects on CRP levels were evident. This is somewhat different from the findings of Brull et al,7 in which an interactive effect of acute prolonged military endurance exercise and CRP genotype was evident for plasma CRP levels. However, their findings were in response to acute and intense endurance military exercise, which places substantially different CV and metabolic demands on individuals than our prolonged moderate-intensity endurance exercise training program. Furthermore, they also studied young military recruits, a population quite different from our sedentary middle-aged to older-aged men and women. However, our finding of no interactive effects of CRP genotype and exercise training on CRP levels may be the result of our minimal sample size.
In summary, our findings support an independent role for the CRP +219G/A variant, and to a lesser extent the −732A/G polymorphism, and the +219/−732 haplotype in the modulation of CRP levels. Regardless of whether CRP is an inflammatory mediator of, or a marker for, CV disease risk, genotype-dependent differences in CRP levels may have important prognostic implications. However, although exercise training resulted in a significant reduction in plasma CRP levels in our population, exercise training did not interact with any of the CRP genotypes to differentially alter CRP levels.
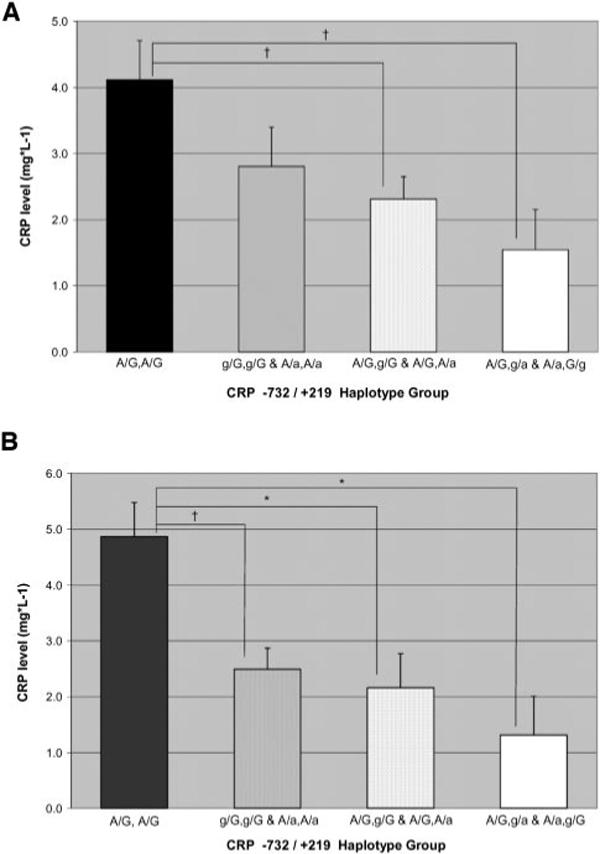
CRP levels at baseline (a) and after aerobic exercise training (b) by CRP −732/+219 haplotype group. †Significant difference between haplotype groups (P≤0.01). *Significant difference between haplotype groups (P≤0.001).
Acknowledgments
This research was supported by the following National Institutes of Health grants: AG17474 (J.M.H.), AG15389 (J.M.H.), AG00980 (T.O.O.), and RR10284 (N.C.R.R.).
References
- 1.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL, Pepys MB. CRP, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA Cohort Study. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 2.Burke GL, Arnold AM, Bild DE, Cushman M, Fried LP, Newman A, Nunn C, Robbins J. Factors associated with healthy aging: the Cardiovascular Health Study. J Am Geriatr Soc. 2001;49:254–262. doi: 10.1046/j.1532-5415.2001.4930254.x. [DOI] [PubMed] [Google Scholar]
- 3.Albert MA, Glynn RJ, Ridker PM. Effect of physical activity on serum C-reactive protein. Am J Cardiol. 2004;93:221–225. doi: 10.1016/j.amjcard.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 5.Szalai AJ, McCrory MA, Cooper GS, Wu J, Kimberly RP. Association between baseline levels of CRP and a dinucleotide repeat polymorphism in the intron of the CRP gene. Genes Immun. 2002;3:14–19. doi: 10.1038/sj.gene.6363820. [DOI] [PubMed] [Google Scholar]
- 6.Zee RY, Ridker PM. Polymorphism in the human CRP gene, plasma CRP concentrations, and the risk of future arterial thrombosis. Atherosclerosis. 2002;162:217–219. doi: 10.1016/s0021-9150(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 7.Brull DJ, Serrano N, Zito F, Jones L, Montgomery HE, Rumley A, Sharma P, Lowe GD, World MJ, Humphries SE, Hingorani AD. Human CRP gene polymorphism influences CRP levels: implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:2063–2069. doi: 10.1161/01.ATV.0000084640.21712.9C. [DOI] [PubMed] [Google Scholar]
- 8.Cao H, Hegele RA. Human CRP 1059G/C polymorphism. J Hum Genetics. 2000;45:100–101. doi: 10.1007/s100380050022. [DOI] [PubMed] [Google Scholar]
- 9.Halverstadt A, Phares DA, Ferrell RE, Wilund KR, Goldberg AP, Hagberg JM. HDL-C, its subfractions, and responses to exercise training are dependent on endothelial lipase genotype. Metabolism. 2003;52:1505–1511. doi: 10.1016/s0026-0495(03)00284-1. [DOI] [PubMed] [Google Scholar]
- 10.Leon AS, Togashi K, Rankinen T, Despres JP, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Association of apolipoprotein E polymorphism with blood lipids and maximal oxygen uptake in the sedentary state and after exercise training in the HERITAGE study. Metabolism. 2004;53:108–116. doi: 10.1016/j.metabol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Dietary guidelines for healthy Am adults A statement for physicians and health professionals by the AHA Nutrition Committee. Circulation. 1988;77:721A–724A. [PubMed] [Google Scholar]
- 12.Wilund KR, Colvin PL, Phares D, Goldberg AP, Hagberg JM. The effect of endurance exercise training on plasma lipoprotein AI and lipoprotein AI:AII concentrations in sedentary adults. Metabolism. 2002;51:1053–1060. doi: 10.1053/meta.2002.33356. [DOI] [PubMed] [Google Scholar]
- 13.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avise JC, Lansman RA, Shade RO. The use of restriction endonucleases to measure mitochondrial DNA sequence relatedness in natural populations. I. Population structure and evolution in the genus Peromyscus. Genetics. 1979;92:279–295. doi: 10.1093/genetics/92.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Levine L, Kwok PY. Fluorescence polarization in homogeneous nucleic acid analysis. Genome Res. 1999;9:492–498. [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Statistical Analysis System (SAS) Changes and Enhancements, Release 8.0. SAS Institute, Inc; Cary, NC: 2001. [Google Scholar]
- 18.Ridker PM. Clinical application of CRP for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 19.Hong MK, Park SW, Lee CW, Kim YH, Kim JH, Song JM, Kang DH, Song JK, Kim JJ, Park SJ. Prospective comparison of coronary artery remodeling between acute coronary syndrome and stable angina in single-vessel disease: correlation between CRP and extent of arterial remodeling. Clin Cardiol. 2003;26:169–172. doi: 10.1002/clc.4960260404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maksimowicz-McKinnon K, Bhatt DL, Calabrese LH. Recent advances in vascular inflammation: CRP and other inflammatory biomarkers. Curr Opin Rheumatol. 2004;16:18–24. doi: 10.1097/00002281-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Vickers MA, Green FR, Terry C, Mayosi BM, Julier C, Lathrop M, Ratcliffe PJ, Watkins HC, Keavney B. Genotype at a promoter polymorphism of the interleukin-6 gene is associated with baseline levels of plasma CRP. Cardiovasc Res. 2002;53:1029–1034. doi: 10.1016/s0008-6363(01)00534-x. [DOI] [PubMed] [Google Scholar]
- 22.Volanakis JE. Human CRP. expression, structure, and function. Mol Immunol. 2001;38:189–197. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 23.Merika M, Thanos D. Enhanceosomes. Curr Opin Genet Dev. 2001;11:205–208. doi: 10.1016/s0959-437x(00)00180-5. [DOI] [PubMed] [Google Scholar]
- 24.LaMonte MJ, Durstine JL, Yanowitz FG, Lim T, DuBose KD, Davis P, Ainsworth BE. Cardiorespiratory fitness and CRP among a tri-ethnic sample of women. Circulation. 2002;106:403–406. doi: 10.1161/01.cir.0000025425.20606.69. [DOI] [PubMed] [Google Scholar]
- 25.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 26.Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–1790. doi: 10.1161/hc1502.107117. [DOI] [PubMed] [Google Scholar]
- 27.Rothenbacher D, Hoffmeister A, Brenner H, Koenig W. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med. 2003;163:1200–1205. doi: 10.1001/archinte.163.10.1200. [DOI] [PubMed] [Google Scholar]
- 28.Isasi CR, Deckelbaum RJ, Tracy RP, Starc TJ, Berglund L, Shea S. Physical fitness and CRP level in children and young adults. Pediatrics. 2003;111:332–338. doi: 10.1542/peds.111.2.332. [DOI] [PubMed] [Google Scholar]
- 29.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 30.Kayaba K, Ishikawa S, Gotoh T, Nago N, Kajii E, Nakamura Y, Kario K. Five-year intra-individual variability in CRP levels in a Japanese population-based study. Jpn Circ J. 2000;64:303–308. doi: 10.1253/jcj.64.303. [DOI] [PubMed] [Google Scholar]
- 31.de Ferranti S, Rifai N. CRP and cardiovascular disease: a review of risk prediction and interventions. Clin Chim Acta. 2002;317:1–15. doi: 10.1016/s0009-8981(01)00797-5. [DOI] [PubMed] [Google Scholar]
- 32.Ikonomidis I, Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation. 1999;100:793–798. doi: 10.1161/01.cir.100.8.793. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma CRP concentration. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 34.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 35.Jialal I, Stein D, Balis D, Grundy SM, Adams-Huet B, Devaraj S. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitivity CRP levels. Circulation. 2001;103:1933–1935. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]


