Abstract
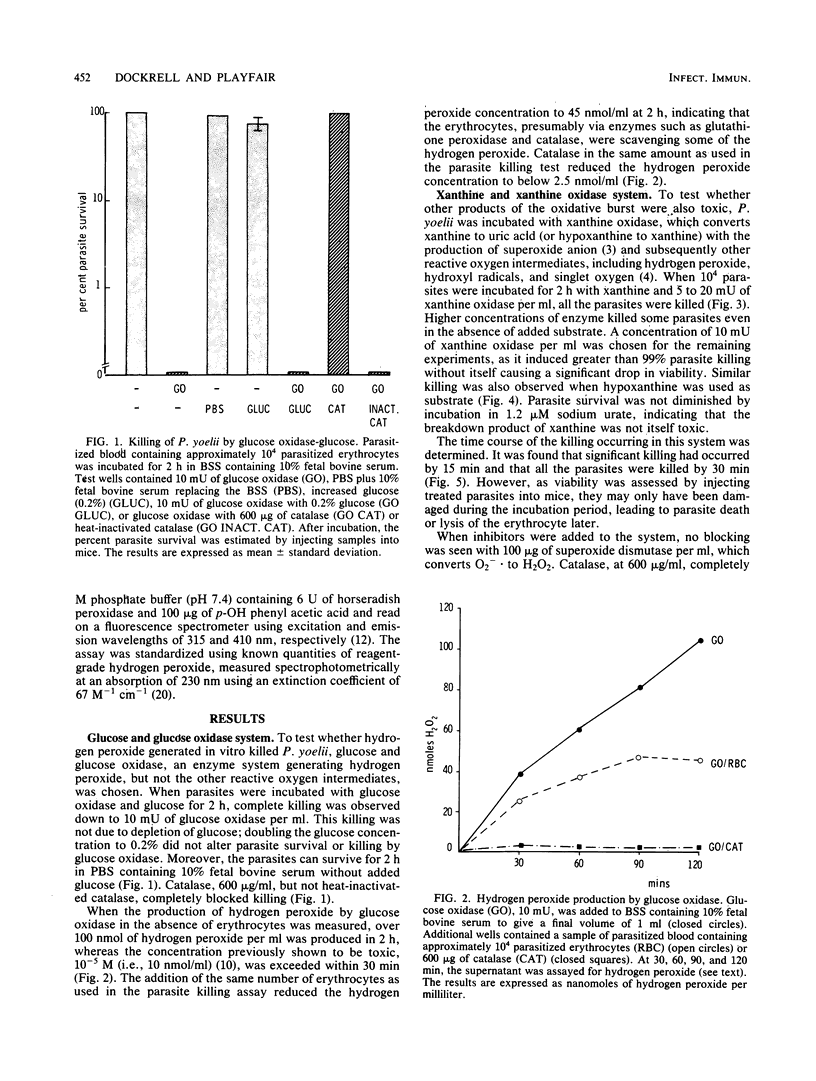
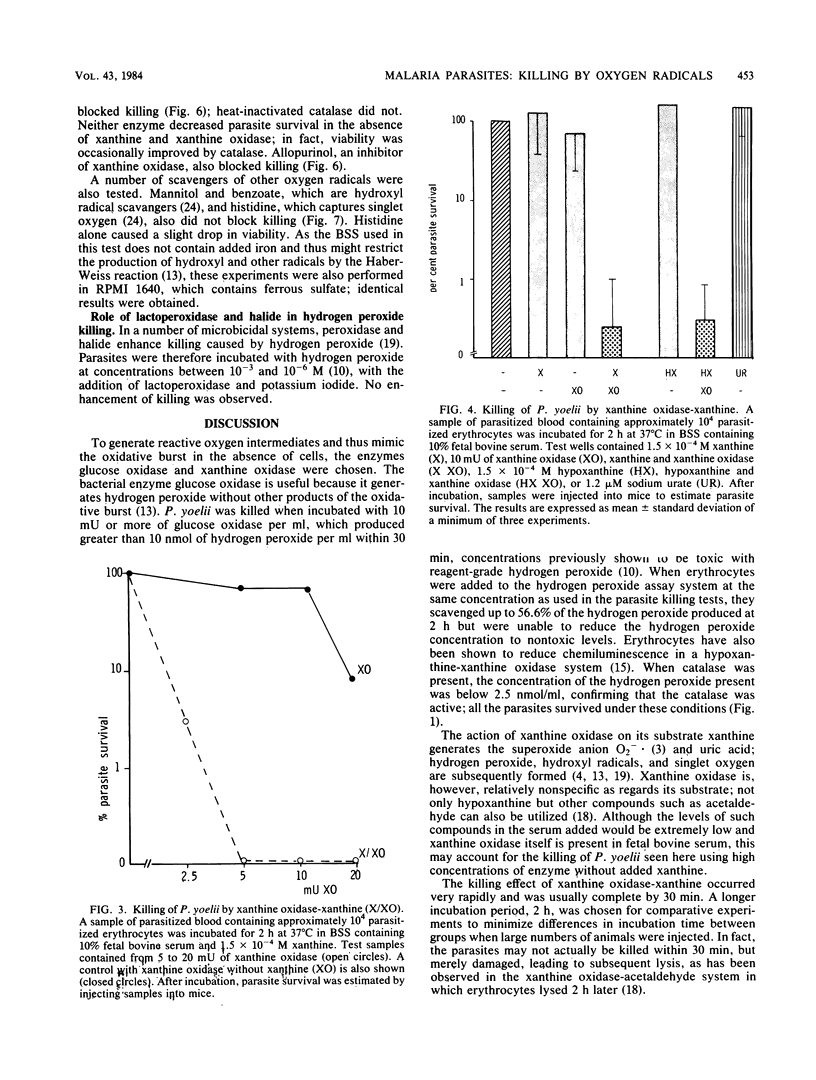
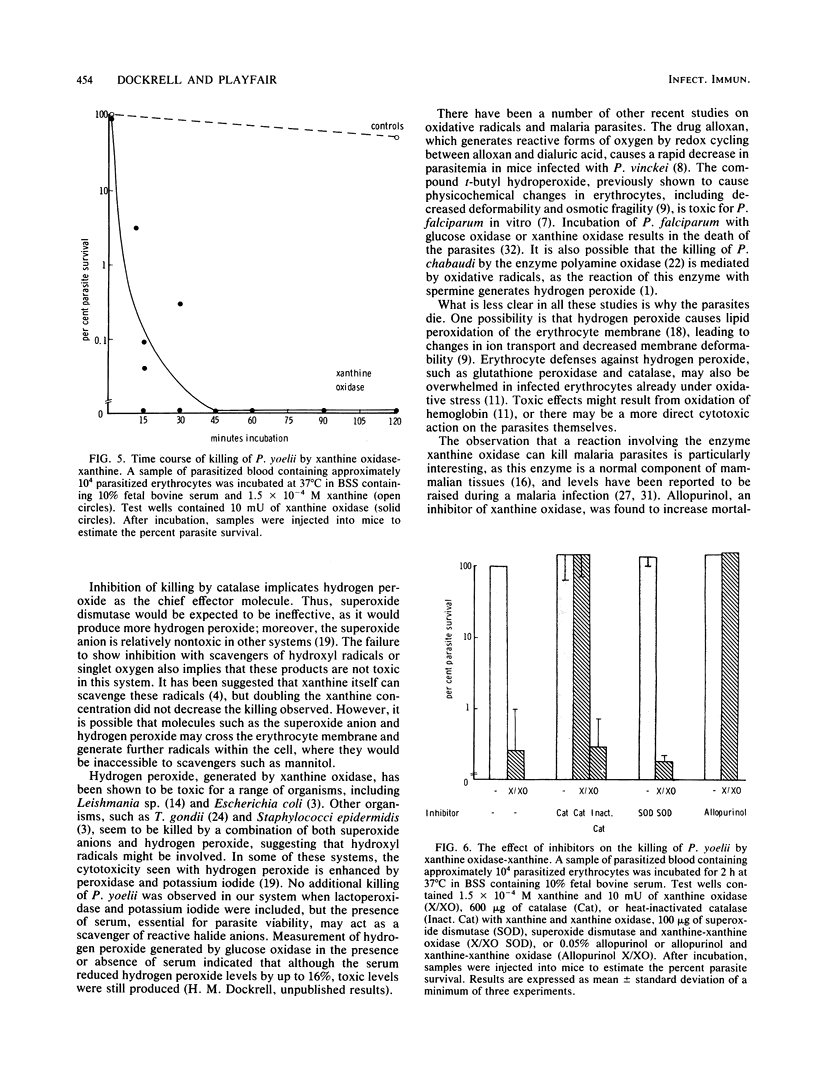
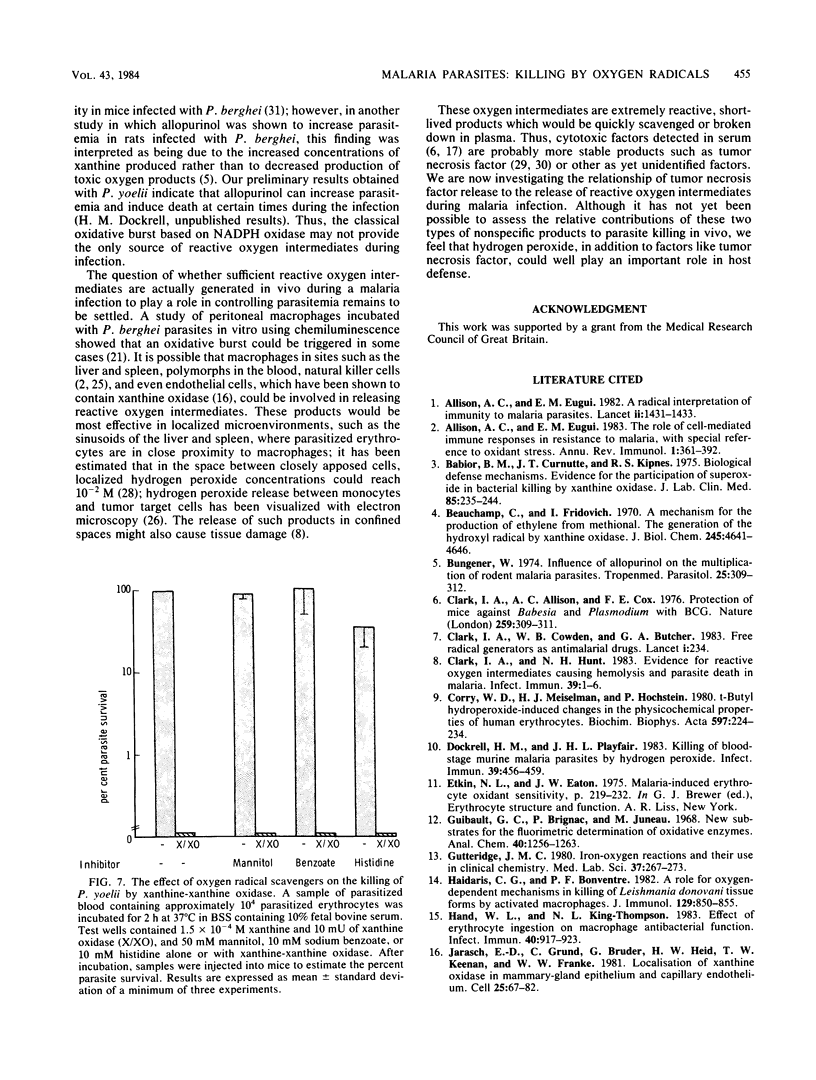
The murine malaria parasite Plasmodium yoelii was killed in vitro when incubated with glucose and glucose oxidase, a system generating hydrogen peroxide, or with xanthine and xanthine oxidase, a system which produces the superoxide anion and subsequently other products of the oxidative burst. Catalase blocked the killing in both cases; superoxide dismutase and scavengers of hydroxyl radicals or singlet oxygen were ineffective in the xanthine oxidase system. Thus, hydrogen peroxide appears to be the main reactive oxygen species killing P. yoelii.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. A radical interpretation of immunity to malaria parasites. Lancet. 1982 Dec 25;2(8313):1431–1433. doi: 10.1016/s0140-6736(82)91330-7. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., Kipnes R. S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975 Feb;85(2):235–244. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Büngener W. Influence of allopurinol on the multiplication of rodent malaria parasites. Tropenmed Parasitol. 1974 Sep;25(3):309–312. [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A. Free oxygen radical generators as antimalarial drugs. Lancet. 1983 Jan 29;1(8318):234–234. doi: 10.1016/s0140-6736(83)92603-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corry W. D., Meiselman H. J., Hochstein P. t-Butyl hydroperoxide-induced changes in the physicochemical properties of human erythrocytes. Biochim Biophys Acta. 1980 Apr 10;597(2):224–234. doi: 10.1016/0005-2736(80)90101-7. [DOI] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbault G. G., Brignac P. J., Jr, Juneau M. New substrates for the fluorometric determination of oxidative enzymes. Anal Chem. 1968 Jul;40(8):1256–1263. doi: 10.1021/ac60264a027. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron-oxygen reactions and their use in clinical chemistry. Med Lab Sci. 1980 Jun;37(3):267–273. [PubMed] [Google Scholar]
- Haidaris C. G., Bonventre P. F. A role for oxygen-dependent mechanisms in killing of Leishmania donovani tissue forms by activated macrophages. J Immunol. 1982 Aug;129(2):850–855. [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Effect of erythrocyte ingestion on macrophage antibacterial function. Infect Immun. 1983 Jun;40(3):917–923. doi: 10.1128/iai.40.3.917-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Akood M. Induction of crisis forms in cultured Plasmodium falciparum with human immune serum from Sudan. Science. 1982 Jun 11;216(4551):1230–1233. doi: 10.1126/science.7043736. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- Makimura S., Brinkmann V., Mossmann H., Fischer H. Chemiluminescence response of peritoneal macrophages to parasitized erythrocytes and lysed erythrocytes from Plasmodium berghei-infected mice. Infect Immun. 1982 Aug;37(2):800–804. doi: 10.1128/iai.37.2.800-804.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981 May 1;153(5):1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Helfand S. L., Werkmeister J., McGarry R., Beaumont T. J., Duwe A. Oxygen intermediates are triggered early in the cytolytic pathway of human NK cells. Nature. 1982 Aug 5;298(5874):569–572. doi: 10.1038/298569a0. [DOI] [PubMed] [Google Scholar]
- Seim S., Espevik T. Toxic oxygen species in monocyte-mediated antibody-dependent cytotoxicity. J Reticuloendothel Soc. 1983 Jun;33(6):417–428. [PubMed] [Google Scholar]
- Sharma O. P., Singh C., Shukla R. P., Sen A. B. Xanthine oxidase in rodent malaria. Indian J Exp Biol. 1978 Jun;16(6):665–667. [PubMed] [Google Scholar]
- Taverne J., Depledge P., Playfair J. H. Differential sensitivity in vivo of lethal and nonlethal malarial parasites to endotoxin-induced serum factor. Infect Immun. 1982 Sep;37(3):927–934. doi: 10.1128/iai.37.3.927-934.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Dockrell H. M., Playfair J. H. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect Immun. 1981 Jul;33(1):83–89. doi: 10.1128/iai.33.1.83-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubaro E., Lotti B., Cavallo G., Croce C., Borelli G. Liver xanthine oxidase increase in mice in three patholgoical models. A possible defence mechanism. Biochem Pharmacol. 1980 Jul 1;29(13):1939–1943. doi: 10.1016/0006-2952(80)90107-0. [DOI] [PubMed] [Google Scholar]
- Wozencraft A. O., Dockrell H. M., Taverne J., Targett G. A., Playfair J. H. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984 Feb;43(2):664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


