Abstract
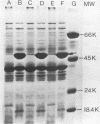
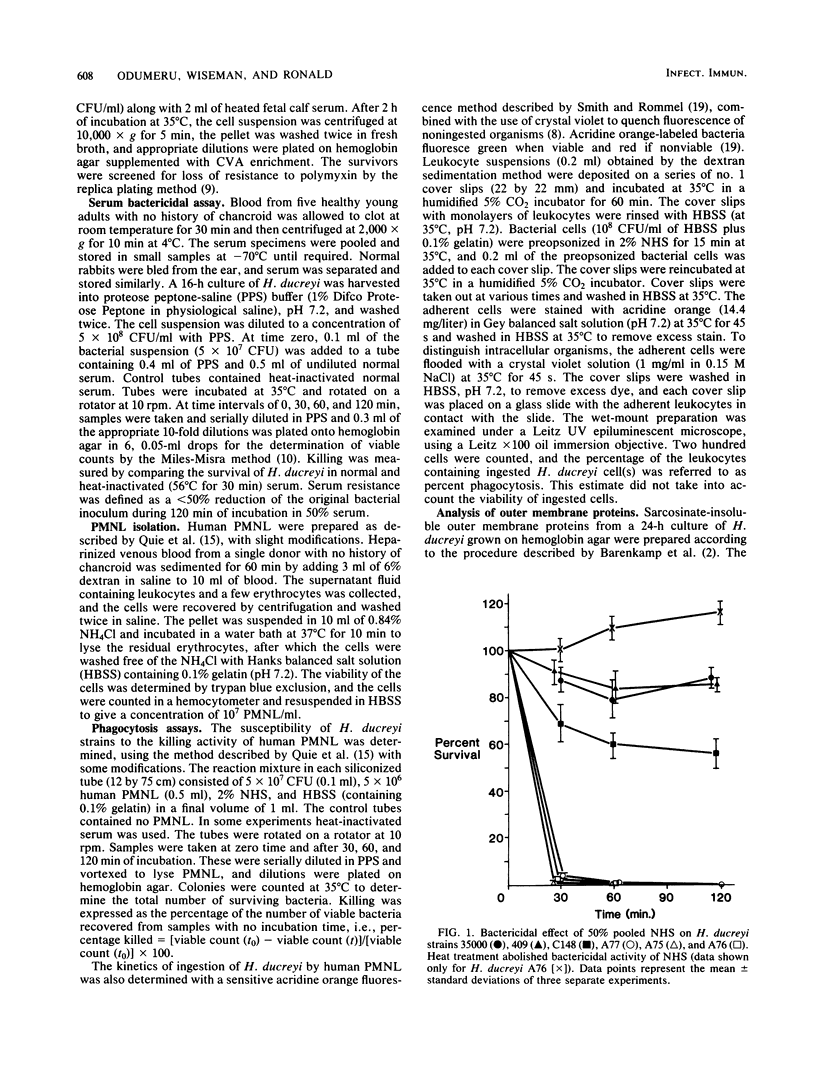
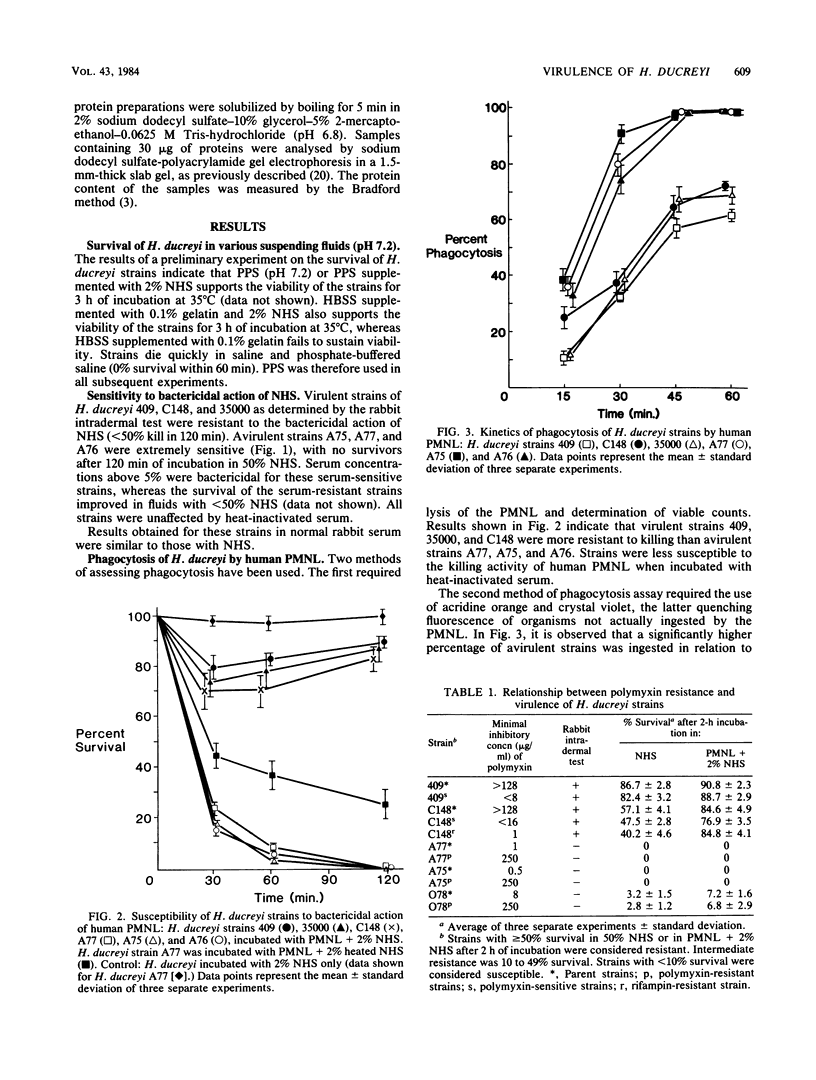
We investigated the susceptibility of virulent and avirulent strains of Haemophilus ducreyi to the bactericidal activity of normal human serum and to phagocytosis and killing by human polymorphonuclear leukocytes (PMNL). Strains were defined as virulent if intradermal inoculation into a rabbit produced a typical necrotic lesion. Nonvirulent strains produced no cutaneous lesions in rabbits. Virulent strains were resistant to the complement-mediated lethal action of normal human and rabbit sera, whereas avirulent strains were susceptible (greater than 95% kill, 60 min). Virulent strains were relatively resistant to phagocytosis and killing by human PMNL, in contrast to the avirulent strains. In past studies polymyxin resistance has been correlated with virulence in H. ducreyi. In our studies, polymyxin resistance could not be correlated with virulence, since polymyxin-sensitive mutants obtained from polymyxin-resistant parent strains remained virulent for rabbits and resistant to bactericidal action of normal serum and phagocytosis and killing by human PMNL. Similarly, polymyxin-resistant mutants obtained from polymyxin-sensitive parent strains remained avirulent for rabbits and susceptible to bactericidal action of normal serum and PMNL. The acquisition of polymyxin resistance was accompanied by the loss of a 47,000-molecular-weight protein. The association of serum resistance and resistance to phagocytosis and killing by human PMNL with virulent strains, as defined by the rabbit intradermal test, suggests that these factors may mediate the pathogenicity of H. ducreyi.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DEACON W. E., SINGER S. Effects of penicillin G in vitro on Hemophilus ducreyi. Public Health Rep. 1956 Nov;71(11):1112–1114. [PMC free article] [PubMed] [Google Scholar]
- Frank M. M. The complement system in host defense and inflammation. Rev Infect Dis. 1979 May-Jun;1(3):483–501. doi: 10.1093/clinids/1.3.483. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCHEL L. H. Serum bactericidal actions. Ann N Y Acad Sci. 1960 Nov 21;88:1265–1272. doi: 10.1111/j.1749-6632.1960.tb20117.x. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Olling S. Sensitivity of gram-negative bacilli to the serum bactericidal activity: a marker of the host-parasite relationship in acute and persisting infections. Scand J Infect Dis Suppl. 1977;(10):1–40. doi: 10.3109/inf.1977.9.suppl-10.01. [DOI] [PubMed] [Google Scholar]
- Pai C. H., DeStephano L. Serum resistance associated with virulence in Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):605–611. doi: 10.1128/iai.35.2.605-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff M. S., Ricupero I., Rahal J. J., Jr Host resistance to Serratia marcescens infection: serum bactericidal activity and phagocytosis by normal blood leukocytes. J Lab Clin Med. 1976 Feb;87(2):206–217. [PubMed] [Google Scholar]
- Smith D. L., Rommel F. A rapid micro method for the simultaneous determination of phagocytic-microbiocidal activity of human peripheral blood leukocytes in vitro. J Immunol Methods. 1977;17(3-4):241–247. doi: 10.1016/0022-1759(77)90106-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]