Abstract
The etiology of Parkinson’s disease (PD) was long thought to be due to environmental factors. Following the discovery of autosomal-dominant mutations in the α-synuclein gene, and later recessive mutations in the DJ-1, Parkin and PINK-1 genes, the field of PD genetics exploded. In 2004, it was discovered that mutations in the PARK8 locus – leucine-rich repeat kinase 2 (LRRK2, Lrrk2) – are the most important genetic cause of autosomal-dominant PD. Lrrk2 substitutions also account for sporadic PD in certain ethnic populations and have been shown to increase the risk of PD in Asian populations. Drug therapies targeting Lrrk2 activity may therefore be beneficial to both familial and sporadic PD patients, hence understanding the role of Lrrk2 in health and disease is critical. This review aims to highlight the research effort concentrated on elucidating the functional biological role of Lrrk2, and to provide some future therapeutic perspectives.
Keywords: GTPase, kinase, LRRK2, models, neurodegeneration, neurogenesis, Parkinson’s disease
Genetics: PARK 8 linkage & discovery of Lrrk2 mutations
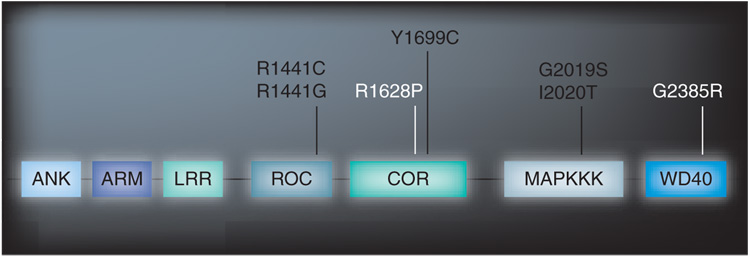
In 2004, mutations in the PARK8 locus; leucine-rich repeat kinase 2 (LRRK2, Lrrk2), were discovered to be the most important genetic cause of autosomal-dominant PD [1]. The PARK8 linkage locus was originally mapped to chromosome 12p11-q13 in the Japanese Sagamihara family, whose diagnosis was l-DOPA-responsive, late-onset parkinsonism that resembled idiopathic PD [2]. This linkage was later confirmed in two other families, A (a German–Canadian kindred) and D (from Western Nebraska), who also have autosomal-dominant, late-onset parkinsonism, and subsequent sequence analysis revealed disease-segregating mutations in LRRK2 [3]. The discovery was established by genetic linkage in other families in which LRRK2 sequencing has revealed additional mutations [1,4–6]. While many substitutions in the LRRK2 gene have been described, only a few have been shown to segregate with disease and are proven pathogenic; Lrrk2 R1441C and G affect the Roc domain, a ‘Ras-like’ part of the protein, the Lrrk2 Y1699C substitution is found in the COR domain and Lrrk2 G2019S and I2020T are within exon 41 at the ‘activation hinge’ of the MAPK domain (Figure 1) [7,8].
Figure 1. Lrrk2 protein showing predicted domains.
Pathogenic substitutions are shown in black and substitutions associated with increase risk shown in white.
The most frequent mutation, Lrrk2 G2019S, originates from a common founder [4], presents with age-dependent penetrance and is responsible for 0.5–2.0% of cases of sporadic PD and 5% of familial parkinsonism in Caucasians [9,10]. Remarkably, it accounts for 29% of Ashkenazi Jews and 37% of North African Arabs with familial parkinsonism and for 13 and 30%, respectively, of patients with seemingly sporadic PD in these two populations [4,9,11]. Furthermore, the Lrrk2 G2385R and R1628P substitutions are risk factors for sporadic PD in Asian populations [12–14].
Pathology of Lrrk2-associated parkinsonism
Lrrk2 G2019S is primarily associated with transitional or brainstem Lewy body pathology, reminiscent of typical, late-onset idiopathic PD [15]. However, one Lrrk2 G2019S case with 4-repeat tauopathy and one case with ubiquitin-positive inclusions, with nigral neuronal loss and gliosis, have also been described [16,17]. Postmortem analysis of affected subjects from other PARK8-linked kindreds, including family A with Lrrk2 Y1699C, family D with Lrrk2 R1441C and the Sagamihara family with Lrrk2 I2020T, have also revealed pleomorphic pathology including neuronal loss without co-existing pathology, and with -synuclein, tau or ubiquitin-positive inclusions [18]. Remarkably, this pleomorphic pathology can occur among carriers of the same pathogenic mutation and even the affected members of the same family can present with different pathological features [3,19]. Overlapping pathology is common to many neurodegenerative diseases and the ability of Lrrk2 mutations to lead to lesions reminiscent of those seen in other disorders, such as Alzheimer’s disease and progressive supranuclear palsy, is intriguing. Whether Lrrk2 exists in Lewy bodies or Lewy neurites, the pathological hallmark of PD, remains c ontentious [20–27].
Lrrk2 structure
The LRRK2 transcript encodes a 2527 amino acid protein that is comprised of 51 exons. Structurally Lrrk2 is a large protein (286 kDa), consistent with that of ROCO proteins [28] with several predicted conserved domains; a leucine-rich repeat (LRR), a Ras in complex proteins (ROC) that belongs to the Rab GTPase superfamily, a C-terminal of Roc (COR) domain, a MAPKKK and a WD40 domain [3]. Whilst the occurrence of a kinase and GTPase domain suggests enzymatic function, the presence of the protein interaction LRR and WD40 domains along with N-terminal armadillo and ankyrin repeats, suggest an additional/alternative role as a scaffolding protein [29]. LRRK1 is the closest human homologue on chromosome 15q26, with 70% homology of Roc, COR and kinase domains. The two proteins differ mostly in the organization of their respective amino-terminus. Lrrk2 possesses several specific repeat stuctures absent in the Lrrk1 homolog. Of note, no PD-causing mutations have been identified in LRRK1 [30].
Anatomical localization of Lrrk2
One of the first steps towards elucidation of the functional role and/or pathogenic mechanisms of Lrrk2 was to establish its normal expression pattern. Outside of the CNS, high expression is found in the lungs, kidney, spleen and leucocytes [3,31–34]. In mice, during embryonic development LRRK2 mRNA expression is not detectable before E15 after which it steadily rises, suggesting it is not an essential developmental gene [31]. Lrrk2 protein is evident by E17 [31] and continues to rise progressively and peaks around P60 [33]. Levels of Lrrk2 remain constant in rodents during aging [34]. To date, studies in brain have consistently described high levels of LRRK2 mRNA in the dorsal striatum and cortex in rodents, with the relative intensity of LRRK2 mRNA signal in other brain structures, including the substantia nigra, generally being lower [35–39]. Radioactive in situ hybridization with oligonucleotide probes [35,36] failed to detect LRRK2 mRNA in the substantia nigra; however, nonradioactive in situ hybridization with riboprobes suggests low levels of LRRK2 mRNA [37,38], which complements reverse transcription-PCR results [36,38]. Lrrk2 protein expression is neuronal rather than glial and in agreement with mRNA studies has been demonstrated to be high in the dorsal striatum and cortex, with expression also found in the olfactory tubercle, nucleus accumbens, hippocampus, cerebellum and, albeit lower, in the substantia nigra [20,21,39,40]. We recently extended these observations to highlight Lrrk2 protein expression in other brain structures, including the septal nuclei, thalamus, hypothalamus, mammillary nuclei, brainstem nuclei and the subventricular zone (SVZ).
Expression of the homolog LRRK1 is similar to that of LRRK2, being widely expressed in brain, especially in the frontal cortex and hippocampus [31,34,41], although the striatum is rich in LRRK2 but devoid of LRRK1 [34]. Given their high homologies regarding domain organization, the similarities in the expression profiles of LRRK1 and LRRK2 may point towards functional redundancy [30,31].
Whilst it is clear that Lrrk2 has a significant presence in dopamine-receptive areas, current evidence suggests that altered dopamine transmission has no effect on LRRK2 mRNA in PD patients in the striatum where dopamine D2 receptor depletion is evident [35,42], or in the substantia nigra where dopamine neurons are lost [42]. Similarly, vesicular monoamine-deficient mice do have altered levels of LRRK2 mRNA [43]. Interestingly, LRRK2 mRNA is increased in l-DOPA induced dyskinetic marmosets after 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine (MPTP) treatment [42]. The effect of altered dopamine neurotransmission on Lrrk2 protein is unknown.
The expression of Lrrk2 in many nondopaminergic areas suggests that it may have a fundamental role in basic cellular function. It remains to be seen if Lrrk2 dysfunction leads to selective vulnerability in specific cellular populations.
Cellular & subcellular localization
When transfected in a variety of cell lines, Lrrk2 is largely excluded from the nucleus and is present in diffuse localization throughout the cytoplasm [20,44–47], possibly in association with mitochondria [44,46]. Although Lrrk2 does not contain any hydrophobic transmembrane domains or obvious mitochondrial or nuclear-targeting sequences [46], subcellular fractionation reveals Lrrk2 in membranous and microsomal fractions [44,46,48]. In addition, Lrrk2 is found in lipid rafts, microdomains on the cellular membrane of membranous organelles for example, golgi apparatus, plasma membrane, synaptic vesicles, mitochondria and endoplasmic reticulum [48]. However, it is thought to be membrane associated rather than membrane integrated [44,46,48]. Similar results were observed in fractionation of rat primary cultures, where Lrrk2 punctate structures particularly colocalize to mitochondria and lysosomal markers [40]. In adult rodent brain tissue, Lrrk2 was also particularly enriched in fractions containing or associated with microsomal, synaptic vesicle and synaptic cytosolic fractions [40].
The association of Lrrk2 with membranous and membrane-bound organelles suggests that Lrrk2 may play an important regulatory role in synaptic function, possibly by regulation of vesicle synthesis or transport and/or regulation of membranous structures. However, this is with the assumption that antibodies to endogenous Lrrk2 protein are specific, and that tagged exogenous Lrrk2 does not mislocalize or aggregate when artificially overexpressed. Clearly, there is a pressing need in the field for better antibodies for detection of endogenous Lrrk2 [31] and a better validation of existing antibodies for specific applications, for example, western blotting, immunoprecipitation and immunohistochemistry.
Functional activity of Lrrk2
Does Lrrk2 form a dimer?
Lrrk2 is unusual since the presence of both a kinase domain and a GTPase domain suggests two distinct enzymes are encoded, and this raises the possibility that they may be functionally linked. Dimerization is a common feature of many protein kinases [49] and Ras GTPases [50], and elucidating the homo- or hetero-dimeric interactions of Lrrk2 may facilitate understanding of how mutations in the different domains can lead to the same disease, as well as aid therapeutic design. In human lymphoblastoid cell lines, endogenous human Lrrk2 appears to dimerize, with the presence of approximately 600 kDa peak and higher molecular weight complex in immunoblots [51]. Several in vitro studies studying exogenous Lrrk2 have reported the ability to dimerize and involve multiple domain swapping [44,51–53]. Whilst support for kinase domain dimeric interactions are lacking [44,51], the Roc domain is able to interact with itself and with the central Roc–COR–kinase region of full-length Lrrk2, an interaction that is strengthened by the WD40 domain [51,53]. Roc–Roc interaction is not nucleotide dependent, but it is weakened by the R1441C pathogenic mutation, indicating that destabilizing dimeric interactions may be important in aberrant Lrrk2 GTPase activity [53]. The caveat is that the I1371V mutation, used to support this hypothesis, is not pathogenic but a benign polymorphism found at appreciable frequency in elderly controls [Owen Ross, Pers. Comm.].
Kinase activity of Lrrk2
Initial in silico modeling suggesting that Lrrk2 mutations may have increased kinase activity and act through a dominant ‘gain-of-function’ placed Lrrk2 kinase activity in the Parkinson’s spotlight [54]. Since abnormal phosphorylation is a common feature in neurodegenerative diseases and Lrrk2 mutations can lead to pleomorphic pathologies, targeting kinase activity represents an attractive ‘druggable’ target not just for PD but for other neurodegenerative disorders.
The first evidence for Lrrk2 mutations augmenting kinase activity was demonstrated using full length human wild-type (WT) and mutant G2019S and R1441C expressed in HEK-293T and SH-SH5Y cells [46]. Since then, the G2019S mutation has been reported to enhance kinase activity compared with WT activity [45,47,55,56]. The I2020T mutation has been reported to increase kinase activity [44,57] but also to reduce kinase activity [55]. Data on kinase alterations by mutation at position R1441 are conflicting, with reports of no change [20,55,58] and increased [47,57,59] for R1441C; and unaltered [55] and increased [57] for R1441G. Finally, the Y1699C mutation is reported as unaltered [20,55] and increased [57].
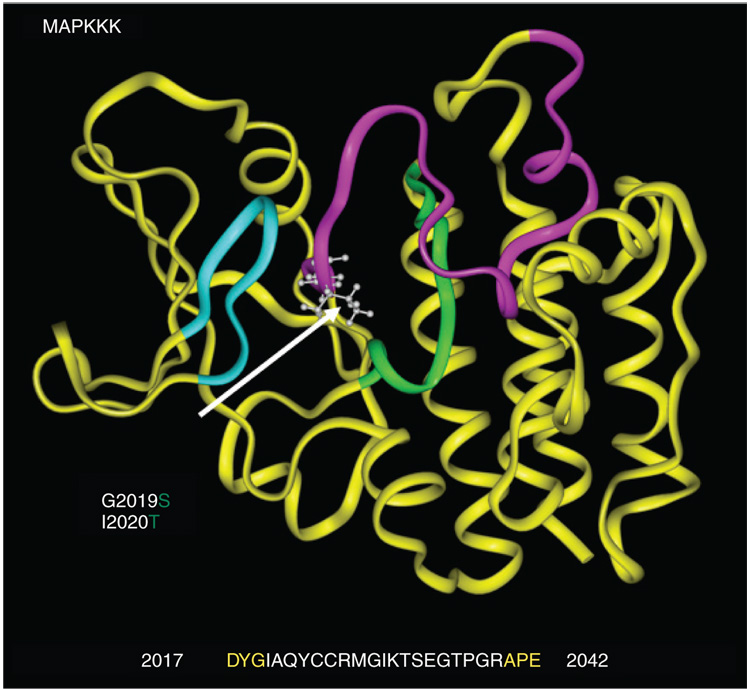
These conflicting data on differential mutant Lrrk2 kinase activity are most likely due to differences in the constructs used (full length and partial and recombinant constructs containing just functional domains), expression systems used (ranging from bacterial expression, expression in different cell lines including HEK293, Cos-7, SH-SY5Y and in transgenic mice via FLAG-tagged or viral constructs) and assay methodology (autophosphorylation and/or myelin basic protein phosphorylation assays and use of artificial peptides). Despite these differences, Lrrk2 G2019S has consistently been shown to be more active than WT Lrrk2. Based on homology to similar serine–threonine protein kinases, the G2019S substitution appears to be within the DYG hinge of the activation segment (Figure 2). This serves to anchor and close a ribbon of protein (the ‘activation segment’) that protects the catalytic site. Hence, the G2019S mutation likely keeps the site open, resulting in increased kinase activity consistent with a dominant gain-of-function [4,54]. The effect of the remaining mutations may be resolved by in vivo animal models bearing the different mutations and the development of more specific antibodies to improve kinase activity measurements.
Figure 2. MAPKKK domain acids 1800–2138.
The kinase active site (green) is centrally located alongside the ATP-binding region (turquoise). The activation segment (magenta) lies between conserved residues DYG and APE. Lrrk2 G2019S and I2020T mutations (indicated by arrow) change highly conserved glycine (G) and isoleucine (I) at the beginning of the activation segment. The activation segment typically blocks substrate access to the catalytic site.
(Figure courtesy of J Johnson, Mayo Clinic).
GTP binding & GTPase activity
The GTPase domain of Lrrk2 shares sequence homology with the Ras-related small GTPase family, including the conservation of amino acids involved in GTP binding and hydrolysis [28]. Ras-GTPases act as molecular switches that cycle between GTP-bound (active) and GDP-bound (inactive) conformations, a process that regulates many diverse cell signaling functions. This process is additionally facilitated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) [59 ].
The functional relationship between the Lrrk2 GTPase binding and kinase domain has been a major research focus with particular attention to the questions:
Can Lrrk2 can bind GTP?
Is Lrrk2 a GTPase?
Does Lrrk2 GTPase activity regulate its kinase activity?
These questions are important since the modulation of kinase activity by targeting the GTPase domain GEFs or GAPS may represent alternative therapeutic targets.
Several groups now have shown that Lrrk2 can bind GTP via GTP-sepharose or GTPγS-binding assays, whereas disruption of the P-loop in the nucleotide-binding pocket of the Roc domain abolishes GTP binding [33,47,53,57–60]. GTPase activity has been more difficult to demonstrate [57,60], presumably owing to the requirement of GEFs and GAPs for in vitro assays; however, optimized experimental conditions have now enabled GTPase activity, albeit low, to be detected [33,53,58,59]. Several groups now agree that Lrrk2 Roc domain mutations do not increase GTP binding [33,58,59], but Roc mutants do reduce GTPase activity [33,58,59] and suggest that Roc domain mutations prolong Lrrk2 in the active GTP-bound state. In support of this, crystal structure studies show that the active GTPase domain of Lrrk2 has a unique dimeric fold and support a prolonged hydrolysis hypothesis, the prediction being that mutations in the ROC domain disrupt this tertiary structure [53].
Functional link between Lrrk2 kinase & GTPase domains
The Roc domain on its own is sufficient for GTPase activity and is independent of kinase activity, since kinase-dead mutants can still bind GTP [33,59,60]. However, the presence of GTPase activity appears to be required for Lrrk2 kinase activity [47,57]. Indeed, Lrrk2-mediated phosphorylation is stimulated by binding of nonhydrolyzable GTP analogs, suggesting that Lrrk2 is activated by MAPKKK intramolecularly by its own GTPase [59]. Furthermore, Roc domain mutations R1441C/G lead to enhanced kinase activity, presumably by prolonging Lrrk2 in the active GTP-bound state [57,59] (although one report did not replicate this finding [58]). It remains to be seen whether GTPase activity can regulate kinase activity in vivo, for which the development of animal models will be imperative, and to subsequently test the therapeutic benefit of targeting GTPase activity.
In vitro overexpression of Lrrk2 causes neurodegereration
Overexpressed Lrrk2 can cause protein aggregate formation and neurodegeneration in vitro. In HEK293 cells, a small portion of cells transfected with WT and mutant G2019S, Y1699C and R1441C contained Lrrk2 aggregates [61]. In human neuroblastoma SH-SY5Y cell lines and in mouse E15/16 primary cortical cultures, neuronal degeneration was observed in WT cultures; however, degeneration was significantly increased in mutant R1441C, G2019S, Y1699C and was accompanied by apoptosis [61]. In agreement with these findings, the formation of Lrrk2 inclusion bodies and the presence of apoptotic cells was more pronounced in mutants R1441C, G2019S and Y1699C compared with WT in Cos-7 cells [20]. Additionally, Lrrk2 potentiated oxidative stress toxicity via hydrogen peroxide in WT and mutants; G2019S, Y1699C and I2020T [57]. In the same experiments using kinase-dead mutants, toxicity induced by Lrrk2 was reduced, suggesting that kinase activity may mediate neurodegeneration in vitro [20,57,61]. GTPase-dead mutants also showed decrease toxicity, again lending further support to the intramolecular control of kinase activity by the GTPase-activity domain [57,61].
A further interesting and supporting observation is that neurite length shortening and reduced branching occurs in rat primary cortical neurons expressing G2019S, R1441C or I2020T mutations when compared with WT, whereas kinase-dead K1906M mutation or Lrrk2 short hairpin RNA-treated cells have increased neurite length [45]. Additionally, in both G21019S and I2020T transfected primary neurons, aggregates were observed that stained positive for phosphorylated tau (which suggests a downstream consequence of abnormal phosphorylation may be disruption of microtubule dynamics) and lysosomal markers (indicative of increased phagocytic, endocytic or autophagic activity and subsequent lysosome targeting) [45]. In a complementary study, transfection of Lrrk2 G2019S (but not WT or kinase-dead) into retinoic acid differentiated SH-SY5Y cells also resulted in reduced neurite length and the formation of autophagic vacuoles in both neuritic and somatic compartments. Treatment with MAPK/ERK inhibitor UO126 reversed reduced autophagy and neurite shortening, implicating this signaling cascade in G2019S-induced pathogenesis [62].
The search for interactors of Lrrk2
Based on homology models, Lrrk2 possesses sequence similarities with tyrosine-like kinases (TLKs), including mixed-lineage kinases and receptor-interacting kinases, which act as serine/threonine kinases but bear homology to tyrosine kinases [29]. TLKs act on the MAPK pathways. Diverse extracellular stimuli provoke MAPK pathways, which are three-tiered cascades comprising of a MAPKKK, MAPKK and the MAPK – each kinase activates the successive kinase through activation-loop phosphorylation [29]. Activation of TLKs may lead to alternate responses, including cell-survival and inflammatory or death-inducing programs that are mediated through the JNK, ERK, MAPK-p38 and NF-κB signaling pathways. Modulation of these pathways may have therapeutic potential in PD [63]. Substrates of MAPKs include transcription factors, mitochondrial proteins and cytosolic proteins.
Protein array studies in PD patient leukocytes have suggested that MAPK activity is altered in PD since phosphorylation of Src, c-JNK and heat shock protein (HSP) 27 is lower in leukocytes from G2019S and idiopathic PD patients compared with healthy controls [32]. Src is involved in the stress response and Hsp27 is thought to help protect cells from stress; therefore, loss of phosphorylation may disrupt their neuroprotective roles. It remains to be seen if these effects are mediated by Lrrk2. Microarray studies in Lrrk2 small interfering RNA-treated SH-SY5Y cells revealed numerous differentially expressed transcripts involved in axonal guidance, nervous system development, cell cycle, growth and differentiation, cell communication MAPKKK cascade and RAS protein signaling transduction [64], although it is important to note that the siRNAs tested were not fully evaluated in this report since no protein knockdown was demonstrated in the SH-SY5Y cell lines. It will be interesting to see whether RNA and protein array-based studies in animal models or in human Lrrk2 brains replicate these findings.
Kinase substrate tracking and elucidation (KESTREL) screening has shown that Lrrk2 may phosphorylate three closely related proteins, ezrin, radixin and moesin (known as ERM proteins), at their analogous residues Thr 567, Thr 564 and Thr 558 [55]. Moesin and radixin are involved in neurite growth cone morphology, motility and process formation in primary cultured neurons [65]. A recent study has demonstrated their critical role in the formation of dendritic filopodia, which are actin-rich protrusions thought to be the precursors of spines during neural development [66]. Given that levels of Lrrk2 are highest amongst spiny neurons of the striatum [26,40], it is tempting to speculate that Lrrk2-mediated phosphorylation of ERM proteins in the striatum may play an important role in spine morphogenesis. However, the original KESTREL assays employed an N-terminally truncated Lrrk2 G2019S protein fragment, and in vivo phosphorylation of ERM proteins by Lrrk2 has yet to be demonstrated.
With regard to Lrrk2 interactors and GTPase activity, a recent publication has shown that Lrrk2 interacts with Rab5 [67]. Rab5 is a small GTPase that acts as a key regulator of endocytic vesicular transport from the plasma membrane to early endosomes [68]. In culture experiments, Lrrk2 overexpression of WT or G2019S and R1441C mutants caused endocytosis deficits that were rescued by Rab5 but not by its inactive form [67]. This supports a role for Lrrk2 in synaptic function by modulating the endocytosis of synaptic vesicles.
An interaction between the Roc domain of Lrrk2 and microtubules α- and β-tubulin has also been reported, although this interaction was not disrupted by the R1441C mutation [69]. Microtubule-dependent transport plays an important role in maintaining neuronal structure and function, and cytoskeletal abnormalities are a prominent feature in neurodegenerative diseases. Interestingly, the Roc domain of another ROCO protein, death-associated protein kinase (DAPk), interacts with actin cytoskeleton cytoskeletal components [70]. Since Lrrk2 colocalizes with β-tubulin in HEK293 cells and primary cortical neurons [40,44] and Lrrk2 G2019S expression in primary cultures leads to aggregation of microtubule-associated protein tau, it is conceivable that Lrrk2 may have a direct or indirect role in cytoskeletal maintenance.
Lrrk2 & the ubiquitin proteasomal degradation pathway
Initial evidence for Lrrk2 involvement in the ubiquitin proteasomal degradation pathway was suggested by the observation that SH-SY5Y cells co-transfected with WT or mutant Lrrk2 and ubiquitin, accumulated increased amounts of polyubiquinated, higher molecular weight Lrrk2 species when treated with proteasomal inhibitor MG-132, as compared with untreated cells [46].
A subsequent co-immunoprecipitation study suggested that Lrrk2 (at the COR domain) interacts with parkin (mediated by the RING2 domain) [61]. Additionally, it was reported that aggregates in Lrrk2 transfected cells were increased by expression with parkin and that parkin increases ubiquitination of aggregated Lrrk2 protein, although these findings have yet to be replicated [71].
Two independent studies identified chaperone Hsp90 to form a complex with Lrrk2 in vitro [44,52]. Supportive evidence in vivo has come from a conditional Lrrk2 G2019S transgenic model in which inhibition of Hsp90 disrupts the association and leads to proteasomal degradation of Lrrk2 [72]. Primary cultures derived from the same mice display axon growth retardation that may be rescued by Hsp90 inhibitors, suggesting that Hsp90 may serve as a target for reducing accumulation of Lrrk2 and its toxic effects.
Lrrk2 in vivo models
In vitro model systems have greatly enhanced our knowledge of Lrrk2 biology and established Lrrk2 kinase activity as a key player in pathological mechanisms; however, to resolve Lrrk2 function in a physiological and pathological context in vivo model systems are needed. Several different approaches have been explored so far; utilizing overexpression/knockout of endogenous Lrrk2 paralogs in invertebrates, and overexpression of human WT and mutant Lrrk2 in invertebrates and in rodents.
Worm models
In the nematode worm Caenorhabditis elegans, knockout of the paralog LRK-1 suggests a role in polarized sorting of synaptic vesicle proteins to axons by excluding them from dendrite-specific transport machinery in the Golgi [73]. LRK-1-knockout worms have a chemosensory deficit, which is interesting since marked olfactory dysfunction is a frequent and early abnormality in PD. C. elegans expressing human WT Lrrk2 have a longer lifespans than G2019S or nontransgenic worms [74]. Human Lrrk2 WT worms also show significantly less vulnerability to the toxin rotenone compared with nontransgenic worms, and surprisingly, G2019S worms also had less vulnerability to rotenone. Conversely, LRK-1 knockdown in worms potentiates rotenone toxicity. These results are comparable to findings for Parkin DJ-1 and PINK1, genes implicated in early-onset parkinsonism, and may suggest Lrrk2 function fits within the same pathway(s). However, a lack of specificity also suggests that C.elegans has a limited repertoire of responses to rotenone-induced oxidative stress and mitochondrial dysfunction.
Fly models
In the fruit fly Drosophila melanogaster, loss- of-function of the LRRK2 paralog ‘dLRRK’ (CG5483) induces locomotive impairment and a reduction in tyrosine hydroxylase (TH) immunostaining within dopaminergic neurons, as well as loss of fertility in females [75]. A second study using the same fly line did not detect motor impairment and also showed that dLRRK loss-of-function flies are resistant to the mitochondrial toxins rotenone and paraquat, but are more sensitive to general oxidative stress, suggesting a role for dLRRK in mitochondria [76]. Overexpression of dLRRKWT or Roc domain substitution R1069C (comparable to Lrrk2 R1441C in humans) using a number of different GAL4 drivers (directed to muscle, whole-body and dopaminergic neurons) produced no deleterious effects [75]. The effects of overexpression of human WT and G2019S Lrrk2 were investigated using GAL4 drivers for the photoreceptor cells (gmr-GAL4) or in dopaminergic neurons (ddc-GAL4) [77]. Progressive retinal degeneration was observed in gmr-GAL4 WT and G2019S flies similar to that shown in other fly neurodegeneration models. In ddc-GAL WT, and to a greater extent ddc-GAL4 G2019S flies, progressive loss of TH neurons, impaired climbing and a decreased locomotor activity was observed, which was responsive to l-DOPA treatment. Furthermore, expression of pan-neuronal (elav-GAL4) of human WT and G2019S Lrrk2 showed increased kinase activity, climbing deficit and a reduction in dopaminergic neurons in the elav-GAL4 G2019S (and to a lesser extent in elav-GAL4 WT), suggesting mutant Lrrk2 toxicity may be selective for dopaminergic neurons.
Rodent models
Invertebrate models provide an excellent rapid test system to investigate gene function and gene–environment interaction; however, there are three major disadvantages that limit their usefulness in PD models:
The LRRK genes are not true orthologues of LRRK2 [78]
They lack an equivalent to the basal ganglia
Their short lifespan leaves the effect of aging difficult to assess
Since Lrrk2-associated disease is typically late onset, the effect of aging is an important consideration. The need for Lrrk2 mammalian animal models is therefore paramount.
Genetic manipulation of neural progenitors in the periventricular cell layer of E16 rat embryos by in utero electroporation with Lrrk2 WT and mutant cDNA expression constructs (followed by 5 days in utero before sacrifice) resulted in dramatic reductions in neurite process length and branchpoint number in G2019S and I2020T mutants, whereas embryos that were electroporated with lentiviral LRRK2 shRNA had increased branchpoint number and a tendency towards increased process length [45], similar to primary culture results described earlier. In adult rodents, adeno-associated viral expression of the kinase domain WT or G2019S in the substantia nigra results in striatal abnormalities, including inclusions containing phosphorylated tau, again in agreement with cell culture results. Although grossly normal, the nigral neurons in G2019S rats displayed caspase-3 activation, indicative of apoptosis [45]. Interestingly, the role of apoptosis in Lrrk2-mediated cell death has recently been suggested to be mitochondrial dependent, involving cytochrome C release and caspase-3 activation, and this is abrogated by genetic ablation of Apaf1, a scaffold protein that participates with procaspase-9 and cytochrome C in formation of the caspase-activating apoptosome [79].
To date, several groups have made reference to LRRK2 transgenic or gene-targeted rodent models within publications, including LRRK2-knockout mice [31,40], bacterial artificial chromosome (BAC) transgenic mice expressing murine FLAG-tagged WT Lrrk2 [33], human Lrrk2 BAC transgenic mice [80] and tetracycline conditional human G2019S Lrrk2 mice [72].
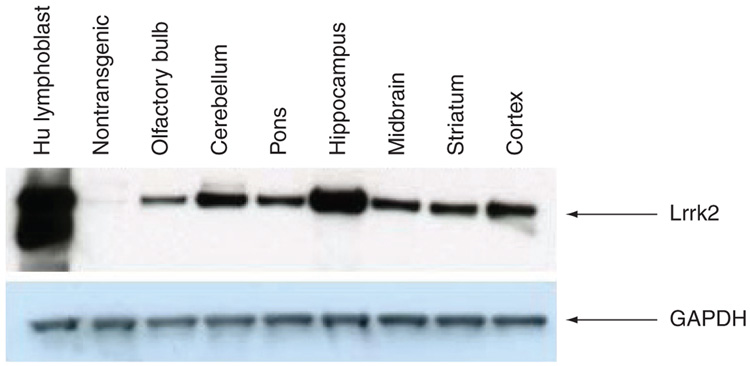
Expression levels in the FLAG-murine Lrrk2 BAC mice are approximately 20-fold greater than endogenous murine Lrrk2, and display both kinase and GTPase activity [33]. Transgenic expression is found in the cortex, amygdala and hippocampus as well as in the ventral tegmental area and the substantia nigra, consistent with previous mRNA and protein descriptions [33]. In human WT Lrrk2 BAC mice, created in our laboratory, we also observe around 20-fold greater endogenous expression levels and see robust transgenic Lrrk2 expression throughout the brain (Figure 3), with highest expression in the hippocampus. An identical pattern of expression is seen in our G2019S and Y1699C lines, although at lower levels (7–11-fold) [MELROSE H, FARRER M, UNPUBLISHED DATA]. No overt phenotype is observed in the Lrrk2 WT mice up to 24 months. In fact, the transgenic mice appear to have improved performance compared with nontransgenic littermates in behavioral tests [MELROSE JANUS, UNPUBLISHED DATA]. We are now pursuing second-hit strategies using environmental toxins and inflammatory agents to investigate the role of Lrrk2 in susceptibility. The mutant mice are still being characterized, but up to 12 months of age no phenotype is observed. Similarly, the conditional Lrrk2 G2019S model, recently used to identify Lrrk2 and Hsp90 interaction, was reported to have no obvious neuropathological or motor abnormalities at 12 months of age, although primary hippocampal neurons derived from the mice displayed retarded axon outgrowth during neuronal morphogenesis [72]. Given that BAC and conditional models recapitulate expression in a physiological manner, and taking into account the age-associated and multifactorial etiology of PD, it is perhaps not surprising that single transgenic mouse models do not recapitulate the phenotypic spectrum of disease. These models are useful to study gene–gene, for example, Lrrk2 and other PD-related proteins, or gene–environment (‘two-hit’ hypotheses) interactions, may be more informative in modeling PD, the variable disease expressivity and penetrance, and to evaluate therapeutic interventions.
Figure 3. Robust physiological expression of Lrrk2 in a mouse model.
Immunoblot to show regional expression of Lrrk2 protein in human BAC transgenic mice using antibody PA0362 (Novus NB110-58771). Expression levels are approximately 20-fold over endogenous mouse Lrrk2.
Lrrk2 & neurogenesis
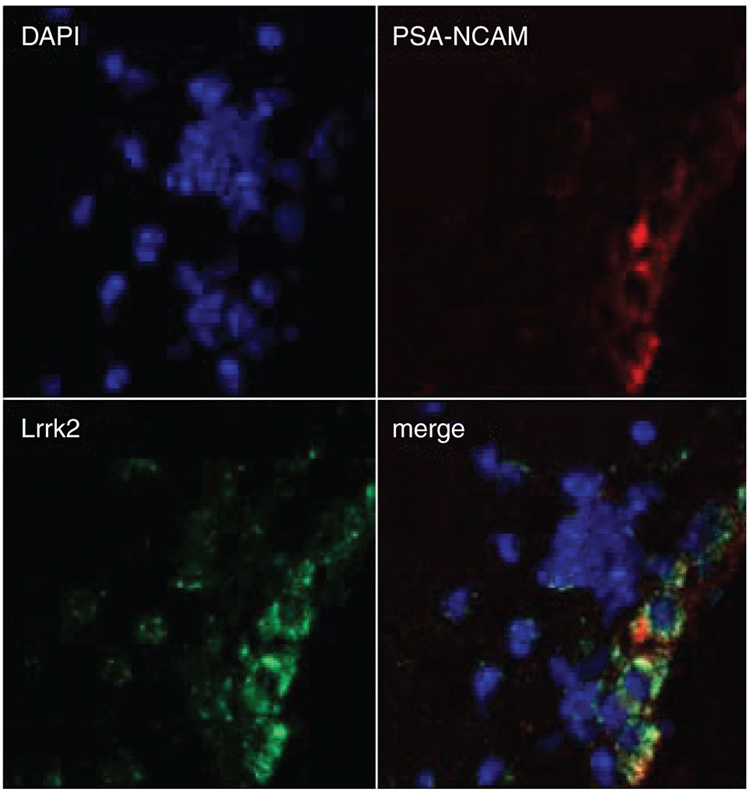
A fascinating observation, recently reported by our group, is the expression of Lrrk2 in the SVZ, an area of neurogenesis in the adult brain (Figure 4) [80]. Cells born in the adult SVZ undergo tangenitial migration along the rostral migratory stream to the olfactory bulb (OB), where they differentiate into neurons [81]. Evidence suggests these new neurons contribute to olfactory function [82–85]. Olfactory dysfunction is an early symptom of PD (and Lrrk2 parkinsonism), and is accompanied by α-synuclein accumulation and Lewy body formation in the OB in the early stages of the disease [15,86–88]. In the adult SVZ and OB, the finely tuned equilibrium in the production of neural precursors, their migration, subsequent differentiation and cell death, is also impaired in α-synuclein mouse models of PD [89,90]. Comparable observations have been made in patients with PD, with a decrease in mitotic cells in both the SVZ and hippocampus (the dentate gyrus subgranular zone being the second major area for adult neurogenesis) [91]. Moreover, an increase in dopaminergic olfactory neurons has been described in PD patients, despite their early and progressive loss in the sense of smell [92].
Figure 4. Lrrk2 is expressed in regions of adult neurogenesis.
Lrrk2 is expressed in chains of migrating neuroblasts in the subventricular zone. Red is polysialic acid–neural cell adhesion molecule (PSA-NCAM) a marker for migrating neuronal precursors, green is Lrrk2.
Adapted from [94].
Preliminary data in our BAC mice expressing human WT and mutant G2019S Lrrk2 reveal both a reduction in the birth of new neurons in the hippocampus and the SVZ as well as a reduction in the number of neurons migrating to the OB [MELROSE H, WINNER B, UNPUBLISHED DATA]. The BAC transgene contains the endogenous human promoter and regulatory sequences and, hence, should accurately reproduce physiological expression. Surprisingly, the human promoter drives very high hippocampal expression in the transgenic mice. The hippocampus is not an area traditionally thought of in PD, however patients can suffer from depression, neuropsychological impairment and cognitive problems (reviewed by [93]). One possibility is that a cumulative reduction in the birth of new neurons caused by aberrant Lrrk2 in the hippocampus may play a role in nonmotor symptoms of disease. Similarly, deficits in the SVZ leading to altered cellular migration may have a role in anosmia in PD patients.
Although the link between neurogenesis and PD has yet to be defined, it is intriguing that both Lrrk2 and α-synuclein have been i mplicated in adult neurogenesis. This new avenue of Lrrk2 research warrants future investigation. In the future, treatment with kinase inhibitors may be neuroprotective, possibly preserving adult neurogenesis and potentially nonmotor symptoms.
Executive summary
Genetics
Mutations in LRRK2 on chromosome 12, known as the PARK 8 locus, cause autosomal dominant Parkinson’s disease (PD).
Pathogenic substitutions are found in the kinase domain (G2019S and I2020T), Ras in complex proteins (Roc) GTPAse domain (R1441C/G) and the c-terminal of Roc (COR) domain (Y1699C).
G2019S is the most common mutation, accounting for 40% of familial PD and 30% of sporadic PD in certain populations.
Substitutions G2385R and R1628P are risk factors in Asian populations.
Pathology
Lrrk2 mutations primarily lead to α-synuclein-immunopositive Lewy body disease. However, pathology may be pleomorphic including neuronal loss, tau and ubiqutin pathology.
Family members with the same mutations can have different pathologies and disparate clinical presentations.
Structure
Lrrk2 protein is 286kDa and is comprised of several domains: a leucine-rich repeat (LRR), a Roc that belongs to the Rab GTPase superfamily, a COR domain, a MAPKKK and a WD40 domain.
Anatomical localization
Lrrk2 is found in dopaminergic-associated areas, including the striatum and substantia nigra, and in nondopaminergic areas, including the hippocampus, cerebellum and thalamus.
Cellular localization
Largely found in cytoplasm and absent from the nucleus. Subcellular fractionation indicates presence in membranous and microsomal fractions indicating a role in synaptic function, possibly by vesicular synthesis and/or transport and membranous structure regulation.
Functional activity
Dimer formation has been shown in vitro, which is likely to be important for both kinase and GTPase function. Mutations may disrupt dimerization properties, as shown for the Roc domain and may provide a parsimonious explanation of how mutations in different domains lead to the same disease.
Lrrk2 binds GTP and has GTPase activity, which appears to regulate kinase activity. Mutations in the GTPase domain lead to enhanced kinase activity.
Augmented kinase activity has been directly shown for the G2019S mutation, and indirectly may be the consequence of other pathogenic mutations.
Kinase function appears to mediate cellular toxicity.
Interactors
Few in vitro interactors found – moesin (actin-binding protein), Rab 5 (regulator of endocytic vesicular transport), α- and β-tubulin (microtubules involved in cytoskeleton and motor transport), Parkin (E3 ligase) and heat shock protein (Hsp)90 (molecular chaperone; dissociation of the Lrrk2–Hsp90 complex results in proteasomal degradation).
In vivo models
Worm models: loss of function of paralog LRK-1 leads to abnormal axonal polarity and chemosensory deficit and susceptibility to the mitochondrial toxin rotenone. Expression of human wild-type and G2019S Lrrk2 leads to increased lifespan and reduced rotenone vulnerability.
Fly models: loss of function of dLRRK2 may lead to motor impairment and loss of tyrosine hydroxylase reactivity in dopaminergic neurons. Resistant to toxins but not oxidative stress. Overexpression of dLrrk2 has no deleterious effects but human Lrrk2 leads to motor impairment and loss of tyrosine hydroxylase staining in dopaminergic neurons.
Rodent models: viral expression of mutant kinase domain in rats leads to striatal abnormalities, tau inclusions and apoptosis. Transgenic/targeted models are not fully characterized but no overt PD-related phenotypes are reported as yet.
Lrrk2 in neurogenesis
Lrrk2 is found in the subventricular zone (SVZ) in migrating neuroblasts. Lrrk2 bacterial artificial chromosome mice show decreased neurogenesis in SVZ and subgranular zone and defective neuronal migration.
Defective neurogenesis may promote the manifestation of nonmotor symptoms.
Future perspective
The need for mammalian models is paramount.
Understanding cell-specific roles/selective vulnerability of Lrrk2, is important for drug design, as is the dimeric function of Lrrk2.
Genetic testing can now inform a clinical diagnosis of PD in patients. It may also be used on a research basis to identify Lrrk2 carriers, to: 1) study the natural history of disease; 2) identify the earliest nonmotor manifestations of disease; 3) find environmental triggers; and, 4) provide the background for neuroprotective trials and therapies based on kinase inhibition.
Future perspective
Parkinson’s disease is probably a multifactorial disorder influenced by a complex combination of gene–gene and gene–environment factors. Aging remains the greatest risk factor and the variable penetrance and pleomorphic pathology in PD suggests that Lrrk2’s normal (versus pathological) function is more likely related to successful (versus unsuccessful) aging of the basal ganglia, rather than being directly causative of disease. Upon this background of susceptibility, other triggers may promote neurodegenerative disease. Genetic screening can now identify asymptomatic and symptomatic Lrrk2 carriers who would benefit from neuroprotection, perhaps in the form of kinase inhibition or kinase-regulating therapy. However, it is important to remember that most Lrrk2-coding substitutions have yet to be proven pathogenic, and even pathogenic mutations have variable penetrance. Until specific therapies are available, we advocate that genetic screening is limited to symptomatic patients as an aid to clinical diagnosis.
In this review, exciting Lrrk2 functional biology and experimental results are interpreted with caution, noting some of the inherent assumptions and limitation of the studies. The role of Lrrk2, and the pathogenicity of amino acid substitutions, may be best understood in the dimeric, perhaps multimeric, context in which the protein most likely operates. The intra- and inter-molecular actions of Lrrk2 will facilitate the search for interacting proteins and small molecule inhibitors, although better antibodies are required for immunoprecipitation and interaction studies. Further development of physiologic mammalian models is also essential in order to validate biological substrates, identify signaling pathways and classify Lrrk2’s specific cellular role(s), for example, in migrating neuroblast versus striatal spiny neurons. The selective vulnerability of the basal ganglia to Lrrk2 dysfunction has yet to be understood. Research to date, and the structure of Lrrk2, suggests the protein complex will have multiple functions. Hence, it is imperative to determine if modulation of ‘Lrrk2 complex’ activity and downstream signaling is safe to manipulate in disease and neurodegenerative conditions, without disrupting its normal, perhaps protective, physiologic role in successful aging. The development of neuroprotective therapies to slow or halt PD, based on Lrrk2 molecular insights has never been more promising. The discovery of Lrrk2 mutations has revolutionized our understanding of PD, from a clinical, genetic and pathological perspective, and is likely to stimulate similar research in related neurodegenerative conditions.
Acknowledgements
I would like to thank Matthew Farrer, Owen Ross and Justus Daechsel for helpful comments and discussion during the preparation of this manuscript.
Footnotes
Financial & competing interests disclosure
Funding support was provided by NIH Grants NIA AG17216 and NINDS NS40256, The Pacific Alzheimer’s Research Foundation and Robert H and Clarice/ML Simpson Foundation Trust Fellowship. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Paisan-Ruiz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Funayama M, Hasegawa K, Kowa H, et al. A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- 3.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 4. Kachergus J, Mata IF, Hulihan M, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am. J. Hum. Genet. 2005;76:672–680. doi: 10.1086/429256. ▪▪ Demonstrates that LRRK2 G2019S accounts for parkinsonism in several families within Europe and North America and describes an ancestral haplotype indicative of a common founder. In addition, the authors show that penetrance of Lrrk2 G2019S disease is age-dependent and highlights the fact that a proportion of clinically typical, late-onset Parkinson’s disease (PD) cases have a genetic basis.
- 5.Funayama M, Hasegawa K, Ohta E, et al. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann. Neurol. 2005;57:918–921. doi: 10.1002/ana.20484. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez D, Paisan Ruiz C, Crawley A, et al. The dardarin G 2019 S mutation is a common cause of Parkinson’s disease but not other neurodegenerative diseases. Neurosci. Lett. 2005;389:137–139. doi: 10.1016/j.neulet.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Mata IF, Kachergus JM, Taylor JP, et al. Lrrk2 pathogenic substitutions in Parkinson’s disease. Neurogenetics. 2005;6:171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 8.Healy DG, Falchi M, O’Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case–control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara L, Warren L, Gibson R, et al. Clinical features of Parkinson disease patients with homozygous leucine-rich repeat kinase 2 G2019S mutations. Arch. Neurol. 2006;63:1250–1254. doi: 10.1001/archneur.63.9.1250. [DOI] [PubMed] [Google Scholar]
- 11.Lesage S, Durr A, Tazir M, et al. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N. Engl. J. Med. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 12. Di Fonzo A, Wu-Chou YH, Lu CS, et al. A common missense variant in the LRRK2 gene Gly2385Arg, associated with Parkinson’s disease risk in Taiwan. Neurogenetics. 2006;7:133–138. doi: 10.1007/s10048-006-0041-5. ▪ Highlights that, in parts of Asia, a common Lrrk2 variant in the WD40 domain increases the likelihood of developing PD.
- 13. Farrer MJ, Stone JT, Lin CH, et al. Lrrk2 G2385R is an ancestral risk factor for Parkinson’s disease in Asia. Parkinsonism Relat. Disord. 2007;13:89–92. doi: 10.1016/j.parkreldis.2006.12.001. ▪ Highlights that, in parts of Asia, a common Lrrk2 variant in the WD40 domain increases the likelihood of developing PD.
- 14.Ross OA, Wu YR, Lee MC, et al. Analysis of Lrrk2 R1628P as a risk factor for Parkinson’s disease. Ann. Neurol. 2008;64(1):88–92. doi: 10.1002/ana.21405. [DOI] [PubMed] [Google Scholar]
- 15.Ross OA, Toft M, Whittle AJ, et al. Lrrk2 and Lewy body disease. Ann. Neurol. 2006;59:388–393. doi: 10.1002/ana.20731. [DOI] [PubMed] [Google Scholar]
- 16.Dachsel JC, Ross OA, Mata IF, et al. Lrrk2 G2019S substitution in frontotemporal lobar degeneration with ubiquitin-immunoreactive neuronal inclusions. Acta Neuropathol. (Berl.) 2007;113:601–606. doi: 10.1007/s00401-006-0178-1. [DOI] [PubMed] [Google Scholar]
- 17.Rajput A, Dickson DW, Robinson CA, et al. Parkinsonism Lrrk2 G2019S, and tau neuropathology. Neurology. 2006;67:1506–1508. doi: 10.1212/01.wnl.0000240220.33950.0c. [DOI] [PubMed] [Google Scholar]
- 18.Taylor JP, Mata IF, Farrer MJ. LRRK2: a common pathway for parkinsonism, pathogenesis and prevention? Trends Mol. Med. 2006;7:7. doi: 10.1016/j.molmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 19. Wszolek ZK, Pfeiffer RF, Tsuboi Y, et al. Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology. 2004;62:1619–1622. doi: 10.1212/01.wnl.0000125015.06989.db. ▪ Describes the strikingly variable pathologic changes in affected individuals with Lrrk2 mutations.
- 20.Greggio E, Jain S, Kingsbury A, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol. Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Miklossy J, Arai T, Guo JP, et al. LRRK2 expression in normal and pathologic human brain and in human cell lines. J. Neuropathol. Exp. Neurol. 2006;65:953–963. doi: 10.1097/01.jnen.0000235121.98052.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Babar A, Siedlak SL, et al. LRRK2 in Parkinson’s disease and dementia with Lewy bodies. Mol. Neurodegener. 2006;1:17. doi: 10.1186/1750-1326-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Siedlak SL, Smith MA, Perry G, Chen SG. LRRK2 protein is a component of lewy bodies. Ann. Neurol. 2006;60:617–618. doi: 10.1002/ana.20928. [DOI] [PubMed] [Google Scholar]
- 24.Giasson BI, Covy JP, Bonini NM, et al. Biochemical and pathological characterization of Lrrk2. Ann. Neurol. 2006;59:315–322. doi: 10.1002/ana.20791. [DOI] [PubMed] [Google Scholar]
- 25.Covy J, Van Deerlin V, Giasson B. Lack of evidence for Lrrk2 in α-synuclein pathological inclusions. Ann. Neurol. 2006;60:618–619. [Google Scholar]
- 26.Melrose H, Kent C, Taylor J, et al. A comparative analysis of Lrrk2 expression in mouse brain and Lewy body disease. Neuroscience. 2007;147(4):1047–1058. doi: 10.1016/j.neuroscience.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Perry G, Zhu X, Babar AK, et al. Leucine-rich repeat kinase 2 colocalizes with α-synuclein in Parkinson’s disease, but not tau-containing deposits in tauopathies. Neurodegener. Dis. 2008;5:222–224. doi: 10.1159/000113708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosgraaf L, Van Haastert PJ. Roc, a Ras/GTPase domain in complex proteins. Biochim. Biophys. Acta. 2003;7:1–3. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 29. Mata IF, Wedemeyer WJ, Farrer MJ, Taylor JP, Gallo KA. LRRK2 in Parkinson’s disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–293. doi: 10.1016/j.tins.2006.03.006. ▪▪ Excellent review that includes homology modeling of Lrrk2, which gives insight into domain organization and the potential functional impact of the pathogenic mutations.
- 30.Taylor JP, Hulihan MM, Kachergus JM, et al. Leucine-rich repeat kinase 1: a paralog of LRRK2 and a candidate gene for Parkinson’s disease. Neurogenetics. 2007;8:95–102. doi: 10.1007/s10048-006-0075-8. [DOI] [PubMed] [Google Scholar]
- 31. Biskup S, Moore DJ, Rea A, et al. Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 2007;8:102. doi: 10.1186/1471-2202-8-102. ▪ Comprehensively evaluates the majority of commercially available Lrrk2 antibodies. Having the benefit of Lrrk2-knockout mice, the authors highlight the problem of cross reactivity with many published antibodies.
- 32.White LR, Toft M, Kvam SN, Farrer MJ, Aasly JO. MAPK-pathway activity, Lrrk2 G2019S, and Parkinson’s disease. J. Neurosci. Res. 2007;85:1288–1294. doi: 10.1002/jnr.21240. [DOI] [PubMed] [Google Scholar]
- 33. Li X, Tan YC, Poulose S, et al. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson’s disease R1441C/G mutants. J. Neurochem. 2007;103(1):238–247. doi: 10.1111/j.1471-4159.2007.04743.x. ▪ Using brain extracts from a FLAG-tagged Lrrk2 mouse model, this paper demonstrates that kinase activity GTPase binding and GTPase hydrolysis occur in vivo.
- 34.Westerlund M, Belin AC, Anvret A, et al. Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: implications for Parkinson’s disease. Neuroscience. 2008;152:429–436. doi: 10.1016/j.neuroscience.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 35.Galter D, Westerlund M, Carmine A, et al. LRRK2 expression linked to dopamine-innervated areas. Ann. Neurol. 2006;59:714–719. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- 36.Melrose H, Lincoln S, Tyndall G, Dickson D, Farrer M. Anatomical localization of leucine-rich repeat kinase 2 in mouse brain. Neuroscience. 2006;139:791–794. doi: 10.1016/j.neuroscience.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Simon-Sanchez J, Herranz-Perez V, Olucha-Bordonau F, Perez-Tur J. LRRK2 is expressed in areas affected by Parkinson’s disease in the adult mouse brain. Eur. J. Neurosci. 2006;23:659–666. doi: 10.1111/j.1460-9568.2006.04616.x. [DOI] [PubMed] [Google Scholar]
- 38.Taymans JM, Van den Haute C, Baekelandt V. Distribution of PINK1 and LRRK2 in rat and mouse brain. J. Neurochem. 2006;98:951–961. doi: 10.1111/j.1471-4159.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- 39.Higashi S, Moore DJ, Colebrooke RE, et al. Expression and localization of Parkinson’s disease-associated leucine-rich repeat kinase 2 in the mouse brain. J. Neurochem. 2006;100(2):368–381. doi: 10.1111/j.1471-4159.2006.04246.x. [DOI] [PubMed] [Google Scholar]
- 40.Biskup S, Moore DJ, Celsi F, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 41.Greggio E, Lewis PA, van der Brug MP, et al. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. J. Neurochem. 2007;102:93–102. doi: 10.1111/j.1471-4159.2007.04523.x. [DOI] [PubMed] [Google Scholar]
- 42.Hurley MJ, Patel PH, Jackson MJ, et al. Striatal leucine-rich repeat kinase 2 mRNA is increased in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned common marmosets (Callithrix jacchus) with l-3, 4-dihydroxyphenylalanine methyl ester-induced dyskinesia. Eur. J. Neurosci. 2007;26:171–177. doi: 10.1111/j.1460-9568.2007.05638.x. [DOI] [PubMed] [Google Scholar]
- 43.Higashi S, Moore DJ, Colebrooke RE, et al. Expression and localization of Parkinson’s disease-associated leucine-rich repeat kinase 2 in the mouse brain. J. Neurochem. 2007;100:368–381. doi: 10.1111/j.1471-4159.2006.04246.x. [DOI] [PubMed] [Google Scholar]
- 44.Gloeckner CJ, Kinkl N, Schumacher A, et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum. Mol. Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 45. Macleod D, Dowman J, Hammond R, et al. The familial parkinsonism gene LRRK2 regulates neurite process morphology. Neuron. 2006;52:587–593. doi: 10.1016/j.neuron.2006.10.008. ▪ First paper to postulate the role of Lrrk2 in neurite outgrowth and branching.
- 46.West AB, Moore DJ, Biskup S, et al. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl Acad. Sci. USA. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith WW, Pei Z, Jiang H, et al. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat. Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 48.Hatano T, Kubo S, Imai S, et al. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum. Mol. Genet. 2007;16:678–690. doi: 10.1093/hmg/ddm013. [DOI] [PubMed] [Google Scholar]
- 49.Pelech S. Dimerization in protein kinase signaling. J. Biol. 2006;5:12. doi: 10.1186/jbiol45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerkhoff E, Rapp U. The Ras–Raf relationship: an unfinished puzzle. Advances in Enzyme Regulation. 2001;41(1):261–267. doi: 10.1016/s0065-2571(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 51.Greggio E, Zambrano I, Kaganovich A, et al. The Parkinson’s disease associated leucine rich repeat kinase 2 (LRRK2) is a dimer that undergoes intra-molecular autophosphorylation. J. Biol. Chem. 2008;283(24):16906–16914. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dachsel JC, Taylor J, Mok SS, et al. Identification of potential protein interactors of Lrrk2. Parkinsonism Relat. Disord. 2008;13(7):382–385. doi: 10.1016/j.parkreldis.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deng J, Lewis PA, Greggio E, et al. Structure of the ROC domain from the Parkinson’s disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc. Natl Acad. Sci. USA. 2008;105:1499–1504. doi: 10.1073/pnas.0709098105. ▪▪ Provides considerable insight into the Lrrk2 Ras in complex protein domain by crystal structure analysis.
- 54.Toft M, Mata IF, Kachergus JM, Ross OA, Farrer MJ. LRRK2 mutations and parkinsonism. Lancet. 2005;365:1229–1230. doi: 10.1016/S0140-6736(05)74809-1. [DOI] [PubMed] [Google Scholar]
- 55. Jaleel M, Nichols RJ, Deak M, et al. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem. J. 2007;405:307–317. doi: 10.1042/BJ20070209. ▪ Highlights the difficulty of kinase measurement and further shows pathogenic Lrrk2 mutants do not necessarily increase kinase activity.
- 56. Luzon-Toro B, Rubio de la Torre E, Delgado A, Perez-Tur J, Hilfiker S. Mechanistic insight into the dominant mode of the Parkinson’s disease-associated G2019S LRRK2 mutation. Hum. Mol. Genet. 2007;16:2031–2039. doi: 10.1093/hmg/ddm151. ▪ Excellent mechanistic paper giving insight into how the G2019S mutation might enhance kinase activity and lead to pathogenicity.
- 57.West AB, Moore DJ, Choi C, et al. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 58.Lewis PA, Greggio E, Beilina A, et al. The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem. Biophys. Res. Commun. 2007;357:668–671. doi: 10.1016/j.bbrc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo L, Gandhi PN, Wang W, et al. The Parkinson’s disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp. Cell Res. 2007;313:3658–3670. doi: 10.1016/j.yexcr.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito G, Okai T, Fujino G, et al. GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry. 2007;46:1380–1388. doi: 10.1021/bi061960m. [DOI] [PubMed] [Google Scholar]
- 61.Smith WW, Pei Z, Jiang H, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc. Natl Acad. Sci. USA. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plowey ED, Cherra SJ, 3rd, Liu YJ, Chu CT. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J. Neurochem. 2008;105:1048–1056. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burke RE. Inhibition of mitogen-activated protein kinase and stimulation of Akt kinase signaling pathways: two approaches with therapeutic potential in the treatment of neurodegenerative disease. Pharmacol. Ther. 2007;114:261–277. doi: 10.1016/j.pharmthera.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Habig K, Walter M, Poths S, Riess O, Bonin M. RNA interference of LRRK2-microarray expression analysis of a Parkinson’s disease key player. Neurogenetics. 2008;9:83–94. doi: 10.1007/s10048-007-0114-0. [DOI] [PubMed] [Google Scholar]
- 65.Paglini G, Kunda P, Quiroga S, Kosik K, Caceres A. Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J. Cell. Biol. 1998;143:443–455. doi: 10.1083/jcb.143.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furutani Y, Matsuno H, Kawasaki M, et al. Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J. Neurosci. 2007;27:8866–8876. doi: 10.1523/JNEUROSCI.1047-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin N, Jeong H, Kwon J, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp. Cell Res. 2008;314(10):2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 68.de Hoop MJ, Huber LA, Stenmark H, et al. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. doi: 10.1016/0896-6273(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 69.Gandhi PN, Wang X, Zhu X, Chen SG, Wilson-Delfosse AL. The Roc domain of leucine-rich repeat kinase 2 is sufficient for interaction with microtubules. J. Neurosci. Res. 2008;86(8):1711–1720. doi: 10.1002/jnr.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen O, Feinstein E, Kimchi A. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 1997;16:998–1008. doi: 10.1093/emboj/16.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dachsel JC, Mata IF, Ross OA, et al. Digenic parkinsonism: investigation of the synergistic effects of PRKN and LRRK2. Neurosci. Lett. 2006;410:80–84. doi: 10.1016/j.neulet.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 72. Wang L, Xie C, Greggio E, et al. The chaperone activity of heat shock protein (Hsp)90 is critical for maintaining the stability of leucine-rich repeat kinase 2. J. Neurosci. 2008;28:3384–3391. doi: 10.1523/JNEUROSCI.0185-08.2008. ▪ Demonstrates that LRRK2 forms a complex with heat shock protein 90 (Hsp90) in vivo and that inhibition of Hsp90 disrupts the association of Hsp90 with LRRK2 and leads to proteasomal degradation of LRRK2.
- 73.Sakaguchi-Nakashima A, Meir JY, Jin Y, Matsumoto K, Hisamoto N. LRK-1, a C. elegans PARK8-related kinase, regulates axonal-dendritic polarity of SV proteins. Curr. Biol. 2007;17:592–598. doi: 10.1016/j.cub.2007.01.074. [DOI] [PubMed] [Google Scholar]
- 74.Wolozin B, Saha S, Guillily M, Ferree A, Riley M. Investigating convergent actions of genes linked to familial Parkinson’s disease. Neurodegener. Dis. 2008;5:182–185. doi: 10.1159/000113697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SB, Kim W, Lee S, Chung J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem. Biophys. Res. Commun. 2007;358:534–539. doi: 10.1016/j.bbrc.2007.04.156. [DOI] [PubMed] [Google Scholar]
- 76.Wang D, Tang B, Zhao G, et al. Dispensable role of Drosophila ortholog of LRRK2 kinase activity in survival of dopaminergic neurons. Mol. Neurodegener. 2008;3:3. doi: 10.1186/1750-1326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Z, Wang X, Yu Y, et al. A Drosophila model for LRRK2-linked Parkinsonism. Proc. Natl Acad. Sci. USA. 2008;105:2693–2698. doi: 10.1073/pnas.0708452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marin I. Ancient origin of the Parkinson disease gene LRRK2. J. Mol. Evol. 2008;67(1):41–50. doi: 10.1007/s00239-008-9122-4. [DOI] [PubMed] [Google Scholar]
- 79.Iaccarino C, Crosio C, Vitale C, et al. Apoptotic mechanisms in mutant LRRK2-mediated cell death. Hum. Mol. Genet. 2007;16:1319–1326. doi: 10.1093/hmg/ddm080. [DOI] [PubMed] [Google Scholar]
- 80.Melrose HL, Kent CB, Taylor JP, et al. A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience. 2007;147:1047–1058. doi: 10.1016/j.neuroscience.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 81.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mandairon N, Sacquet J, Jourdan F, Didier A. Long-term fate and distribution of newborn cells in the adult mouse olfactory bulb: influences of olfactory deprivation. Neuroscience. 2006;141:443–451. doi: 10.1016/j.neuroscience.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 83.Saino-Saito S, Sasaki H, Volpe BT, et al. Differentiation of the dopaminergic phenotype in the olfactory system of neonatal and adult mice. J. Comp. Neurol. 2004;479:389–398. doi: 10.1002/cne.20320. [DOI] [PubMed] [Google Scholar]
- 84.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule Neurons: role of olfaction. J. Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gheusi G, Cremer H, McLean H, et al. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc. Natl Acad. Sci. USA. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berendse HW, Booij J, Francot CM, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann. Neurol. 2001;50:34–41. doi: 10.1002/ana.1049. [DOI] [PubMed] [Google Scholar]
- 87.Sobel N, Thomason ME, Stappen I, et al. An impairment in sniffing contributes to the olfactory impairment in Parkinson’s disease. Proc. Natl Acad. Sci. USA. 2001;98:4154–4159. doi: 10.1073/pnas.071061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 89.Winner B, Lie DC, Rockenstein E, et al. Human wild-type α-synuclein impairs neurogenesis. J. Neuropathol. Exp. Neurol. 2004;63:1155–1166. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- 90.Winner B, Rockenstein E, Lie DC, et al. Mutant α-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol. Aging. 2008;29(6):913–925. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 92.Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov. Disord. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- 93.Berg D. Biomarkers for the early detection of Parkinson’s and Alzheimer’s disease. Neurodegener. Dis. 2008;5:133–136. doi: 10.1159/000113682. [DOI] [PubMed] [Google Scholar]
- 94.Melrose HL, Kent CB, Taylor JP, et al. A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience. 2007;147(4):1047–1058. doi: 10.1016/j.neuroscience.2007.05.027. [DOI] [PubMed] [Google Scholar]