Abstract
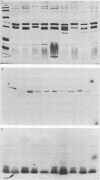
The antigen-specific basis of human serum immunoglobulin G antibody response to complicated gonococcal infection was studied in 13 patients by using the Western blot technique for transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to nitrocellulose paper. Of 13 patients (8 with disseminated gonococcal infection, 4 with pelvic inflammatory disease, 1 with gonococcal epididymitis), 12 reacted with protein I antigens and 9 with lipopolysaccharide (LPS). Sera from eight patients reacted with both protein I and LPS, whereas sera from four reacted only with protein I, and one sera reacted with LPS alone. One serum with antibody to both protein I and LPS by Western blot analysis was tested for bactericidal activity before and after adsorption of antibody to LPS. Removal of antibody to LPS reduced the bactericidal titer of this serum from 1:100 to 1:50, indicating that antibody to both antigens may be bactericidal for Neisseria gonorrhoeae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Swanson J., Holmes K. K., Kraus S. J., Gotschlich E. C. Quantitative determination of antibody to gonococcal pili. Changes in antibody levels with gonococcal infection. J Clin Invest. 1973 Nov;52(11):2896–2909. doi: 10.1172/JCI107486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. K., Counts G. W., Beaty H. N. Disseminated gonococcal infection. Ann Intern Med. 1971 Jun;74(6):979–993. doi: 10.7326/0003-4819-74-6-979. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Goldenberg D. L. Clinical manifestations of disseminated infection caused by Neisseria gonorrhoeae are linked to differences in bactericidal reactivity of infecting strains. Ann Intern Med. 1981 Aug;95(2):175–178. doi: 10.7326/0003-4819-95-2-175. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom E. G., Knapp J. S., Buchanan T. B. Serology of Neisseria gonorrhoeae: W-antigen serogrouping by coagglutination and protein I serotyping by enzyme-linked immunosorbent assay both detect protein I antigens. Infect Immun. 1982 Jan;35(1):229–239. doi: 10.1128/iai.35.1.229-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Ochs H. D., Buchanan T. M. Immunoglobulin class responsible for gonococcal bactericidal activity of normal human sera. J Immunol. 1979 May;122(5):1771–1779. [PubMed] [Google Scholar]
- Siegel M., Olsen D., Critchlow C., Buchanan T. M. Gonococcal pili: safety and immunogenicity in humans and antibody function in vitro. J Infect Dis. 1982 Mar;145(3):300–310. doi: 10.1093/infdis/145.3.300. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Ciak J., Boslego J., McChesney D. G., Brinton C. C., Zollinger W. Antigenic specificity of antibodies in vaginal secretions during infection with Neisseria gonorrhoeae. J Infect Dis. 1980 Jul;142(1):23–31. doi: 10.1093/infdis/142.1.23. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Boslego J. W., Ciak J., McChesney D., Brinton C. C., Wood S., Takafuji E. Gonococcal pilus vaccine. Studies of antigenicity and inhibition of attachment. J Clin Invest. 1981 Oct;68(4):881–888. doi: 10.1172/JCI110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner P. J., Handsfield H. H., Holmes K. K. Low antibiotic resistance of gonococci causing disseminated infection. N Engl J Med. 1973 Jun 7;288(23):1221–1222. doi: 10.1056/NEJM197306072882308. [DOI] [PubMed] [Google Scholar]