Abstract
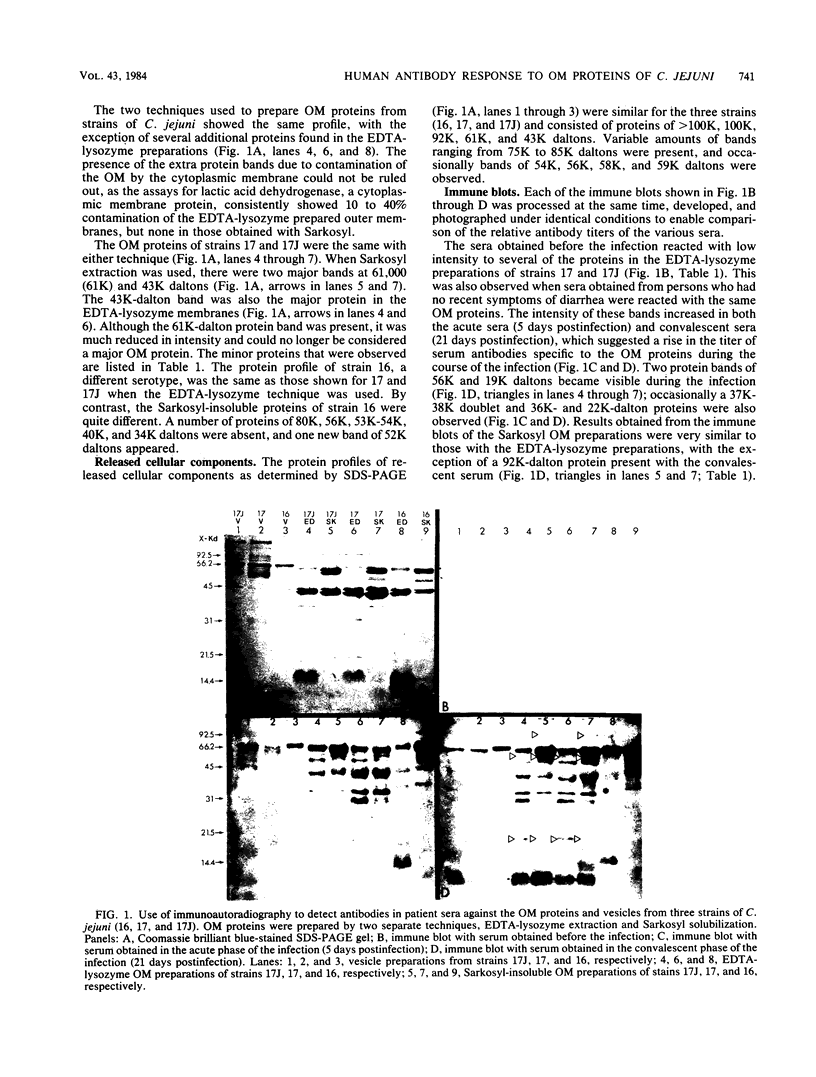
Two techniques were used to isolate outer membrane proteins from Campylobacter jejuni, EDTA-lysozyme extraction and sodium-N-lauroylsarcosinate (Sarkosyl) solubilization. The protein profiles of the two preparations were similar, with a few additional bands in the EDTA-lysozyme preparations. The major outer membrane protein was 43,000 (43K) daltons, and there were 8 to 10 minor bands ranging from 92K to 14K daltons. There was no difference in the protein profile of a strain causing an infection (strain 17) and the resulting stool isolate (strain 17J). Sera collected before the infection and during the acute and convalescent stages were used with Western blotting and immunoautoradiographic techniques to determine the antigenicity of outer membrane proteins. A number of antigenic proteins were detected before the infection by their reaction with preinfection serum (61K, 51K, 43K, 40K, 34K, and 31K daltons), and three additional bands appeared during the infection when acute and convalescent sera were used (92K, 56K, and 19K daltons). Furthermore, an area of the gel at less than 14.4K daltons that did not stain with Coomassie brilliant blue became visible in the immune blots when the convalescent serum was used.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Wells J. G., Feldman R. A., Pollard R. A., Allen J. R. Campylobacter enteritis in the United States. A multicenter study. Ann Intern Med. 1983 Mar;98(3):360–365. doi: 10.7326/0003-4819-98-3-360. [DOI] [PubMed] [Google Scholar]
- Bradbury W. C., Marko M. A., Hennessy J. N., Penner J. L. Occurrence of plasmid DNA in serologically defined strains of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1983 May;40(2):460–463. doi: 10.1128/iai.40.2.460-463.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gankema H., Wensink J., Guinée P. A., Jansen W. H., Witholt B. Some characteristics of the outer membrane material released by growing enterotoxigenic Escherichia coli. Infect Immun. 1980 Aug;29(2):704–713. doi: 10.1128/iai.29.2.704-713.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Outer membrane characteristics of Campylobacter jejuni. Infect Immun. 1982 Dec;38(3):898–906. doi: 10.1128/iai.38.3.898-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen K. I., Prescott J. F., Dohoo I. R. Pathogenicity of Campylobacter jejuni isolates from animals and humans. Infect Immun. 1982 Oct;38(1):46–52. doi: 10.1128/iai.38.1.46-52.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Mug-Opstelten D., Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim Biophys Acta. 1978 Apr 4;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Congi R. V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983 Aug;2(4):378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- Smibert R. M. The genus Campylobacter. Annu Rev Microbiol. 1978;32:673–709. doi: 10.1146/annurev.mi.32.100178.003325. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., De Grandis S. A., Karmali M. A., Fleming P. C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981 May;19(5):831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]