Abstract
The hallmark of gonorrhea is an intense inflammatory response that is characterized by polymorphonuclear leukocytes (PMNs) with intracellular gonococci. A redundancy of defenses may protect Neisseria gonorrhoeae from phagocyte-derived reactive oxygen species. Here we showed that a gonococcal catalase (kat) mutant in strain MS11 was more sensitive to H2O2 than mutants in cytochrome c peroxidase (ccp), methionine sulfoxide reductase (msrA), or the metal-binding protein (mntC) of the MntABC transporter. kat ccp and kat ccp mntC mutants were significantly more sensitive to H2O2 than mutants in any single factor. None of the mutants showed increased susceptibility to murine PMNs. Recovery of the mntC and kat ccp mntC mutants from the lower genital tract of BALB/c mice, but not the kat or kat ccp mutants, was significantly reduced relative to wild-type bacteria. Interestingly, unlike the MS11 kat mutant, a kat mutant of strain FA1090 was attenuated during competitive infection with wild-type FA1090 bacteria. The FA1090 kat mutant and MS11 mntC mutant were also attenuated in mice that are unable to generate a phagocytic respiratory burst. We conclude that inactivation of three well-characterized antioxidant genes (kat, ccp, and mntC) does not increase gonococcal susceptibility to the phagocytic respiratory burst during infection and that gonococcal catalase and the MntC protein confer an unidentified advantage in vivo. In the case of catalase, this advantage is strain specific. Finally, we also showed that an msrA mutant of strain MS11 demonstrated delayed attenuation in BALB/c but not C57BL/6 mice. Therefore, MsrA/B also appears to play a role in infection that is dependent on host genetic background.
Neisseria gonorrhoeae is a facultative anaerobic bacterium with no environmental or animal reservoir outside of humans. N. gonorrhoeae is maintained in the human population on mucosal surfaces, including the urethra, cervix, pharynx, and rectum. Tissues of the upper reproductive tract of both males and females can also be infected. As is typical of the pyogenic cocci, N. gonorrhoeae induces an intense inflammatory response during symptomatic infections that is characterized by numerous polymorphonuclear leukocytes (PMNs) with intracellular gram-negative diplococci (21).
The mechanism(s) by which the gonococcus evades oxidative killing by phagocytes is of particular interest based on evidence that a proportion of intracellular gonococci survives and even multiplies within the PMNs (5, 6, 34, 42, 54, 55) despite the induction of an intracellular respiratory burst (42, 58). Several factors protect N. gonorrhoeae from exposure to oxidative stress in vitro (reviewed in reference 43). A nonenzymatic quenching mechanism that is based on the accumulation of intracellular manganese through the MntABC transporter protects N. gonorrhoeae from H2O2 and superoxide anion (52), and high levels of catalase protect gonococci from H2O2 and exposure to paraquat (20, 25, 47). Cytochrome c peroxidase (Ccp) also increases gonococcal resistance to H2O2 (53), and methionine sulfoxide reductase (MsrA/B), which repairs methionine sulfoxide residues on oxidatively damaged proteins, confers increased resistance to H2O2 and extracellularly generated reactive oxygen species (ROS) (45). Several other gonococcal factors that detoxify H2O2 and/or ROS have been identified (8, 41, 50, 59).
Many physiological factors can affect interactions between N. gonorrhoeae and PMNs. These factors include iron concentration, O2 tension, pH (17), lactate (3), and the presence of CMP neuraminic acid (18, 38, 58). Environmental factors also regulate the expression of genes that protect N. gonorrhoeae from H2O2 and ROS in vitro (43). The balance of these physiological factors is difficult to reproduce in vitro. Infection of estradiol-treated BALB/c mice with N. gonorrhoeae causes a localized inflammatory response as evidenced by elevated numbers of PMNs and macrophages in vaginal and cervical tissue from infected mice (48). We recently reported that a catalase (kat) mutant of N. gonorrhoeae strain FA1090 can establish experimental murine infection despite the induction of a vigorous PMN response. High numbers of catalase-deficient gonococci were seen within PMNs and there was no significant difference in the number of wild-type or kat mutant gonococci recovered (46). That report was the first demonstration that catalase-deficient gonococci can persist during periods of inflammation in an in vivo system. We did not use the sensitive method of competitive infection to assess colonization of the kat mutant, however, or test the possibility that functionally redundant factors may mask any attenuation due to the absence of catalase.
Here we examined the contribution of four well-characterized antioxidant factors toward N. gonorrhoeae resistance to oxidative killing by phagocytes in the mouse infection model. To this end, we constructed single, double, and triple mutants in the kat, ccp, msrA, and/or mntC genes in the same strain background. Mutants that showed the most sensitivity to H2O2 in vitro were tested for the capacity to colonize BALB/c mice during competitive infection with the wild-type parent strain. Mutants that were attenuated in BALB/c mice were tested in mice that are deficient in the Phox91 subunit of the NADPH oxidase complex to determine if evasion of phagocytic respiratory burst was the basis of the attenuation.
MATERIALS AND METHODS
Bacterial strains.
A description of the bacteria used in this study is provided in Table 1. N. gonorrhoeae strains MS11 and FA1090 have been extensively characterized in the male volunteer model of experimental urethritis (9, 40, 51). The kat, ccp, msrA, and mntC genes of strain MS11 were isolated by PCR amplification of MS11 chromosomal DNA using primers Kat2R and Kat2F (for kat), ccpR and ccpF (for ccp), msrR and msrF (for msrA), and mntR and mntF (for mntC) (Table 2). The primers were designed to allow amplification of the 10-bp neisserial DNA uptake sequence (13) found downstream of each gene. PCR primers were designed based on information from the N. gonorrhoeae FA1090 genome sequence database (http://www.genome.ou.edu/gono.html). The resultant PCR products (1,697 bp [kat], 1,464 bp [ccp], 1,337 bp [mntC], and 1,708 bp [msrA]) were ligated to the PCR Blunt vector (Invitrogen) and electroporated into Escherichia coli Top10. Kmr transformants were screened for the desired clones and confirmed by nucleotide sequence analysis. Cloned genes were digested with a restriction enzyme that cut within a unique site in each open reading frame (NdeI for kat, HindIII for ccp, BsgI for mntC, and SspI for msrA). The linearized plasmids were ligated with a chloramphenicol acetyltransferase (cat) gene contained on an Agel and Xbal fragment from pGCC5 (for ccp and msrA), an erythromycin resistance gene (Emr) amplified from pGCC3 with the primers pG3EmR and pG3EmF (for kat), or a nonpolar aphA-3 kanamycin resistance (Kmr) cassette contained on an 840-bp SmaI fragment from pUC18Km (31) (for mntC). Plasmids with the insertionally inactivated genes were cut at the restriction site incorporated into the respective primers (Table 2) to generate linear fragments, which were then transformed into strain MS11. Transformants were selected on agar with the appropriate antibiotics to isolate single mutants GP301, GP302, GP303, and GP304 (Table 1). Double mutants GP311 and GP314 were generated by transforming linear plasmid DNA that contained insertionally inactivated ccp and msrA genes, respectively, into the kat mutant GP301. Double mutant GP315 was generated by introducing the ccp::Kmr gene into mutant GP303. Mutants with inactivated mntC genes and kat (GP316) or ccp (GP317) genes were isolated after transformation of linearized plasmids carrying the kat::Emr or ccp::Cmr genes into GP304 bacteria. The triple mutant GP318 (mntC kat ccp) was constructed by introducing a ccp::Cmr gene into mutant GP316.
TABLE 1.
N. gonorrhoeae strains used in this study
| Strain | Description | Reference |
|---|---|---|
| MS11 | Wild type, PorB.1B, Smr | 51 |
| FA1090 | Wild type, PorB.1B, Smr | 9 |
| GP301 | kat::Emr (strain MS11) | This study |
| GP302 | ccp::Cmr (strain MS11) | This study |
| GP303 | msrA::Cmr (strain MS11) | This study |
| GP304 | mntC::Kmr (strain MS11) | This study |
| GP311 | kat::Emrccp::Cmr (strain MS11) | This study |
| GP314 | kat::EmrmsrA::Cmr (strain MS11) | This study |
| GP315 | msrA::Cmr, ccp::Kmr (strain MS11) | This study |
| GP316 | mntC::Kmr, kat::Emr (strain MS11) | This study |
| GP317 | mntC::Kmrccp::Cmr (strain MS11) | This study |
| GP318 | mntC::Kmrkat::Emrccp::Cmr (strain MS11) | This study |
| GP500 | katΔ47-1384::aphA-3 (strain FA1090) | 47 |
| GP506 | GP500 complemented with katMS11 | This study |
TABLE 2.
Nucleotide primers used in this study
| Primer | Sequence (5′-3′)a |
|---|---|
| Kat2Rb | TGAGTACTGACCAACCTGAGAAAAGGA |
| Kat2Fb | TGAGTACTAAAAAACGCCCACTGAAAC |
| pG3EmR | TGACTGCGCTTACCCTTCCT |
| pG3EmF | AGGCAGGAGAGGGATTCGTC |
| ccpRc | ACGCGTCGACCTCCCATAAGGAATACACG |
| ccpFc | ACGCGTCGACAGCCGATGGTAATGGTAGC |
| mntRc | ACGCGTCGACACAGGAAAACAGTTATGAAAC |
| mntFc | ACGCGTCGACTGAATGTAAACCGGTGAGG |
| msrRc | ACGCGTCGACCGTCCACACAGGAAACATC |
| msrFc | ACGCGTCGACGCGATGCTGTCTGAACAAATC |
Enzyme restriction sites (underlined) were included in these primers for cloning purposes.
Primer contains a ScaI restriction site.
Primer contains a SalII restriction site.
The kat mutant GP500 (kat::aphA3) is in N. gonorrhoeae strain FA1090 and was described previously (47). For complementation of GP500, a wild-type copy of the MS11 kat gene without its own promoter was cloned into the PCR Blunt vector using primers that produced ScaI sites as described previously (58). The resultant plasmid was digested with ScaI and the 1,697-kb fragment that contains the kat gene was cloned into the ScaI site of pGCC4 (30). An 8.6-kb fragment that contains the kat gene and sequences that correspond to a nonessential region between the lctP and aspC genes on the gonococcal chromosome was transformed into kat mutant GP500. Transformants were selected on GC agar with 1 μg/ml of Em, and a colony that exhibited catalase activity upon exposure to a few drops of H2O2 was passaged and frozen as strain GP506. The occurrence of the desired allelic exchange in GP506 bacteria was confirmed by PCR. All transformations were performed by the method of Gunn and Stein (19). Neisserial Insertional Complementation System vectors pGCC3, pGCC4, and pGCC5 (29, 30) were provided by H. S. Seifert, Northwestern University.
Culture conditions and growth curves.
All Neisseria strains were cultured in 7% CO2 at 37°C on GC agar or in GC broth (GCB) supplemented with Kellogg's supplement and 12 μM Fe(NO3)3 (22). Luria agar with Em (300 μg/ml), Km (50 μg/ml), Cm (50 μg/ml), tetracycline (50 μg/ml), or ampicillin (100 μg/ml [for Luria both] or 200 μg/ml [for Luria plates]) was used for plasmid maintenance or to isolate E. coli carrying recombinant plasmids. GC agar with Em (0.5 μg/ml [for FA1090] or 3 μg/ml [MS11]), Km (50 μg/ml), or Cm (10 μg/ml) was used to isolate mutants following allelic exchange. GC agar containing vancomycin, colistin, nystatin, trimethoprim, and streptomycin sulfate (GC-VNTS agar), GC agar with streptomycin (Sm; 100 μg/ml), or GC agar with Sm and Cm, Km, or Em at the above concentrations was used to culture vaginal mucus in mouse infection experiments. VCNT supplement and all media were from Difco. All other antibiotics were from Sigma. Growth kinetics of wild-type and mutant gonococci were determined by culturing bacteria in supplemented GCB with 5 mM NaHCO3 at 37°C with aeration and measuring the change in absorbance at 600 nm (A600) over time. For cocultures (in vitro competition assays), similar numbers of wild-type MS11 and mutant gonococci were inoculated into supplemented GCB, and aliquots were cultured on GC agar with and without the appropriate antibiotic selection at hourly time points through mid-stationary phase. The number of CFU on GC with Em (for GP301 or GP311), Km (for GP304, GP500, or GP506), or Cm (for GP303) was subtracted from the number of CFU on GC agar without antibiotics (total CFU) to determine the relative number of wild-type and mutant gonococci over time.
Catalase activity.
Catalase activity was measured in whole-cell lysates of stationary-phase cultures grown in supplemented GCB as described previously (47) except that sonication was used to break bacterial cells (level 3, 15 s on and 30 s off, 3 min total) and bacteria were suspended in 50 mM potassium phosphate (monobasic) buffer before lysis. Catalase activity was expressed as units/mg of protein using the following formula: units/mg = (ΔA240/min × 1,000)/(43.6 × mg of enzyme/ml of reaction mixture). Isopropyl-β-d-thiogalactopyranoside (IPTG; 0.1 mM; Sigma) was used to induce transcription of the kat gene in strain GP506.
Sensitivity to H2O2 and inducers of ROS.
Wild-type and mutant gonococci were cultured aerobically in a 5% CO2 incubator or anaerobically overnight in an anaerobic jar in the presence of 2 mM nitrite (27). H2O2 and paraquat sensitivities were determined via a disc diffusion assay (47). Zones of growth inhibition were calculated by determining the diameter (in mm) of the region in which no bacteria grew minus the diameter of the disc. All experiments were performed at least twice to test reproducibility.
Experimental murine infection.
Six- to 8-week-old female BALB/c mice (National Cancer Institute), C57BL/6J mice, and B6.129S6-Cybbtm1Din/J mice, which is a Phox-deficient mouse line in the C57BL/6J background (Jackson Laboratories), were treated with 17β-estradiol and antibiotics to promote long-term colonization by N. gonorrhoeae as described previously (23, 24). For noncompetitive infections, 106 CFU of wild-type MS11 or GP318 (kat ccp mntC) gonococci were inoculated intravaginally into separate groups of mice (n = 8 mice/group), and vaginal mucus was quantitatively cultured every other day on GC-VCNTS agar. For competitive infections, groups of five to eight mice were inoculated with a mixed suspension that contained similar numbers of wild-type and mutant bacteria, and the relative recovery of the mutant over time was determined using the appropriate selective agar as described previously (58). Competitive indices (CI) were calculated as the ratio of mutant to wild-type bacteria recovered (output) divided by the ratio of mutant to wild-type bacteria in the inoculum (input). All animal experiments were conducted in the laboratory animal facility at the Uniformed Services University, which is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care under a protocol approved by the University's Institutional Animal Care and Use Committee.
PMN assays.
Murine PMNs were elicited by peritoneal lavage, and opsonophagocytic killing of N. gonorrhoeae was performed via a tumbling tube assay as described previously (16, 58). Results were expressed as percent survival {100 × [(number of CFU recovered at 90 min)/(number of CFU recovered at time zero)]} (4). Induction of the phagocytic respiratory burst in PMNs incubated with wild-type or mutant gonococci was measured via a luminol- and isoluminol-enhanced chemiluminescence (CL) assay (4, 58). Experiments were performed on three separate occasions with similar results. The Phox-deficient and Phox-sufficient phenotypes of B6.129S6-Cybbtm1Din/J and C57BL/6J mice were confirmed by measuring the CL response of PMNs from these mice upon stimulation with 10 ng/ml phorbol myristate acetate and by nitroblue tetrazolium staining (36).
Quantitative reverse transcription-PCR (RT-PCR).
A modification of the conditions used by Packiam et al. (33) was used to measure in vivo expression of the wild-type kat gene in strain FA1090 and a recombinant kat gene in the complemented mutant GP506 in vivo. Mice were infected with wild-type, GP500 kat mutant, or GP506-complemented mutant bacteria that had been cultured in the presence or absence of 0.1 mM IPTG. Total RNA was extracted from wild-type inoculum suspensions and from vaginal swab suspensions from infected and uninfected mice using the Qiagen mini RNAeasy isolation kit. All preparations were treated twice with DNase I (6 U, RNase free; Ambion) and stored at −70°C until use. cDNA was synthesized with SuperScript III reverse transcriptase (Invitrogen) per the manufacturer's instructions. Control samples containing nuclease-free water instead of reverse transcriptase were tested in parallel to rule out contaminating DNA as the source of any PCR product. The SYBR Green Master Mix kit (ABI) was used to perform real-time PCR assays. cDNA reaction mixtures (20 μl) were diluted to a final volume of 100 μl with nuclease-free double-distilled water. Two microliters of the diluted cDNA template and 1:2 and 1:4 dilutions thereof were subjected to PCR amplification in the Applied Biosystems 7500 Real-Time PCR system in a total volume of 25 μl containing 12.5 μl SYBR Green Master Mix, 1 μl of each primer (0.4 μM, final concentration), and 8.5 μl double-distilled H2O. Reaction conditions were 10 min at 95°C, 40 15-s cycles of 95°C, and 1 min at 60°C. Data were analyzed using the Sequence Detector v.1.7a software (ABI). The cycle threshold (CT) was defined as the cycle number that corresponded to the point at which the amplification plot of the samples was linear. The comparative CT method (ΔΔCT) was used to measure kat expression relative to that of rmp, which encodes the reduction-modifiable membrane protein as the active reference control (normalizer). Quantification of relative kat expression was based on the difference between the CT values of the normalizer (rmp) and the CT values of individual samples: ΔCT = CT(rmp) − CT(sample). The difference between the ΔCT value of the wild-type or GP506 samples and the ΔCT value of the wild-type inoculum (ΔΔCT) was used to obtain an absolute value for the difference in kat mRNA levels between samples (2ΔΔCT). Results are expressed in arbitrary units to reflect this difference.
Data analysis.
An unpaired t test was used to evaluate differences in susceptibility to H2O2, paraquat, and PMN killing and to compare the average duration of recovery for mice. All statistical analyses were performed with SPSS software (Chicago, IL).
RESULTS
In vitro analysis of mutant phenotypes.
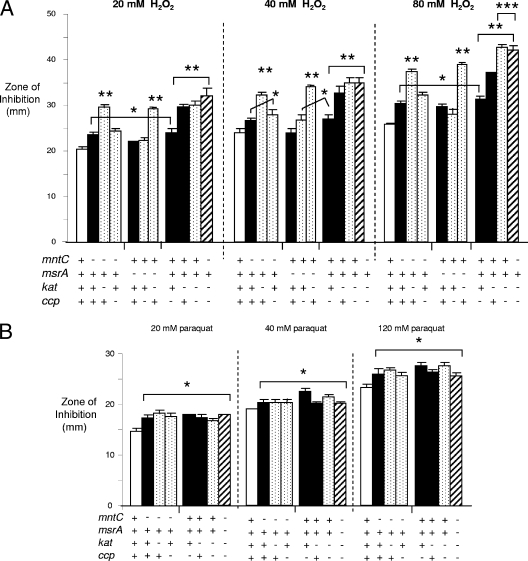
The sensitivities of MS11 mutants with an insertionally inactivated kat, ccp, msrA, or mntC gene and different combinations thereof to H2O2 or paraquat were measured to confirm the expected phenotypes and to determine if loss of more than one factor had an additive effect. We were particularly interested in the sensitivity of the mutants under anaerobic conditions, since the O2 tension of the female genital tract is reduced (35, 56). Similar to findings reported by others (26, 45, 47, 52), the kat, mntC, and msrA single mutants were more sensitive to H2O2 than the wild-type strain under aerobic conditions (P < 0.05). Consistent with the fact that ccp is only expressed during O2-limited growth (53), the ccp mutant was not more sensitive under aerobic conditions (data not shown). When cultured anaerobically, the kat, ccp, msrA, and mntC mutants showed a dose-dependent H2O2 sensitivity that was greater than that of the wild-type strain, although the H2O2 sensitivity phenotype of the msrA mutant was not consistently different over all doses tested (Fig. 1A). Compared to the other single mutants, the kat mutant GP301 was more sensitive to H2O2 at a statistically higher level (P < 0.001).
FIG. 1.
Sensitivity of antioxidant mutants to H2O2 and paraquat. Wild-type MS11 and mutant gonococci were tested for sensitivity to increasing concentrations of H2O2 (A) or paraquat (B) in a disc diffusion assay under anaerobic conditions in the presence of 2 mM nitrite. Results for the wild-type strain (open bars), single mutants (black bars), double mutants (stippled bars), and the triple mutant GP318 (striped bar) are shown and represent the average zone of inhibition and standard deviation as calculated from the results of triplicate assays. The experiment was repeated once to test reproducibility and the results were similar. *, P < 0.05 (compared to wild-type strain); **, P < 0.001 (compared to wild-type strain); ***, P < 0.05 (compared to kat mutant GP301).
Inactivation of both the kat and ccp genes (mutant GP311), but not the kat and msrA genes (mutant GP314) or kat and mntC genes (mutant GP316) (Fig. 1A), caused a higher level of H2O2 sensitivity than that exhibited by the single kat mutant GP301 when examined at the highest concentration tested (80 mM H2O2) (P < 0.05). The H2O2 sensitivity of mutant GP318, which carries three inactivated genes, kat, ccp, and mntC, was similar to that of the kat ccp double mutant (Fig. 1A). Inactivation of the ccp gene increased the H2O2 sensitivity of the mntC mutant to the level conferred by inactivation of ccp alone when tested at 80 mM H2O2 (P < 0.007). Inactivation of ccp along with msrA (mutant GP315) did not have an additive effect over mutation of either single gene. We concluded that when gonococci are cultured anaerobically, catalase is the most important factor for protecting against H2O2 and that mutation of ccp but not mntC or msrA increases the H2O2 sensitivity of the kat mutant. Additionally, loss of ccp can elevate the H2O2 sensitivity of an mntC mutant, but not an msrA mutant, when cultured anaerobically.
Paraquat, which induces the production of intracellular ROS, was more toxic to the kat mutant GP301 and mntC mutant GP304 than wild-type MS11 bacteria under aerobic conditions, as reported for mutants in these factors in other strains (47, 52) (data not shown). When tested anaerobically, the mntC, ccp, and kat single mutants, the mntC kat, mntC ccp, and kat ccp double mutants, and the mntC ccp kat triple mutant were equally more sensitive to paraquat than the wild-type strain (P < 0.05) (Fig. 1B). We conclude that inactivation of kat or mntC causes strain MS11 to be less able to tolerate intracellular ROS under anaerobic conditions. However, in contrast to sensitivity to exogenously added H2O2, mutation of these factors or of msrA along with kat or mntC does not have an additive effect.
Assessment of in vivo fitness.
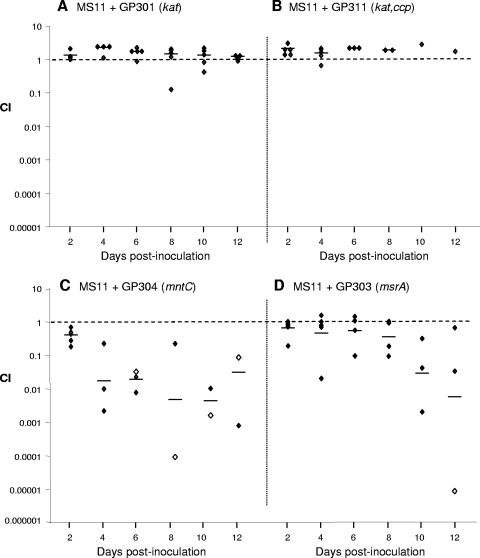
We next tested the capacity of mutants with the highest degree of H2O2 sensitivity in vitro to establish genital tract infection in female mice. Based on the likelihood that inactivation of the mntC, ccp, and kat genes would have the most profound effect in vivo, we inoculated groups of mice with 106 CFU of wild-type MS11 bacteria or the kat ccp mntC mutant GP318 and measured the number of gonococci recovered over 12 days. We found no difference in the duration of infection, with mice colonized with MS11 or GP318 bacteria for an average of 8.3 or 7.5 days, respectively (range, 2 to 12 days [wild type] or 4 to 12 days [mutant]). There was also no difference in the number of bacteria recovered over time (Fig. 2A). We therefore next tested the capacity of the mntC kat ccp mutant to compete with the wild-type strain in vivo, which is a more sensitive technique than noncompetitive infection. Groups of mice were inoculated with mixed suspensions containing similar numbers of GP318 and MS11 bacteria, and the ratio of mutant to wild-type bacteria among vaginal isolates was compared to that of the inoculum. Significantly reduced recovery of GP318 bacteria occurred relative to the wild-type strain over time, with a 10-fold decrease in the mean CI detected 2 days after inoculation. By day 8, a 1,000-fold decrease in the mean CI was detected, and high numbers of wild-type bacteria but no GP318 bacteria were recovered from a majority of mice on days 8 and 10 postinoculation (Fig. 2B). No differences in the growth rate (Fig. 2C) or ratio of mutant to wild-type gonococci (Fig. 2D) were observed when wild-type and GP318 mutant bacteria were cultured together under aerobic conditions.
FIG. 2.
A kat ccp mntC mutant is attenuated during competitive infection with the wild-type strain. The fitness of the triple kat ccp mntC mutant GP318 was compared to that of the wild-type strain in noncompetitive and competitive infections of estradiol-treated BALB/c mice. (A) For noncompetitive infections, groups of mice (n = 8) were inoculated with wild-type MS11 or mutant GP318 bacteria, and vaginal swab suspensions were quantitatively cultured over time. There was no difference in the number of wild-type or mutant gonococci recovered from either group. (B) For competitive infections, a mixed suspension containing similar numbers of wild-type MS11 and mutant GP318 gonococci were inoculated into mice. The relative recovery of the mutant over time was determined using GC agar with Sm (total number) and GC agar with Sm and Cm (mutant) as described in Materials and Methods. Results shown are the CI for individual mice at each time point. A CI of <1.0 indicates a decrease in the ratio of mutant to wild-type gonococci compared to that of the inoculum and thus decreased fitness. Horizontal bars represent the geometric mean. Open symbols represent mice from which no mutants were recovered; the limit of detection (1 CFU per 100 μl of swab suspension) was used as the number of mutant CFU recovered in these cases. (C) The growth kinetics of wild-type MS11 and mutant GP318 when incubated separately in GC broth under aerobic conditions or together was measured by the change in the A600 over time. (D) Ratio of mutant to wild-type gonococci recovered from the mixed broth culture shown in panel C divided by the ratio at time zero. The values shown (in vitro CI) are approximately 1.0 at each time point tested, which indicates the mutant has no advantage or disadvantage compared to the wild-type strain when cultured under these conditions in vitro.
To determine which gene(s) contributed to the attenuated phenotype of mutant GP318, competitive infections were performed between wild-type MS11 bacteria and the kat mutant GP301, the kat ccp double mutant GP311, and the mntC mutant GP304. Despite the clear advantage that is afforded by catalase when gonococci are exposed to H2O2 in vitro, the absence of catalase alone or of both catalase and Ccp did not cause attenuation in vivo, as evidenced by an average CI of 1.0 over the 10-day experiment (Fig. 3A and B). Emr vaginal isolates (GP301 and GP311) on the primary isolation plates were flooded with H2O2, and no bubbles were observed; therefore, the kat mutation did not revert in vivo. We also detected expression of ccp in vaginal swab suspensions from mice infected with wild-type gonococci by RT-PCR (data not shown). This result is consistent with the O2-limited environment of the mouse vagina and rules out the possibility that lack of expression of the wild-type gene during competitive infections with the kat ccp mutant was responsible for our not seeing a difference in the survival of Ccp-deficient gonococci in the mouse model.
FIG. 3.
Inactivation of mntC or msrA but not kat or kat and ccp in strain MS11 causes attenuation in BALB/c mice. Female estradiol-treated BALB/c mice were inoculated with similar numbers of wild-type MS11 gonococci mixed with GP301(kat) (A), GP311 (kat ccp) (B), GP304 (mntC) (C), or GP303 (msrA) (D) bacteria. The relative recovery of each mutant over time was determined using GC agar with Sm (total number) and GC agar with Sm plus Em (GP301), Cm (GP311 or GP303), or Km (GP304) as described in Materials and Methods. Results shown are the CI for individual mice at each time point. Horizontal bars represent the geometric mean. A CI of <1.0 indicates a decrease in the ratio of mutant to wild-type gonococci compared to that of the inoculum and thus decreased fitness. Open symbols represent mice from which no mutants were recovered; the limit of detection (1 CFU per 100 μl of swab suspension) was used as the number of mutant CFU recovered in these cases. Mutants GP303 and GP304 were tested twice in competitive infections with the wild-type strain (n = 5 to 8 mice per experiment), and the results were reproducible.
In contrast to the kat and kat ccp mutants, the mntC mutant GP304 was attenuated relative to the wild-type strain. The relative recovery of GP304 bacteria decreased over time, with a 60- to 100-fold decrease in the mean CI detected by days 4 to 10 postinoculation (Fig. 3C). Repeat experiments showed similar results in terms of the degree of attenuation of the mntC mutant. Mutants GP301, GP311, and GP304 showed no growth advantage or disadvantage compared to the parent strain MS11 when cultured in GCB in vitro (data not shown). These results are consistent with inactivation of mntC being responsible for the attenuated phenotype of the mntC kat ccp triple mutant in vivo.
Strain-specific differences in the catalase mutants.
We previously reported that a kat mutant in strain FA1090 colonized female BALB/c mice and persisted during a vigorous PMN influx. A nonsignificant, but dose-dependent, trend toward reduced duration of infection by the kat mutant was observed when compared to wild-type gonococci inoculated into separate groups of mice (46). Therefore, here we utilized the more sensitive technique of competitive infection to compare the fitness of GP500 bacteria relative to wild-type FA1090 bacteria in vivo. Interestingly, and in contrast to the MS11 kat mutant, the FA1090 kat mutant GP500 was dramatically attenuated relative to strain FA1090, with a 100- to 10,000-fold decrease in CI on days 2 to 6 postinoculation. No kat mutant bacteria were recovered from five of six mice by day 6 of infection, in contrast to high numbers of wild-type gonococci (103 to >105 CFU/100 μl of vaginal swab suspension) recovered at this time point (Fig. 4A).
FIG. 4.
An FA1090 kat mutant is attenuated during competitive infection with the wild-type strain. Female estradiol-treated BALB/c mice were inoculated with similar numbers of wild-type FA1090 bacteria mixed with GP500 (kat) or GP506 (complemented mutant) gonococci. The relative recovery of each strain was determined over time by culture on GC media with Sm (total number) or GC with Sm and Km (GP500 and GP506) as described in Materials and Methods. Results shown are the CI for individual mice at each time point. Horizontal bars represent the geometric mean. A CI of <1.0 indicates a decrease in the ratio of mutant to wild-type gonococci compared to that of the inoculum and thus decreased fitness. CI values of >1.0 indicate increased fitness. Open symbols represent mice from which no mutants were recovered; the limit of detection (1 CFU per 100 μl of swab suspension) was used as the number of mutant CFU recovered in these cases. The experiment was repeated and gave reproducible results. No fitness difference was observed when wild-type and kat mutant bacteria were cocultured in vitro.
Previous attempts to fully complement mutant GP500 were unsuccessful due to unstable expression (46) or production of an aberrant catalase protein by the recombinant kat gene (47). Here, we successfully complemented mutant GP500 by integrating a wild-type copy of the MS11 kat gene into a noncoding region of the chromosome as described in Materials and Methods. Complemented strain GP506 produced bubbles upon exposure to H2O2 and wild-type levels of catalase (Table 3) when cultured in the presence of IPTG. The H2O2 resistance of GP506 bacteria was also similar to that of the wild-type strain, and strain GP506 produced a catalase protein with the same migration as the wild-type species on activity gels (data not shown). When tested in competitive infections with the parent strain FA1090, GP506 bacteria showed no evidence of attenuation, and in fact, the complemented mutant demonstrated a fitness advantage relative to the wild-type strain as indicated by a 10-fold increase in the mean CI within 2 days after inoculation (Fig. 4B).
TABLE 3.
Catalase expression in mutant and complemented strains
| Strain | Catalase activity (units/mg) ± SD |
|---|---|
| FA1090 | 556 ± 32 |
| GP500 | 0 |
| GP506 with IPTG | 628 ± 6 |
| GP506 without IPTG | 13 ± 4 |
| MS11 | 567 ± 27 |
| GP301 | 0 |
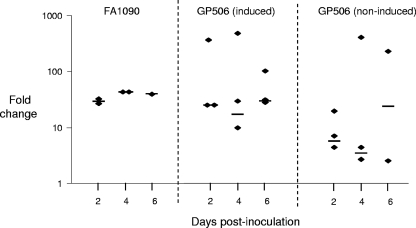
The kat gene used to complement strain GP506 lacks its native promoter region and is under the control of the lac promoter present in the complementation vector (30, 44). The GP506 bacteria used to inoculate mice were cultured on medium with IPTG, but we did not administer IPTG to the mice to maintain expression of the kat gene over the course of infection. Therefore, to confirm that expression of the kat gene in strain GP506 occurred in vivo, quantitative RT-PCR was performed on vaginal washes from mice inoculated with wild-type, GP500, or GP506 bacteria cultured with or without IPTG. No PCR product was amplified from vaginal washes from mice infected with the kat mutant GP500 when kat-specific primers were used. Wild-type bacteria expressed the kat gene in vivo at a 10-fold-higher level than that expressed by in vitro-grown bacteria (Fig. 5, left panel). These results suggest kat expression is upregulated in the murine genital tract. In vivo expression of the kat gene in the complemented mutant was similar to wild-type expression levels when preinduced cultures were used to inoculate the mice (Fig. 5, center panel). A lower level of kat transcription was detected in mice inoculated with uninduced GP506 bacteria; however, expression was 5- to 33-fold higher than that of the kat gene expressed by in vitro-grown wild-type FA1090 bacteria (Fig. 5, right panel). We also observed variability in kat expression among mice inoculated with strain GP506 regardless of IPTG induction, with some mice expressing almost 1,000-fold more kat than that expressed by in vitro-grown wild-type bacteria. It is therefore possible that the observed in vivo fitness advantage of strain GP506 relative to the parent strain may be in part due to increased transcription of the recombinant kat gene when in the murine lower genital tract. The identity of the inducing factor is not known. Although N. gonorrhoeae does not produce a lactose permease, it is possible that vaginal lactose may induce expression from the lac promoter used in the complementation vector. However, we found no difference in the level of catalase produced by strain GP506 when cultured on media with no lactose and with 0.05 mM to 80 mM lactose (data not shown), and thus we ruled out this hypothesis. We conclude that an IPTG-like substance must be present in the murine genital tract that induces expression of the complementing kat gene in strain GP506 during infection.
FIG. 5.
In vivo expression of the kat gene in wild-type bacteria and the complemented kat mutant. Mice were inoculated with wild-type FA1090 (left panel), the complemented mutant GP506 cultured in IPTG (center panel), or GP506 bacteria cultured without IPTG (right panel). Vaginal washes from individual mice in each group were collected on days 2, 4, and 6 postinoculation, and RT-PCR was performed to measure expression of the wild-type or recombinant kat genes. Expression of rmp served as the active reference control (normalizer). Results are expressed as the fold difference compared to expression of the native kat gene in the wild-type FA1090 inoculum. The geometric mean is represented by the horizontal bar. No kat transcript was detected in vaginal washes from mice infected with the kat mutant GP500. Samples to which no reverse transcriptase was added were tested in parallel to control for contaminating DNA, and no PCR products were amplified from these samples.
Susceptibility to killing by PMNs from BALB/c mice.
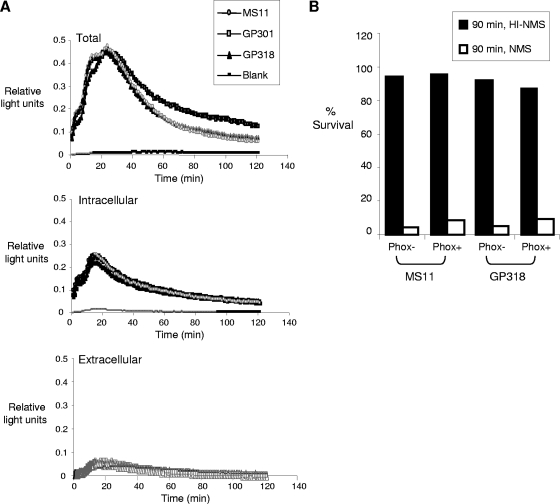
To determine whether any of the mutants were more susceptible to PMN killing in vitro, we compared the susceptibility of MS11 wild-type and mutant gonococci to PMNs isolated from BALB/c mice. All the MS11 mutants, including the kat ccp mntC mutant GP318, induced a CL response that was primarily intracellular, and there was no difference in the magnitude or kinetics of the CL response following exposure of PMNs to wild-type or mutant gonococci (Fig. 6A). As reported previously (58), wild-type MS11 gonococci were significantly killed by murine PMNs when opsonized with normal mouse serum and killing was abrogated by using serum heated to 56°C for 30 min. None of the MS11 mutants, including the mntC kat ccp triple mutant, showed increased susceptibility to PMN killing in vitro when cultured aerobically or under anaerobic conditions prior to the assay to express the ccp gene (data not shown). The CL data rule out the possibility that differences in the mutants' capacities to stimulate the phagocytic respiratory burst might influence the results. We conclude that none of the four factors tested, alone or in combination with one or two other factors, confers detectable resistance to PMN killing in vitro, including MntC, the loss of which attenuated strain MS11 in vivo.
FIG. 6.
Interactions of wild-type and mutant gonococci with murine PMNs. (A) CL response of PMNs from BALB/c mice after incubation with wild-type MS11, kat mutant GP301, and the mntC kat ccp mutant GP318. The total (top panel), intracellular (middle panel), and extracellular (bottom panel) CL responses upon exposure to bacteria (with no added phorbol myristate acetate) were measured as described previously (4, 58). (B) Opsonophagocytic killing of wild-type or GP318 (mntC kat ccp) mutant gonococci by PMNs from C57BL/6J (Phox-sufficient) and B6.129S6-Cybbtm1Din/J (Phox-deficient) mice. Results are expressed as percent survival. The experiment was performed twice and the results were similar. HI-NMS, heat-inactivated normal mouse serum; NMS, normal mouse serum.
Investigation of attenuated phenotypes in Phox-deficient mice.
To further test the basis for the attenuation of the MS11 mntC (GP304) and FA1090 kat (GP500) mutants we performed competitive infections in mice that carry a mutation in the 91-kDa subunit of the NADPH oxidase complex (Phox-deficient) and C57BL/6 (wild-type, Phox-sufficient) mice. We predicted that if increased susceptibility to phagocyte-derived ROS produced were responsible for the reduced recovery of these mutants, the mutants would not show an attenuated phenotype in Phox-deficient mice. We found that the recoveries of both GP304 and GP500 bacteria were markedly reduced in Phox-deficient mice relative to their respective wild-type parent strains, and there was no difference in the mean CI when results from the Phox-deficient and wild-type C57BL/6 backgrounds were compared (Fig. 7). We also tested the susceptibility of wild-type MS11 and the mntC kat ccp triple mutant GP318 to PMNs from Phox-deficient and normal C57BL/6 mice. Both wild-type MS11 and GP318 bacteria were killed by PMNs from either mouse strain, and there was no difference in the degree of killing with respect to the strain of mice from which the PMNs were isolated (Fig. 6B). We conclude that the MS11 mntC and FA1090 kat mutants are attenuated in vivo for reasons that are unrelated to increased susceptibility to the phagocytic respiratory burst.
FIG. 7.
The FA1090 kat mutant and MS11 mntC mutants are attenuated in Phox-deficient and wild-type C57BL/6J mice. FA1090 kat mutant GP500 and the MS11 mntC mutant GP504 were tested in B6.129S6-Cybbtm1Din/J (Phox-deficient) and C57BL/6J (Phox-sufficient) mice by competitive infection with the respective parent strains. Experiments were performed as for Fig. 3 and 4. Results shown are the CI for individual mice at each time point. Horizontal bars represent the geometric means. A CI of <1.0 indicates a decrease in the ratio of mutant to wild-type gonococci compared to that of the inoculum and thus decreased fitness. Open symbols represent mice from which no mutants were recovered; the limit of detection (1 CFU per 100 μl of swab suspension) was used as the number of mutant CFU recovered in these cases.
Delayed attenuation of an msrA mutant in BALB/c mice.
Inactivation of the msrA gene in strain MS11 singly or in combination with ccp or kat did not elevate H2O2 sensitivity to the level shown by the kat mutant alone at any concentration of H2O2 tested. Like mutants in mntC, kat, and ccp, the msrA mutant was not more sensitive to killing by isolated PMNs from BALB/c mice in vitro. We wished to test the msrA mutant in vivo, however, based on reports that msrA mutants in Mycoplasma genitalium (11) and Helicobacter pylori (1) were attenuated in animal infection models. Interestingly, msrA mutant GP303 showed significantly reduced recovery from BALB/c mice relative to wild-type MS11 bacteria as infection progressed beyond 8 days, with a mean 50-fold decrease in recovery on day 10 postinoculation (Fig. 3D). No MsrA/B-deficient bacteria were recovered from one mouse that was colonized with >105 CFU of wild-type gonococci on day 12 (Fig. 3D). We found no difference in the growth kinetics of wild-type and msrA mutant bacteria when cultured in broth through late stationary phase under aerobic conditions (data not shown), and the delayed attenuation phenotype was reproduced in a second competitive infection experiment. We next performed experiments with C57BL/6 mice and Phox-deficient mice to determine if the basis of the attenuation was related to the phagocytic respiratory burst. Surprisingly, mutant GP303 was not attenuated in the C57BL/6 background in each of two experiments (data not shown). The msrA mutant was equally susceptible to killing by PMNs from Phox-deficient mice compared to C57BL/6 mice (data not shown); however, without Phox-deficient mice in the BALB/c background, we were unable to more definitively test the role of NADPH oxidase in challenging the msrA mutant during infection.
DISCUSSION
The capacity of N. gonorrhoeae to persist in inflamed urogenital tissues is a remarkable feature of its pathogenesis. The mechanism by which N. gonorrhoeae evades oxidative killing by PMNs is not yet known, and a functional redundancy may mask the phenotype of mutants in one or a few of these factors. Others reported that a kat mutant and a kat ccp mutant of gonococcal strain 1291 were not more sensitive to killing by human PMNs (42). Here we constructed a series of mutants in strain MS11 that allowed us to test four well-characterized factors known to protect N. gonorrhoeae from oxidative stress in vitro. We found that catalase was clearly the most important factor in protecting against H2O2 and that only Ccp had an additive effect over catalase alone. Despite their in vitro phenotypes, the MS11 kat and kat ccp mutants showed no evidence of attenuation in a murine model of genital tract infection. In contrast, a kat ccp mntC mutant was attenuated in vivo, as was an mntC single mutant. Neither the mntC nor mntC kat ccp mutant showed increased susceptibility killing by PMNs from normal mice, and the mntC kat ccp mutant was not less susceptible to PMNs from mice that produce a defective NADPH oxidase. We further ruled out the possibility that inactivation of mntC increases susceptibility to phagocyte-derived ROS during genital tract infection by performing infection studies in Phox-deficient mice.
The basis for the attenuated phenotype of the MS11 mntC mutant in vivo is not known. Unfortunately we have been unable to introduce a wild-type copy of the mntC gene into the mutants GP304 and GP318, and therefore we have not definitively confirmed that the disadvantage shown in vivo is due to the absence of MntC. An impaired capacity to transport manganese and zinc (7, 28) through the MntABC system may impose an in vivo growth defect to the mntC mutant, since such metals may be needed as cofactors for enzymes that are important for gonococcal survival in vivo. We did not observe a difference in growth between wild-type FA1090 and the mutants that lack mntC when cultured in GCB or on GC agar. This result is in contrast to the report by Tseng et al. (52) in which an mntC mutant had a reduced growth rate when cultured on medium that had a fivefold-lower concentration of manganese than GC agar. It is unlikely that supplemented GC agar or broth provides the same balance of minerals found in the genital tract, and therefore we cannot rule out that reduced growth of the mntC mutant in vivo is responsible for the observed attenuated phenotype.
Interestingly, Mn2+ uptake mutants of Streptococcus pneumoniae (28a) were attenuated in murine respiratory and/or systemic infection models, the basis of which is not yet known. Epithelial cells can also produce ROS (39), and a gonococcal mntC mutant showed decreased intracellular survival in cervical epithelial cells (28).Other sources of ROS that might challenge gonococci that lack MntC include reactions with metal ions, including iron within heme-containing proteins released from cells (10), and H2O2-producing commensal flora (14). We believe the latter explanation is unlikely since we rarely isolate H2O2-positive commensal vaginal bacteria from mice and because we have shown H2O2-producing human lactobacilli do not challenge wild-type gonococci or mutants that lack catalase or catalase and Ccp in the mouse model (32). Finally, recent evidence suggests the gonococcal MntABC transporter plays a role in biofilm formation (28), and therefore, inactivation of mntC may affect colonization of N. gonorrhoeae in the mouse model. The mean CI in competitive infection experiments with wild-type and mntC mutant bacteria was not as dramatic as that obtained in experiments with wild-type and kat ccp mntC mutant gonococci, and thus it is possible that the kat ccp mntC mutant may have a greater in vivo survival disadvantage than that exhibited by the mntC single mutant. Competitive infections between the mntC kat ccp triple mutant and the mntC single mutant would allow investigation of this hypothesis. We did not pursue this line of investigation, based on our in vitro and in vivo evidence that none of these genes contributes to evasion of PMN killing.
An unexpected but interesting finding was the strain-specific attenuation associated with the kat gene. A catalase-deficient mutant of FA1090, but not MS11, resulted in an in vivo growth or survival disadvantage that was unrelated to increased susceptibility to the phagocytic respiratory burst. We previously reported that the FA1090 kat mutant was more susceptible to PMN killing than wild-type FA1090 (46). We have since been unable to reproduce these data, which we believe reflects the high degree of variability in the PMN killing assay. We also found no correlation between PMN influx, colonization load, and clearance of the kat mutant bacteria in the previous study, a result which is consistent with PMN killing not challenging catalase-deficient gonococci in vivo. As with the MS11 mntC mutant, the reason for the attenuation of the FA1090 kat mutant is not known, but it could be due to increased susceptibility to ROS produced by sources other than phagocytes.
The demonstration that loss of catalase is attenuating in one strain but not another is intriguing. MS11 is a serum intermediate strain that was originally isolated from the endocervix of an uncomplicated infection (51), and FA1090 is a serum-resistant strain isolated from a case of disseminated gonococcal infection (9). Others have reported that strain FA1090 is more sensitive to H2O2 than strains F62 and 28BI but that the three different strains have similar levels of catalase activity (2). We found that exposure of MS11 and FA1090 bacteria to H2O2 resulted in similar zones of inhibition (data not shown) and that the level of catalase activity in the two strains was the same (Table 3). We have detected other strain differences in the mouse model. For example, mutation of the lactate permease gene (lctP) conferred a growth or survival disadvantage in the murine genital tract to strain F62 (15), but not strain MS11 (H. Wu and A. E. Jerse, unpublished data). These observations underscore the importance of interpreting results in the context of the strain being studied and are evidence that evolutionary differences that affect the impact of specific adaptation genes on bacterial growth or survival in vivo have occurred.
Finally, we have presented evidence here that MsrA/B, which acts to repair oxidative damage rather than directly neutralize oxidative factors, enhances gonococcal survival late during infection of BALB/c mice. This attenuation was not observed in C57BL/6 mice. We consider these results intriguing based on our recent discovery that N. gonorrhoeae induces a vaginal PMN influx and proinflammatory cytokines and chemokines in BALB/c but not C57BL/6 mice (M. Packiam, R. R. Ingalls, and A. E. Jerse, Abstr. 16th Int. Pathog. Neisseriaceae Conf., Rotterdam, Netherlands, abstr. P097, 2008). There may therefore be a link between the msrA phenotype and the inflammatory response. Studies with PMNs from normal and Phox-deficient mice did not support the hypothesis that phagocyte-derived ROS challenge the msrA mutant in vivo; however, this protein may have other protective roles. Mycobacterium tuberculosis MsrA conferred increased resistance to nitrosative stress to msrA mutant E. coli (49), and Mycobacterium smegmatis MsrA was implicated in increased survival of M. smegmatis within macrophages by a mechanism that does not involve direct protection from H2O2 or reactive nitrogen intermediates (12). Our recent demonstration that macrophages are recruited to genital tract tissue in mice infected with N. gonorrhoeae at later time points than those at which we detect PMNs (48) is potentially consistent with the delayed attenuation of the gonococcal msrA mutant. Alternatively, a deficiency in MsrA/B may have pleiotropic effects by causing the accumulation of many different functionally impaired proteins, including adhesins (57).
In summary, several reports have shown that gonococci are killed by PMNs, primarily by oxygen-independent mechanisms (5, 37, 42). It is still not known, however, how gonococci evade the phagocytic respiratory burst. Our study was not exhaustive, as other factors are reported to protect the gonococcus from ROS in vitro, including thiol-disulfide oxidoreductase (Sco) (41), azurin (59), bacterioferritin (8), and newly described peroxidase-induced genes of unknown function (50). It is conceivable that the layers of functional redundancy in this well-adapted pathogen may be too thick to strip away by genetic mutation. Alternatively, the gonococcus may utilize a novel mechanism of evading phagocytic ROS. Finally, our findings suggest there are interesting roles for the MntABC transporter and the MsrA/B protein in vivo that as of yet are undefined.
Acknowledgments
We thank Cara Olsen for help with the statistical analyses and Lotisha Garvin for assistance with manuscript preparation.
This work was supported by the National Institutes of Health grant number RO1 AI42053. A.A.S.-G. was supported by NRSA grant F31 AI10494.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 29 December 2008.
REFERENCES
- 1.Alamuri, P., and R. J. Maier. 2004. Methionine sulphoxide reductase is an important antioxidant enzyme in the gastric pathogen Helicobacter pylori. Mol. Microbiol. 531397-1406. [DOI] [PubMed] [Google Scholar]
- 2.Alcorn, T. M., H. Y. Zheng, M. R. Gunther, D. J. Hassett, and M. S. Cohen. 1994. Variation in hydrogen peroxide sensitivity between different strains of Neisseria gonorrhoeae is dependent on factors in addition to catalase activity. Infect. Immun. 622138-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britigan, B. E., D. Klapper, T. Svendsen, and M. S. Cohen. 1988. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J. Clin. Investig. 81318-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bylund, J., M. Samuelsson, L. V. Collins, and A. Karlsson. 2003. NADPH-oxidase activation in murine neutrophils via formyl peptide receptors. Exp. Cell. Res. 28270-77. [DOI] [PubMed] [Google Scholar]
- 5.Casey, S. G., W. M. Shafer, and J. K. Spitznagel. 1986. Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect. Immun. 52384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey, S. G., D. R. Veale, and H. Smith. 1979. Demonstration of intracellular growth of gonococci in human phagocytes using spectinomycin to kill extracellular organisms. J. Gen. Microbiol. 113395-398. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C. Y., and S. A. Morse. 2001. Identification and characterization of a high-affinity zinc uptake system in Neisseria gonorrhoeae. FEMS Microbiol. Lett. 20267-71. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. Y., and S. A. Morse. 1999. Neisseria gonorrhoeae bacterioferritin: structural heterogeneity, involvement in iron storage and protection against oxidative stress. Microbiology 1452967-2975. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, M. S., J. G. Cannon, A. E. Jerse, L. M. Charniga, S. F. Isbey, and L. G. Whicker. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169532-537. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, M. S., Y. Chai, B. E. Britigan, W. McKenna, J. Adams, T. Svendsen, K. Bean, D. J. Hassett, and P. F. Sparling. 1987. Role of extracellular iron in the action of the quinone antibiotic streptonigrin: mechanisms of killing and resistance of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 311507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhandayuthapani, S., M. W. Blaylock, C. M. Bebear, W. G. Rasmussen, and J. B. Baseman. 2001. Peptide methionine sulfoxide reductase (MsrA) is a virulence determinant in Mycoplasma genitalium. J. Bacteriol. 1835645-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas, T., D. S. Daniel, B. K. Parida, C. Jagannath, and S. Dhandayuthapani. 2004. Methionine sulfoxide reductase A (MsrA) deficiency affects the survival of Mycobacterium smegmatis within macrophages. J. Bacteriol. 1863590-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elkins, C., C. E. Thomas, H. S. Seifert, and P. F. Sparling. 1991. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J. Bacteriol. 1733911-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eschenbach, D. A., P. R. Davick, B. L. Williams, S. J. Klebanoff, K. Young-Smith, C. M. Critchlow, and K. K. Holmes. 1989. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J. Clin. Microbiol. 27251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exley, R. M., H. Wu, J. Shaw, M. C. Schneider, H. Smith, A. E. Jerse, and C. M. Tang. 2007. Lactate acquisition promotes successful colonization of the murine genital tract by Neisseria gonorrhoeae. Infect. Immun. 751318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer, S. H., and R. F. Rest. 1988. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect. Immun. 561574-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabig, T. G., S. I. Bearman, and B. M. Babior. 1979. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood 531133-1139. [PubMed] [Google Scholar]
- 18.Gill, M. J., D. P. McQuillen, J. P. van Putten, L. M. Wetzler, J. Bramley, H. Crooke, N. J. Parsons, J. A. Cole, and H. Smith. 1996. Functional characterization of a sialyltransferase-deficient mutant of Neisseria gonorrhoeae. Infect. Immun. 643374-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn, J. S., and D. C. Stein. 1996. Use of a non-selective transformation technique to construct a multiply restriction/modification-deficient mutant of Neisseria gonorrhoeae. Mol. Gen. Genet. 251509-517. [DOI] [PubMed] [Google Scholar]
- 20.Hassett, D. J., L. Charniga, and M. S. Cohen. 1990. recA and catalase in H2O2-mediated toxicity in Neisseria gonorrhoeae. J. Bacteriol. 1727293-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook, E. W., and H. H. Handsfield. 1999. Gonococcal infections in the adult, p. 451-466. In K. K. Holmes, P. F. Sparling, P.-A. Mardh, W. S. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill, New York, NY.
- 22.Jerse, A. E. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 675699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jerse, A. E., E. T. Crow, A. N. Bordner, I. Rahman, C. N. Cornelissen, T. R. Moench, and K. Mehrazar. 2002. Growth of Neisseria gonorrhoeae in the female mouse genital tract does not require the gonococcal transferrin or hemoglobin receptors and may be enhanced by commensal lactobacilli. Infect. Immun. 702549-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerse, A. E., N. D. Sharma, A. N. Simms, E. T. Crow, L. A. Snyder, and W. M. Shafer. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 715576-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, S. R., B. M. Steiner, D. D. Cruce, G. H. Perkins, and R. J. Arko. 1993. Characterization of a catalase-deficient strain of Neisseria gonorrhoeae: evidence for the significance of catalase in the biology of N. gonorrhoeae. Infect. Immun. 611232-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, S. R., B. M. Steiner, and G. H. Perkins. 1996. Cloning and characterization of the catalase gene of Neisseria gonorrhoeae: use of the gonococcus as a host organism for recombinant DNA. Infect. Immun. 642627-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knapp, J. S., and V. L. Clark. 1984. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 46176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim, K. H., C. E. Jones, R. N. van den Hoven, J. L. Edwards, M. L. Falsetta, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2008. Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect. Immun. 763569-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.McAllister, L. J., H. J. Tseng, A. D. Ogunniyi, M. P. Jennings, A. G. McEwan, and J. C. Paton. 2004. Molecular analysis of the psa permease complex of Streptocccus pneumoniae. Mol. Microbiol. 53889-901. [DOI] [PubMed] [Google Scholar]
- 29.Mehr, I. J., C. D. Long, C. D. Serkin, and H. S. Seifert. 2000. A homologue of the recombination-dependent growth gene, rdgC, is involved in gonococcal pilin antigenic variation. Genetics 154523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehr, I. J., and H. S. Seifert. 1998. Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation and DNA repair. Mol. Microbiol. 30697-710. [DOI] [PubMed] [Google Scholar]
- 31.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 1755899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muench, D. F., D. J. Kuch, H. Wu, A. A. Begum, S. J. Veit, M.-E. Pelletier, A. A. Soler-Garcia, and A. E. Jerse. H2O2-producing lactobacilli inhibit gonococci in vitro but not during experimental genital tract infection. J. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 33.Packiam, M., D. M. Shell, S. V. Liu, Y. B. Liu, D. J. McGee, R. Srivastava, S. Seal, and R. F. Rest. 2006. Differential expression and transcriptional analysis of the alpha-2,3-sialyltransferase gene in pathogenic Neisseria spp. Infect. Immun. 742637-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons, N. J., A. A. Kwaasi, J. A. Turner, D. R. Veale, V. Y. Perera, C. W. Penn, and H. Smith. 1981. Investigation of the determinants of the survival of Neisseria gonorrhoeae within human polymorphonuclear phagocytes. J. Gen. Microbiol. 127103-112. [DOI] [PubMed] [Google Scholar]
- 35.Rashad, A. L., W. L. Toffler, N. Wolf, K. Thornburg, E. P. Kirk, G. Ellis, and W. E. Whitehead. 1992. Vaginal pO2 in healthy women and in women infected with Trichomonas vaginalis: potential implications for metronidazole therapy. Am. J. Obstet. Gynecol. 166620-624. [DOI] [PubMed] [Google Scholar]
- 36.Rest, R. F. 1997. Measurement of the human neutrophil respiratory burst during phagocytosis and killing of bacteria, p. 502-522. In V. L. Clark, S. P. Colowick, and P. M. Bavoid (ed.), Bacterial pathogenesis. Academic Press, New York, NY.
- 37.Rest, R. F., S. H. Fischer, Z. Z. Ingham, and J. F. Jones. 1982. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect. Immun. 36737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rest, R. F., and J. V. Frangipane. 1992. Growth of Neisseria gonorrhoeae in CMP-N-acetylneuraminic acid inhibits nonopsonic (opacity-associated outer membrane protein-mediated) interactions with human neutrophils. Infect. Immun. 60989-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochelle, L. G., B. M. Fischer, and K. B. Adler. 1998. Concurrent production of reactive oxygen and nitrogen species by airway epithelial cells in vitro. Free Radic. Biol. Med. 24863-868. [DOI] [PubMed] [Google Scholar]
- 40.Schneider, H., J. M. Griffiss, J. W. Boslego, P. J. Hitchcock, K. M. Zahos, and M. A. Apicella. 1991. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J. Exp. Med. 1741601-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seib, K. L., M. P. Jennings, and A. G. McEwan. 2003. A Sco homologue plays a role in defence against oxidative stress in pathogenic Neisseria. FEBS Lett. 546411-415. [DOI] [PubMed] [Google Scholar]
- 42.Seib, K. L., M. P. Simons, H. J. Wu, A. G. McEwan, W. M. Nauseef, M. A. Apicella, and M. P. Jennings. 2005. Investigation of oxidative stress defenses of Neisseria gonorrhoeae by using a human polymorphonuclear leukocyte survival assay. Infect. Immun. 735269-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seib, K. L., H. J. Wu, S. P. Kidd, M. A. Apicella, M. P. Jennings, and A. G. McEwan. 2006. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol. Mol. Biol. Rev. 70344-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skaar, E. P., B. Lecuyer, A. G. Lenich, M. P. Lazio, D. Perkins-Balding, H. S. Seifert, and A. C. Karls. 2005. Analysis of the Piv recombinase-related gene family of Neisseria gonorrhoeae. J. Bacteriol. 1871276-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skaar, E. P., D. M. Tobiason, J. Quick, R. C. Judd, H. Weissbach, F. Etienne, N. Brot, and H. S. Seifert. 2002. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc. Natl. Acad. Sci. USA 9910108-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soler-Garcia, A. A., and A. E. Jerse. 2007. Neisseria gonorrhoeae catalase is not required for experimental genital tract infection despite the induction of a localized neutrophil response. Infect. Immun. 752225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soler-Garcia, A. A., and A. E. Jerse. 2004. A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals. Microb. Pathog. 3755-63. [DOI] [PubMed] [Google Scholar]
- 48.Song, W., S. Condron, B. T. Mocca, S. J. Veit, D. Hill, A. Abbas, and A. E. Jerse. 2008. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17β-estradiol-treated mice. Vaccine 265741-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St. John, G., N. Brot, J. Ruan, H. Erdjument-Bromage, P. Tempst, H. Weissbach, and C. Nathan. 2001. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc. Natl. Acad. Sci. USA 989901-9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stohl, E. A., A. K. Criss, and H. S. Seifert. 2005. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol. Microbiol. 58520-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swanson, J., K. Robbins, O. Barrera, D. Corwin, J. Boslego, J. Ciak, M. Blake, and J. M. Koomey. 1987. Gonococcal pilin variants in experimental gonorrhea. J. Exp. Med. 1651344-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 401175-1186. [DOI] [PubMed] [Google Scholar]
- 53.Turner, S., E. Reid, H. Smith, and J. Cole. 2003. A novel cytochrome c peroxidase from Neisseria gonorrhoeae: a lipoprotein from a gram-negative bacterium. Biochem. J. 373865-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veale, D. R., H. Finch, and H. Smith. 1976. Penetration of penicillin into human phagocytes containing Neisseria gonorrhoeae: intracellular survival and growth at optimum concentrations of antibiotic. J. Gen. Microbiol. 96353-363. [DOI] [PubMed] [Google Scholar]
- 55.Veale, D. R., M. Goldner, C. W. Penn, J. Ward, and H. Smith. 1979. The intracellular survival and growth of gonococci in human phagocytes. J. Gen. Microbiol. 113383-393. [DOI] [PubMed] [Google Scholar]
- 56.Wagner, G., L. Bohr, P. Wagner, and L. N. Petersen. 1984. Tampon-induced changes in vaginal oxygen and carbon dioxide tensions. Am. J. Obstet. Gynecol. 148147-150. [DOI] [PubMed] [Google Scholar]
- 57.Wizemann, T. M., J. Moskovitz, B. J. Pearce, D. Cundell, C. G. Arvidson, M. So, H. Weissbach, N. Brot, and H. R. Masure. 1996. Peptide methionine sulfoxide reductase contributes to the maintenance of adhesins in three major pathogens. Proc. Natl. Acad. Sci. USA 937985-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu, H., and A. E. Jerse. 2006. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect. Immun. 744094-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, H. J., K. L. Seib, J. L. Edwards, M. A. Apicella, A. G. McEwan, and M. P. Jennings. 2005. Azurin of pathogenic Neisseria spp. is involved in defense against hydrogen peroxide and survival within cervical epithelial cells. Infect. Immun. 738444-8448. [DOI] [PMC free article] [PubMed] [Google Scholar]