Abstract
Apoptin, a protein from the chicken anemia virus, has attracted attention because it specifically kills tumor cells while leaving normal cells unharmed. The reason for this tumor selectivity is unclear and depends on subcellular localization, as apoptin resides in the cytoplasm of normal cells but in the nuclei of transformed cells. It was shown that nuclear localization and tumor-specific killing crucially require apoptin's phosphorylation by an as yet unknown kinase. Here we elucidate the pathway of apoptin-induced apoptosis and show that it essentially depends on abnormal phosphatidylinositol 3-kinase (PI3-kinase)/Akt activation, resulting in the activation of the cyclin-dependent kinase CDK2. Inhibitors as well as dominant-negative mutants of PI3-kinase and Akt not only inhibited CDK2 activation but also protected cells from apoptin-induced cell death. Akt activated CDK2 by direct phosphorylation as well as by the phosphorylation-induced degradation of the inhibitor p27Kip1. Importantly, we also identified CDK2 as the principal kinase that phosphorylates apoptin and is crucially required for apoptin-induced cell death. Immortalized CDK2-deficient fibroblasts and CDK2 knockdown cells were markedly protected against apoptin. Thus, our results not only decipher the pathway of apoptin-induced cell death but also provide mechanistic insights for the selective killing of tumor cells.
Targeted therapies have been the “holy grail” of cancer research for a long time. Initial successes such as the discovery of the role of estrogen signaling in the growth of breast cancer paved the way to the clinical use of antiestrogens. The importance of Her2/Neu for supporting cell proliferation and resistance to anticancer agents led to the development of herceptin and related pathway inhibitors (7, 45). In more narrow fields, retinoids allowed for a marked improvement in the therapy of acute promyelocytic leukemia (17), while imatinib has shown remarkable potency against chronic myelogenous leukemia (50). Despite these examples of new therapies, cancer remains the second most deadly group of diseases in the industrialized world, and the development of new targeted anticancer therapies is badly needed.
Apoptin is a small viral protein that was originally identified in chicken anemia virus and shows remarkable anticancer toxicity and selectivity (3, 48, 59). The tumor-selective cell death induced by apoptin has been demonstrated in a great number of tumor cells of different origins by the use of several techniques, including overexpression or introduction of cell-permeative apoptin constructs (8, 13, 24, 51). Unlike the case in tumor cells, apoptin does not induce apoptosis in a variety of normal cells, including human endothelial cells, hepatocytes, and hematopoietic stem cells. The mechanism by which apoptin is able to distinguish between tumor and normal cells is unknown but seems to correlate with its cellular localization. In primary cells, apoptin is retained in the cytoplasm, whereas in transformed cells it migrates into the nucleus. Recently, it was shown that apoptin is phosphorylated by an unknown kinase at Thr-108 specifically in transformed cells, which facilitates its nuclear localization and tumor-specific activity (55). The responsible kinase was largely undetectable in lysates of primary normal cells, whereas tumor cell lysates harbored high intrinsic levels of this kinase activity. Furthermore, an apoptin T108E mutant mimicking constitutive phosphorylation readily entered the nucleus and also killed normal cells (55). These results implied that phosphorylation of apoptin is a key regulatory mechanism of the apoptin-mediated cell death pathway.
Many experimental and clinical anticancer drugs interfere either directly or indirectly with cell cycle progression or signaling pathways that promote cell survival (11, 19). A major survival mechanism is mediated by the Akt/protein kinase B (PKB) pathway (12, 43). Akt, a serine/threonine kinase, is activated at the cell membrane by the 3-phosphoinositide-dependent protein kinase 1 (PDK1) after both enzymes have been recruited to the lipid messenger phosphatidylinositol 3,4,5-phosphate generated by phosphatidylinositol 3′-kinase (PI3-K) (16). An important function of Akt is the maintenance of cell survival and inhibition of apoptosis. Among other functions, Akt inactivates several proapoptotic molecules, such as the Bcl-2 protein Bad and the Forkhead transcription factor FKHRL, and triggers the activation of the antiapoptotic transcription factor NF-κB (15). Akt also modulates the functions of numerous substrates regulating cell proliferation, such as glycogen synthase kinase 3, the cyclin-dependent kinase (CDK) inhibitors p21Cip1/Waf1 and p27Kip1, and mammalian target of rapamycin (28, 33).
There is increasing evidence that in addition to the cytoplasm, all components of the PI3-K/Akt pathway can reside in the nucleus, where they may still exert largely undefined functions. In this context, Akt has been found to regulate cell cycle progression at the G1/S and G2/M phases by either directly or indirectly phosphorylating various substrates, including p21Waf1, p27Kip1, cyclin D1, and CDK2 (28, 33, 39). Furthermore, while the role of the PI3-K/Akt pathway in cell survival is well established, there are some exceptions where PI3-K and Akt are obviously involved in promotion of cell death (2, 37, 47, 57). We recently reported that apoptin's toxicity depends on the hyperactivation of the PI3-K/Akt pathway. Thus, we observed that apoptin's interaction with PI3-K and a consequent nuclear translocation of Akt are necessary for apoptin's toxicity (40, 42). Apparently, therefore, apoptin can hijack the PI3-K/Akt pathway and direct its action toward the induction of apoptosis.
While the mechanism of action of apoptin remains largely unclear, we hypothesized that the nuclear translocation of Akt might be involved in the tumor-selective subcellular localization and proapoptotic activity of apoptin. Here we elucidated by various means that apoptin triggers the nuclear translocation and activation of Akt, resulting in the subsequent activation of CDK2. Akt-mediated activation of CDK2 was caused by two mechanisms, including the direct phosphorylation of CDK2 and the phosphorylation-induced degradation of its inhibitor p27Kip1. Furthermore, we demonstrate that cyclin A- but not cyclin E-associated CDK2 is responsible for apoptin phosphorylation as well as tumor-specific localization and proapoptotic activity.
MATERIALS AND METHODS
Cell culture and reagents.
MCF-7 and PC3 cells and CDK2-expressing (wild type [WT]), CDK2−/−, and Skp2−/− immortalized murine embryonic fibroblasts (MEFs) were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 100 μg/ml penicillin, and 0.1 μg/ml streptomycin (Gibco BRL, Grand Island, NY). PC3 cells overexpressing Bcl-2 (25) were kindly provided by M. B. Cohen (University of Iowa, Iowa City). Primary CDK2−/− MEFs (6) were obtained from P. Kaldis (National Cancer Institute, Frederick, MD) and immortalized using a temperature-sensitive adenoviral large T antigen as described previously (41). Immortalized Skp2−/− fibroblasts were a kind gift from N. P. Malek (Hannover Medical School, Hannover, Germany). Primary human mammary epithelial cells (HMECs) were maintained in mammary epithelial basal medium supplemented according to the supplier's instructions (Clonetics, San Diego, CA). The following antibodies were used: rabbit anti-Akt, rabbit anti-phospho-Akt Ser-473, mouse anti-phospho-Ser, and mouse anti-phospho-threonine proline-specific antibody, from Cell Signaling, Danvers, MA; anti-CDK1, anti-CDK2, anti-cyclin A, anti-cyclin E, and anti-Bcl-2, from Santa Cruz Biotechnologies, Santa Cruz, CA; anti-p27Kip1-phospho-Thr-157, rabbit anti-hemagglutinin, Cy3-conjugated goat anti-rabbit secondary antibody, antitubulin, and antiactin, from Sigma, St. Louis, MO; anti-Skp2 and anti-Cks1, from Zymed Laboratories, San Francisco, CA; and anti-phospho-histone H1 and horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies, from Upstate, Lake Placid, NY. Rabbit antiapoptin was a kind gift from D. A. Jans (Monash University, Clayton, Victoria, Australia). The PI3-K inhibitors wortmannin and LY294002 and the proteasome inhibitor MG115 were purchased from Calbiochem (San Diego, CA) and used at concentrations of 5 nM, 1.5 μM, and 2 μM, respectively. Roscovitine, Z-VAD-FMK, and PD98059 were obtained from Alexis Biochemicals (Lausanne, Switzerland) and used at concentrations of 5 μM, 50 μM, and 50 μM, respectively.
Recombinant proteins, plasmids, and transfections.
Eukaryotic expression constructs encoding N-terminally green fluorescent protein (GFP)-tagged apoptin and various deletion mutants have been described (52) and were kindly provided by D. A. Jans. Tat-apoptin was a kind gift from M. Tavassoli (King's College, London, United Kingdom) and was expressed in Escherichia coli and purified as described previously (24). Recombinant glutathione S-transferase (GST)-apoptin was bacterially expressed and purified on glutathione-Sepharose beads (40). Adenoviral constructs encoding a constitutively active form of Akt with a myristoylated N terminus and a dominant-negative form of Akt containing a point mutation in its catalytic domain (K179M) as well as T308A and S473A substitutions of the phosphorylation sites were kindly provided by K. Walsh (Whitaker Cardiovascular Institute, Boston, MA). An adenoviral nuclear Akt construct was a gift from M. A. Sussman (San Diego State University Heart Institute, CA). Viral transductions were performed as described previously (18). A constitutively active and a kinase-dead mutant (K111Q/D205N/D223N) of PDK1 were obtained from A. J. Halayko (University of Manitoba, Winnipeg, Manitoba, Canada). A kinase-dead version of PKCɛ containing the double mutation K436R and A159E was kindly provided by E. Kardami (University of Manitoba). The CDK2 T160A mutant vector and GST-CDK2 WT vectors were described earlier (23, 53) and were obtained from D. Morgan (University of California, San Francisco) and T. Hunter (University of California, San Diego), respectively. The target-specific small interfering RNA (siRNA) for p27 was purchased from Santa Cruz Biotechnologies, and the plasmid encoding CDK2 siRNA was purchased from Millipore Corporation (Bedford, MA). Target-specific siRNAs for Akt and PI3-K p85 were purchased from Upstate, and the siRNAs against Skp2 and Cks1 were purchased from Invitrogen (Burlington, Ontario, Canada). Transfections were performed using Lipofectamine or Oligofectamine according to the manufacturer's protocol (Invitrogen).
Cell death assays.
Cell death was determined by measurement of hypodiploid nuclei by the Nicoletti method (54). Briefly, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and then resuspended in a hypotonic buffer (0.1% sodium citrate, 0.1% Triton X-100, 0.5 mg/ml RNase A) containing 40 μg/ml propidium iodide. Cell nuclei were then incubated for 10 min at 37°C and subsequently analyzed by flow cytometry.
Immunoprecipitation.
Cells were washed twice with cold PBS, lysed with ice-cold lysis buffer (50 mM Tris-HCl, pH 7.5, 1% Nonidet P-40, 150 mM NaCl, 1 mM Na3VO4, 2 mM EGTA, protease inhibitor cocktail), incubated for 30 min on ice, and centrifuged for 10 min at 4°C. Immunoprecipitations were performed with the indicated antibodies, and the immune complexes were captured with protein A-agarose beads (Amersham Biosciences, Piscataway, NJ). After three washes with cell lysis buffer, bead-bound proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot analysis according to standard protocols.
GST pull-down assays.
GST-tagged apoptin or control GST bound to glutathione-Sepharose beads (Amersham) was incubated with cell lysates for 1 h at 4°C. Following three washes of the beads with lysis buffer, protein complexes were eluted by boiling in SDS sample buffer, separated by SDS-PAGE, and analyzed by Western blotting.
Cell fractionation.
Cytoplasmic, nuclear, and mitochondrial fractions were separated by differential centrifugation. Briefly, cells were treated with apoptin, washed with PBS, and resuspended for 10 min on ice in lysis buffer containing 10 mM Tris-HCl, pH 7.8, 1% Nonidet P-40, 10 mM β-mercaptoethanol, and protease inhibitors. The nuclear fraction was recovered by centrifugation at 600 × g for 5 min. The supernatant was centrifuged at 10,000 × g for 30 min to obtain the mitochondrial fraction (pellet) and the cytosolic fraction (supernatant). The mitochondrial fraction was further lysed in 10 mM Tris-HCl, pH 7.4, 300 mM NaCl, 1% Triton X-100, and 5 mM EDTA.
Immunocytochemistry.
Cells were fixed in 4% paraformaldehyde in PBS, permeabilized in 0.2% Triton X-100, and stained with either Akt- or CDK2-directed antibodies followed by their respective secondary antibodies conjugated to Cy3. The fluorescence signal was acquired by multilaser confocal microscopy.
In vitro kinase assays.
In vitro kinase assays using GST-apoptin, Tat-apoptin, and histone H1 as substrates were done using a nonradioactive assay. Briefly, recombinant CDK2 bound with either cyclin E (10 ng) or cyclin A (500 ng) and CDK1 bound with cyclin B (10 ng; all purchased from Millipore) or the immunoprecipitated CDK2 WT or mutant proteins were used in a kinase reaction mix with 5 μg of GST-apoptin, Tat-apoptin, or histone H1 as a substrate in the presence of 200 μM ATP in a kinase assay buffer (25 mM Tris-HCl, pH 7.5, 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2, and phosphatase inhibitor cocktail). After incubation for 45 min at 30°C, the reaction products were resolved by SDS-PAGE and detected by immunoblotting with their respective phospho-specific antibodies.
RESULTS
Akt translocates to the nucleus during apoptin-induced cell death.
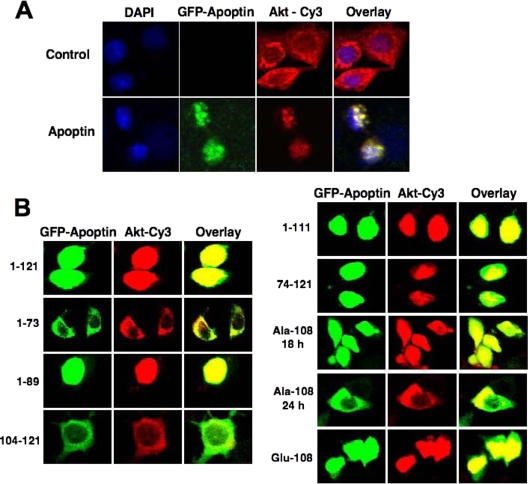
Akt is generally regarded as a survival and proliferation-promoting kinase. In the presence of apoptin, however, Akt acts as a pro-cell-death molecule, as its inhibition severely interferes with cell death pathways triggered by apoptin (40, 42). To learn more about the altered apoptotic properties of Akt, we tested if apoptin affects the cellular localization of Akt and thus might redirect it to different targets. As shown in Fig. 1A, in the absence of apoptin, Akt was localized mainly in the cytoplasm of MCF-7 breast cancer cells but became nuclear in apoptin-expressing cells. Time course experiments revealed that upon transfection with the apoptin construct, the majority of Akt was transferred to the nucleus within 12 h, a time that clearly preceded induction of cell death (data not shown). Similar results were obtained with PC3 prostate cancer cells.
FIG. 1.
Akt translocates to the nucleus during apoptin-induced cell death. (A) MCF-7 cells were transfected with GFP-tagged apoptin or a vector control. At 18 h posttransfection, the localization of Akt in either the absence or presence of apoptin in MCF-7 cells was detected by confocal microscopy after immunostaining with anti-Akt and Cy3-conjugated secondary antibody. DAPI (4′,6-diamidino-2-phenylindole) was used to counterstain nuclei, and the images were overlaid to determine the Akt localization within the cell. (B) MCF-7 cells were transfected with different apoptin mutants, and the localization of Akt and apoptin was detected by immunocytochemistry.
To determine whether apoptin/PI3-K interaction is necessary for Akt nuclear translocation, we investigated the localization of Akt in cells expressing different apoptin deletion mutants. As shown in Fig. 1B, Akt translocated to the nucleus only in cells expressing full-length apoptin (aa 1 to 121), an N-terminally truncated mutant (aa 74 to 121), or two mutants (aa 1 to 89 and aa 1 to 111) lacking the second nuclear localization signal (NLS) of apoptin. In contrast, cells expressing the apoptin mutants 1-73 and 104-121 did not cause Akt nuclear translocation, and the mutants themselves did not accumulate in the nucleus. This behavior correlates with the ability of the apoptin mutants to interact with the p85 subunit of PI3-K and to induce apoptosis (42). Also, upon transfection of a Glu-108 mutant, which mimics phosphorylation of the Thr-108 residue of apoptin (55), Akt efficiently translocated to the nucleus. Interestingly, in the presence of the phosphorylation-deficient Ala-108 mutant, Akt translocated to the nucleus together with apoptin at 18 h posttransfection, but it did not efficiently accumulate there, as at later time points considerable amounts of Akt were still detected in the cytoplasm together with apoptin. This is in accordance with a previous study (52) showing that the apoptin T108A mutant is still able to enter the nucleus. Thus, these results imply that apoptin might act as a shuttle molecule for Akt, mediating its nuclear transport via a piggyback interaction.
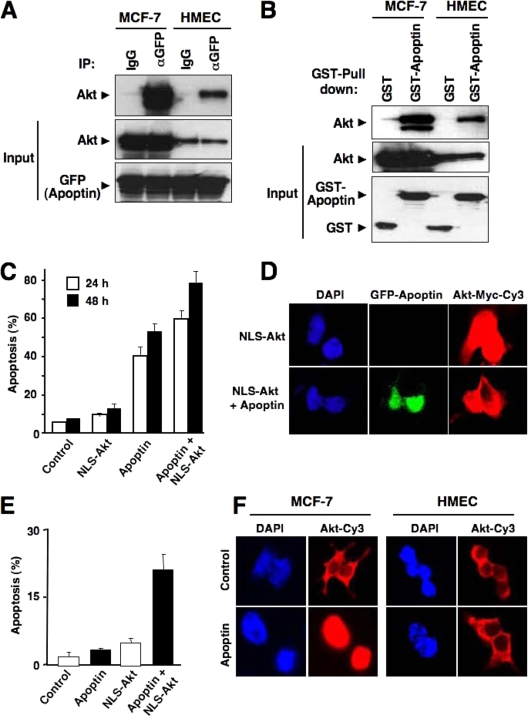
To investigate whether Akt and apoptin indeed physically interact with each other, we performed coimmunoprecipitation assays using cell lysates from apoptin-transfected cells as well as in vitro pull-down assays with recombinant apoptin. When apoptin was expressed as a GFP fusion protein, a GFP-specific antibody clearly precipitated endogenous Akt from transformed MCF-7 cells but also from primary HMECs (Fig. 2A). These results were further supported by in vitro pull-down assays revealing that recombinant GST-apoptin but not the GST control strongly bound Akt from MCF-7 cells and HMECs (Fig. 2B).
FIG. 2.
Apoptin interacts with Akt. (A) MCF-7 cells and HMECs were transfected with GFP-tagged apoptin. The interaction of endogenous Akt with apoptin was detected by immunoblotting with Akt antibody and immunoprecipitation with either control immunoglobulin G (IgG) or GFP antibody at 18 h posttransfection. (B) GST pull-down assay was performed using control GST or GST-apoptin immobilized on agarose beads and incubated with extracts prepared from either MCF-7 cells or HMECs. The interaction of Akt with apoptin was assessed by immunoblotting with anti-Akt antibody. (C and D) PC3 cells were infected with either adenoviral Myc-tagged NLS-Akt or a control virus in the presence or absence of GFP-apoptin. (C) Apoptotic cell death was measured by flow cytometric detection of hypodiploid DNA at 24 h or 48 h posttransfection. Data represent the means of four independent experiments. (D) The localization of NLS-Akt was detected after 18 h by anti-Myc-tag antibody followed by Cy3-marked secondary antibody. (E) Effect of NLS-Akt on apoptin-induced apoptosis in primary HMECs. Cells were transduced with an NLS-Akt or control virus as described for panel C. Cells were then treated with cell-permeative Tat-apoptin (1 μM) or left untreated. The percentage of apoptosis was determined after 30 h of treatment. (F) Apoptin triggers nuclear translocation of endogenous Akt in tumor cells but not in nontransformed cells. MCF-7 cells and HMECs were treated with Tat-apoptin for 18 h or left untreated. The differential localization of Akt was detected by immunostaining with anti-Akt and Cy3-conjugated secondary antibody. Nuclei were stained with DAPI.
To determine whether nuclear Akt alone is sufficient to induce cell death even in the absence of an apoptotic stimulus, we infected PC3 cells in the absence and presence of apoptin with an NLS-Akt adenoviral vector to enforce nuclear localization of Akt (Fig. 2C and D). Nuclear Akt alone was marginally toxic by itself compared to the control adenoviral vector. Coexpression of NLS-Akt together with apoptin, however, significantly sensitized PC3 cells to apoptin-induced cell death (Fig. 2C). Also, HMECs became susceptible toward apoptin-induced cell death when Akt was targeted to the nucleus, although cell death levels remained lower for the nontransformed cells than for tumor cells (Fig. 2E). Furthermore, when MCF-7 cells were treated with Tat-apoptin, which can enter cells due to an attached human immunodeficiency virus Tat protein transduction domain (24), endogenous Akt revealed a nuclear translocation (Fig. 2F). Interestingly, under the same conditions, endogenous Akt remained exclusively cytoplasmic in HMECs following apoptin stimulation.
CDK2-cyclin A activity is elevated during apoptin-induced cell death.
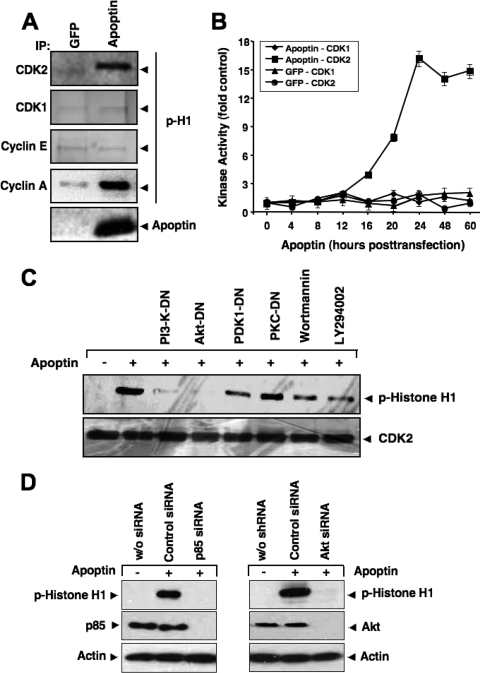
While searching for potential nuclear targets of Akt during apoptin's proapoptotic signaling, we measured the activation of two major cyclin-dependent kinases, CDK1 and CDK2, using an in vitro kinase assay with histone H1 as the substrate. PC3 cells were transfected to express either GFP or GFP-apoptin, and CDK activity in immunoprecipitates was measured at 24 h posttransfection, using CDK1- or CDK2-specific antibodies. CDK2 but not CDK1 activity was elevated in the presence of apoptin, as indicated by histone H1 phosphorylation (Fig. 3A). By coimmunoprecipitation, we next determined whether active CDK2 was associated with cyclin E or cyclin A. As shown in Fig. 3A, cyclin A- but not cyclin E-associated CDK2 showed enhanced phosphorylation of histone H1, suggesting that only cyclin A/CDK2 plays a role in apoptin-induced cell death. Control experiments utilizing GFP revealed that induction of CDK2 activity was specific for apoptin. CDK2 activity was already detectable at 16 h and peaked 24 h after transfection with apoptin (Fig. 3B).
FIG. 3.
CDK2 is activated during apoptin-induced cell death. (A) CDK2, CDK1, cyclin E, and cyclin A were immunoprecipitated from MCF-7 cells transfected with either GFP or GFP-apoptin at 24 h posttransfection and used in an in vitro kinase assay with histone H1 as the substrate. The level of histone H1 phosphorylation was detected by immunoblotting with a phospho-specific antibody against histone H1. (B) The kinase activities of CDK1 and CDK2 at different times after transfection with either GFP or apoptin were measured and plotted. Immunoblot signals were quantified against the respective controls, using a Storm scanner and accompanying software. (C) PC3 cells were transfected with apoptin alone or together with different dominant-negative mutants of PI3-K, Akt, PDK1, or PKCɛ. Kinase activity of immunoprecipitated CDK2 was measured using histone H1 as the substrate 24 h after transfection. Total CDK2 levels were determined by Western blotting. The PI3-K inhibitors wortmannin (5 nM) and LY294002 (1.5 μM) were applied 1 h before cell lysis. (D) Knockdown of PI3-K and Akt abolishes apoptin-induced CDK2 activation. PC3 cells were transfected with either control siRNA, a PI3-K p85-specific siRNA (left), or an Akt-specific siRNA (right). RNA interference resulted in an almost complete knockdown of p85 and Akt expression. At 72 h posttransfection, cells were treated with Tat-apoptin or left untreated. The kinase activity of immunoprecipitated CDK2 was determined by using histone H1 as the substrate 24 h after apoptin treatment. The level of histone H1 phosphorylation was detected by immunoblotting with a phospho-specific antibody against histone H1. Actin served as a loading control.
We next tested whether the increased CDK2 activity during apoptin-induced apoptosis was dependent on upstream PI3-K/Akt activation. Figure 3C shows that CDK2 was activated only when the PI3-K/Akt pathway remained intact. Prevention of Akt activation by wortmannin and LY294002, two pharmacological PI3-K inhibitors, strongly reduced CDK2 activation. In addition, expression of dominant-negative and kinase-dead mutants of upstream PI3-K, PDK1, or Akt almost completely abolished apoptin-induced CDK2 activity, whereas expression of an unrelated dominant-negative PKCɛ mutant had no effect (Fig. 3C). Similar results were obtained by RNA interference-mediated knockdown of PI3-K p85 as well as of Akt, which completely abolished apoptin-induced histone H1 phosphorylation (Fig. 3D). These results therefore suggest that apoptin-induced CDK2 activation requires an intact PI3-K/Akt pathway.
CDK2-cyclin A activity is required for apoptin-triggered cell death.
To test whether CDK2 is required for apoptin-induced cell death, we first inhibited CDK2 activity by using the CDK-specific inhibitor roscovitine. Figure 4A shows that in the presence of the CDK2 inhibitor, PC3 cells were strongly resistant to apoptin-induced cell death. The background levels of cell death seen with the combination of CDK2 inhibitor and apoptin expression might be attributed to a slight toxicity of roscovitine itself. We next inhibited the expression of CDK2 by transfection with a plasmid encoding a CDK2-specific siRNA and tested the effect on apoptin-induced cell death. Western blot analysis confirmed that the CDK2-specific but not control siRNA largely reduced CDK2 expression and, moreover, significantly prevented apoptosis induction by apoptin (Fig. 4B and C). More importantly, these results were substantiated in another experimental system employing cell-permeative Tat-apoptin. Whereas Tat-apoptin induced potent apoptosis in immortalized CDK2-proficient MEFs, immortalized cells from CDK2-deficient mice were largely apoptin resistant (Fig. 4D). Thus, these results suggest that CDK2 is required for apoptin-induced cell death.
FIG. 4.
CDK2-cyclin A activity is required for apoptin-triggered cell death. (A) The effect of CDK2 inhibitor on apoptin's toxicity was measured by transfecting PC3 cells with GFP-apoptin in the presence or absence of the CDK2 inhibitor roscovitine (CDK2-I). Apoptosis was measured by flow cytometric detection of hypodiploid DNA after 24 and 48 h. (B) PC3 cells were transfected with plasmids for either CDK2 siRNA or control siRNA. After 48 h, CDK2 expression was detected by immunoblotting. (C) PC3 cells were transfected with GFP or GFP-apoptin and a CDK2-specific or control siRNA plasmid. Apoptosis was quantified after 24 and 48 h by measurement of the formation of hypodiploid DNA. (D) Immortalized CDK2-deficient murine fibroblasts and the respective WT control cells were treated with cell-permeative Tat-apoptin (1 μM) for either 24 or 48 h, and apoptosis was then evaluated by flow cytometry. Data represent the averages of four independent experiments. In both cell populations, cell death in the absence of apoptin did not exceed 4%. (E) CDK2 kinase activity was measured 24 h after transfection of PC3 cells with apoptin alone, with cotreatment with the CDK2 inhibitor roscovitine (CDK2-I) or the caspase inhibitor Z-VAD-FMK, or with overexpression of Bcl-2. (F) Levels of cytosolic cytochrome c were detected by cellular fractionation and immunoblotting with PC3 cells transfected with apoptin alone, with cotreatment with CDK2 inhibitor, or with Bcl-2 overexpression. Actin served as a loading control.
We and others have previously shown that apoptin's death signaling converges at the mitochondrial death pathway (14, 41). To further investigate whether CDK2 activation occurs upstream or downstream of caspases, we measured CDK2 activity in cells incubated with the broad-spectrum caspase inhibitor Z-VAD-FMK. CDK2 activation was completely blocked by roscovitine but not by the caspase inhibitor (Fig. 4E). Moreover, expression of Bcl-2, although preventing cytochrome c release from mitochondria, did not affect CDK2 activation (Fig. 4E and F). These results therefore indicate that CDK2 activation occurs upstream of the mitochondrial death pathway.
Cyclin A-associated CDK2 is the apoptin kinase that regulates its nuclear localization in cancer cells.
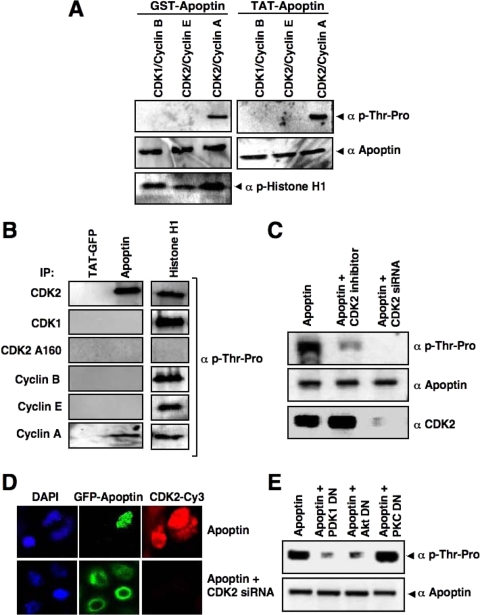
Apoptin phosphorylation at Thr-108 has previously been reported to be critical for its apoptotic activity and tumor cell-specific nuclear localization (52, 55). The apoptin Thr-108 residue lies within a consensus phosphorylation site for proline-dependent kinases such as CDKs. To test whether CDK2 may directly phosphorylate apoptin, we performed in vitro kinase assays, using recombinant GST-apoptin and Tat-apoptin as substrates. As shown in Fig. 5A, immunoblotting with a phospho-threonine-proline-specific antibody revealed that apoptin can be phosphorylated by active recombinant CDK2-cyclin A. Interestingly, neither active recombinant CDK2-cyclin E nor CDK1-cyclin B was able to phosphorylate apoptin in vitro, although both kinase complexes efficiently phosphorylated histone H1. We further confirmed CDK2-mediated phosphorylation of apoptin by using immunoprecipitated CDK2, CDK1, cyclin A, cyclin E, and cyclin B, with inactive CDK2 (CDK2 T160A) as a negative control. Apoptin was phosphorylated only by the immune complex of active CDK2 and cyclin A, not by other combinations of CDKs and cyclins (Fig. 5B). These data correlate with the activation of cyclin A-associated CDK2, and not other CDKs, in the presence of apoptin.
FIG. 5.
CDK2 is the specific apoptin kinase. (A) A nonradioactive in vitro kinase assay was performed with recombinant GST-apoptin and Tat-apoptin as substrates, using active CDK1-cyclin B, CDK2-cyclin E, or CDK2-cyclin A. Apoptin phosphorylation was detected by immunoblotting using an antibody against phosphorylated threonine-proline residues. Total apoptin levels were detected by apoptin antibody. Histone H1 was used as a positive control. (B) Active CDK2, CDK1, cyclin B, cyclin E, and cyclin A were immunoprecipitated from PC3 cells with their respective antibodies and the CDK2 T160A mutant was immunoprecipitated with anti-hemagglutinin antibody, and the immunoprecipitates were used in a kinase assay with Tat-GFP, Tat-apoptin, or histone H1 as the substrate. Phosphorylation was detected as described in the legend to Fig. 4A. (C) PC3 cells were transfected with GFP-apoptin alone or in the presence of the CDK2 inhibitor or the CDK2-targeting siRNA. GFP-apoptin was immunoprecipitated at 24 h posttransfection with anti-GFP antibodies. The phosphorylation of apoptin in the immunoprecipitates was detected by immunoblotting using anti-phospho-Thr-Pro antibodies. Total apoptin and CDK2 levels are indicated. (D) The effect of CDK2 inhibition on apoptin's localization was demonstrated in PC3 cells transfected with GFP-apoptin in the absence or presence of a CDK2-targeting siRNA plasmid. After 24 h, cells were analyzed by confocal laser scanning microscopy. (E) PI3-K/Akt signaling is required for apoptin phosphorylation. PC3 cells were transfected with GFP-apoptin in the presence or absence of a dominant-negative mutant of PDK1, Akt, or PKCɛ. The levels of apoptin phosphorylation were determined at 24 h posttransfection as described for panel C. Total apoptin levels were detected with anti-apoptin antibodies.
We next investigated whether activated CDK2 phosphorylates apoptin in vivo by two different approaches. First, we inhibited CDK2 activity in PC3 cells by using roscovitine and transfected the cells to express apoptin. Twenty-four hours later, apoptin was immunoprecipitated and its phosphorylation status was assessed using the phospho-Thr-Pro-specific antibody. Figure 5C shows that apoptin was phosphorylated in the presence of active CDK2, whereas the level of phosphorylation was significantly reduced in the presence of the CDK2 inhibitor. Second, we tested the effect on apoptin phosphorylation by inhibiting the expression of CDK2, using a specific siRNA. Figure 5C shows that apoptin phosphorylation was almost completely abrogated in cells with downregulated CDK2 expression, implying that CDK2 is the apoptin kinase.
We further investigated the role of CDK2 in controlling apoptin's nuclear localization by inhibiting CDK2 expression in PC3 cells with siRNAs. In the presence of CDK2, apoptin was localized exclusively in the nucleus, but interfering with CDK2 expression by using siRNA severely impaired apoptin's nuclear accumulation (Fig. 5D). Similar data were obtained with MCF-7 cells (not shown). Furthermore, upstream activation of PI3-K/Akt was necessary for CDK2-mediated phosphorylation of apoptin, as the phosphorylation of apoptin by CDK2 was strongly reduced in the presence of dominant-negative mutants of PDK1 and Akt but not upon coexpression of an unrelated PKCɛ dominant-negative mutant (Fig. 5E). Our results therefore strongly suggest that CDK2 is the kinase controlling phosphorylation and nuclear accumulation of apoptin in cancer cells.
p27Kip1 is phosphorylated during apoptin-induced cell death.
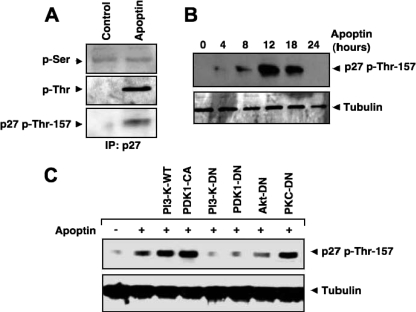
We have recently shown that Akt phosphorylates CDK2 at Thr-39 (39). Since Akt localizes mostly to the nucleus during apoptin-induced cell death, we also investigated the role of other possible nuclear targets for Akt. p27Kip1, a negative regulator of the cell cycle at the G1/S phase, has an Akt consensus motif for phosphorylation. Because p27Kip1 is a potent inhibitor of CDK2, we tested whether p27Kip1 is a nuclear target of Akt during apoptin-induced cell death. p27Kip1 was immunoprecipitated from lysates of cells expressing apoptin or the GFP control. p27Kip1 phosphorylation was then monitored using phospho-serine- or phospho-threonine-specific antibodies. p27Kip1 threonine phosphorylation increased in the presence of apoptin, but apoptin had no effect on serine phosphorylation (Fig. 6A). We confirmed the phosphorylation of p27Kip1 by using an antibody against phosphorylated Thr-157, which represents a potential Akt phosphorylation site. The kinetics revealed that phosphorylation of p27Kip1 at Thr-157 occurred soon after Akt nuclear translocation. A significant increase in the Thr-157 phosphorylation of p27Kip1 was seen 12 h after transfection and then declined after 24 to 30 h (Fig. 6B). Phosphorylation of p27Kip1 at Thr-157 was strongly dependent on Akt activation and its upstream regulators PI3-K and PDK1. Cotransfection of cells with apoptin and a dominant-negative mutant of PI3-K, PDK1, or Akt markedly decreased Thr-157 phosphorylation of p27Kip1. In contrast, phosphorylation of Thr-157 remained unaffected by dominant-negative PKCɛ (Fig. 6C).
FIG. 6.
p27Kip1 is phosphorylated during apoptin-induced cell death. (A) PC3 cells were transfected with the GFP control or GFP-apoptin. After 12 h, the phosphorylation of p27Kip1 was detected by phospho-Ser- or phospho-Thr-specific antibodies after immunoprecipitation with anti-p27Kip1 antibody. Threonine phosphorylation was also detected with an antibody against Thr-157-phosphorylated p27Kip1. (B) p27Kip1 phosphorylation after apoptin transfection at different time points was detected by immunoblotting with phospho-Thr-157-specific p27Kip1 antibody. Tubulin was used as a loading control. (C) Cells were transfected with apoptin alone or cotransfected with either WT PI3-K, constitutively active PDK1 (CA), PI3-K dominant-negative (DN) vector, PDK1-DN, Akt-DN, or PKCɛ-DN vector. After 16 h, cells were lysed and phosphorylated p27Kip1 was detected by Western blotting.
Akt-mediated phosphorylation enhances degradation of p27Kip1 via the proteasomal pathway.
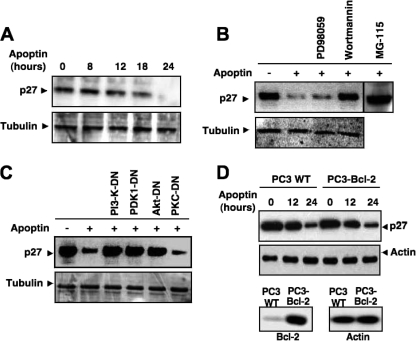
Previous studies have shown that p27Kip1 phosphorylation targets it for proteasomal degradation (62). To determine the functional significance of p27Kip1 phosphorylation by Akt, we checked the protein levels of p27Kip1 before and after apoptin transfection. Strikingly, we found a strong decrease in the level of p27Kip1 in apoptin-expressing cells (Fig. 7A and B). The kinetics of p27Kip1 downregulation revealed that at about 24 h posttransfection, p27Kip1 was completely absent in PC3 cells. The decrease in the level of p27Kip1 protein was entirely dependent on p27Kip1 phosphorylation by Akt, as the protein level was restored to the control level in apoptin-expressing cells that had been pretreated with the PI3-K inhibitor wortmannin but not with the MEK inhibitor PD98059 (Fig. 7B). Inhibition of p27Kip1 degradation was also seen upon coexpression of PI3-K-DN, PDK1-DN, or Akt-DN but not of PKC-DN (Fig. 7C). In contrast, overexpression of Bcl-2 failed to prevent p27Kip1 degradation, suggesting that it was not dependent on the mitochondrial caspase cascade (Fig. 7D). Rather, the decrease in the protein level of p27Kip1 was due to proteasomal degradation, as treatment with the proteasome inhibitor MG115 restored the p27Kip1 level even in the presence of apoptin (Fig. 7B).
FIG. 7.
Akt-mediated phosphorylation enhances degradation of p27Kip1 via the proteasomal pathway. (A) Levels of p27Kip1 in GFP-apoptin-transfected PC3 cells were monitored for 24 h posttransfection by Western blotting. Tubulin was used as a loading control. (B) PC3 cells, transfected with apoptin or left untransfected, were incubated with the MEK inhibitor PD98059, the PI3-K inhibitor wortmannin, or the proteasomal inhibitor MG115. Protein levels of p27Kip1 were detected by immunoblotting. (C) Roles of PI3-K and Akt in p27Kip1 stability. Protein levels of p27Kip1 were detected by immunoblotting of lysates of PC3 cells 24 h after transfection with apoptin and the indicated dominant-negative mutant of PI3-K, Akt, PDK1, or PKCɛ. (D) Bcl-2 does not affect degradation of p27Kip1. WT and Bcl-2-overexpressing PC3 cells were treated with Tat-apoptin for the indicated times, and the protein level of p27Kip1 was evaluated by immunoblotting. Actin was used as a loading control. The status of Bcl-2 expression and expression of an actin control in the two cell lines are shown in the lower panels.
We next wished to investigate the functional role of p27Kip1 in apoptin-induced cell death and therefore blocked its expression with a p27Kip1-specific siRNA. Transfection of apoptin into p27Kip1 siRNA-expressing cells revealed that p27Kip1 downregulation sensitized cells to apoptin-induced cell death (Fig. 8A). The proteasomal degradation of phosphorylated p27 is controlled by the F-box protein Skp2, an E3 ubiquitin ligase, and the accessory protein Cks1, which was initially identified as a binding protein of Cdc2 (9, 20). Consequently, the targeted deletion of Cks1 (58) or Skp2 (46) leads to marked increases of the p27Kip1 level. To further assess the role of p27Kip1 in apoptin-induced cell death, we therefore downregulated both components of the p27Kip1 degradation machinery. Using two different siRNAs, Skp2 as well as Cks1 expression was efficiently suppressed in PC3 cells, correlating with a marked increase in p27Kip1 level (Fig. 8B). Importantly, the knockdown of either Skp2 or Cks1 resulted in a significantly reduced sensitivity to apoptin-induced apoptosis (Fig. 8C). Moreover, similar to p27Kip1-deficient MEFs, Skp2-deficient MEFs were protected against apoptin-induced cell death compared to WT cells (Fig. 8D). These results therefore strongly suggest that p27Kip1 is functionally involved in cell death triggered by apoptin.
FIG. 8.
p27Kip1 is involved in apoptin-induced apoptosis. (A) Downregulation of p27Kip1 protects against apoptin-induced apoptosis. PC3 cells were transfected with a GFP control or GFP-apoptin in the presence and absence of a p27Kip1-specific siRNA. (Left) Apoptosis was measured by flow cytometric detection of hypodiploid DNA at the indicated time points. The data represent the means from three independent experiments. (Right) The efficiency of p27Kip1 downregulation by the specific or scrambled control siRNA was investigated at 36 h posttransfection by immunoblotting with a p27Kip1-specific antibody. Tubulin served as a loading control. (B to D) Apoptin-induced apoptosis is inhibited in cells with suppressed expression of Skp2 and Cks1, two components of the p27 degradation machinery. (B) PC3 cells were transfected with either a control siRNA, two different Skp2 siRNAs (left panels), or two different Cks1 siRNAs (right panels). The protein levels of Skp2, Cks1, p27, and actin were analyzed by immunoblotting at 72 h post-siRNA transfection. (C) PC3 cells transfected with either control siRNA, Skp2 siRNA no. 2, or Cks1 siRNA no. 2 were treated with Tat-apoptin (1 μM) at 36 h posttransfection, and the percentage of apoptosis was measured after 24 h of further incubation. (D) Immortalized Skp2-deficient murine fibroblasts and the respective WT control cells were treated with Tat-apoptin, and the percentage of apoptosis was evaluated after 24 and 48 h of treatment. Data represent the averages of three independent experiments.
DISCUSSION
Apoptin has been reported to induce the selective death of tumor cells derived from diverse tumors, whereas it does not induce apoptosis in nontransformed cells (3, 48, 59). The manner by which apoptin distinguishes between tumor and normal cells remains largely unclear. Apoptin's nuclear localization and phosphorylation by a so far unknown kinase have been proposed to be crucial for the tumor-selective activity of apoptin (55). In an attempt to elucidate the mechanism of the tumoricidal activity of apoptin, we previously identified components of the PI3-K/Akt pathway as interaction partners of apoptin (39, 42). In this study, we provide for the first time a molecular mechanism for the unexpected proapoptotic role of the PI3-K/Akt pathway by demonstrating that CDK2 is activated downstream of Akt and subsequently mediates the phosphorylation and nuclear retention of apoptin.
We observed that active Akt, if translocated to the nucleus, stimulates rather than represses apoptosis induced by apoptin and certain anticancer agents (40, 42). In the presence of apoptin, Akt may target alternate substrates or pathways that may lead to the aberrant activation of CDK2, disturbance of cell cycle progression, and cell death. This suggests that the outcome of CDK2 activation may vary depending on the type of stimulus and the temporospatial signaling features. Interestingly, a recent report suggested that the transient activation of Akt supports cell survival, whereas its sustained activation leads to apoptosis (63). Several other molecules have been assigned a dual role in both cell survival and cell death. For example, NF-κB, the nuclear orphan receptor Nur77, mitogen-activated protein kinases, and even caspases have been implicated in both processes (4, 35, 36, 38, 65). Furthermore, there is growing evidence that certain cyclin/CDK complexes might control not only cell proliferation but also cell death (22).
The interaction of Akt with apoptin enhances the nuclear localization of Akt. Recent studies indicated that under certain conditions active Akt could be translocated to the nucleus with the help of cytosolic proteins, as Akt lacks an inherent NLS motif (31, 49). We observed that Akt translocation was a rather early event that clearly preceded the onset of apoptin-induced cell death. Moreover, the interaction of Akt with apoptin was crucial for its nuclear translocation, as shown by the following two complementary lines of evidence: (i) Akt was translocated to the nucleus only in the presence of those apoptin mutants that had the ability to interact with PI3-K, and (ii) Akt showed nuclear colocalization only with apoptin mutants that themselves accumulated in the nucleus. Furthermore, we showed that the enforced nuclear translocation of Akt by transfecting cells with an NLS-fused Akt form induced no significant apoptosis on its own but enhanced apoptin-triggered death.
Our results certainly do not exclude the possibility that nuclear Akt may have a prosurvival function under some conditions. For example, it was reported that the tumor suppressor PML prevents cell growth by dephosphorylating and inactivating Akt inside the nucleus (61). Other researchers reported that nuclear but not cytoplasmic Akt interacts with Ebp1, an inhibitor of apoptotic DNA fragmentation, and enhances its antiapoptotic action (1). There are several reported nuclear targets of Akt, including FOXO3a, Nur77, and p21Cip1/Waf1. We previously found that Akt can phosphorylate CDK2 itself at Thr-39. In this study, we report that the CDK inhibitor p27Kip1 is an additional nuclear target involved in apoptin-induced cell death. p27Kip1 was strongly downregulated during apoptin-induced cell death, an event that required Akt activation. Our results show that Akt phosphorylates p27Kip1 at Thr-157 and targets it for proteasomal degradation. Previously, it was shown that p27Kip1 phosphorylation at Thr-187 by CDKs triggers its ubiquitination by the SCF/Skp2 ubiquitin ligase complex and promotes p27Kip1 degradation (62). During apoptin-induced cell death, however, we found no increased levels of p27Kip1 phosphorylated at Thr-187. It was also reported that p27Kip1 phosphorylation at Ser-10, Thr-157, or Thr-198 by the PI3-K/Akt pathway affects its nuclear import and causes the cytoplasmic accumulation of p27Kip1 (34, 56). However, the nuclear localization of p27Kip1 was not altered during apoptin-induced cell death. Akt might further control expression of p27Kip1 at the mRNA level via the inactivation of the transcription factor FOXO3a (44). Though we observed the phosphorylation of FOXO3a by Akt during apoptin-induced cell death, we did not detect transcriptional changes of p27Kip1 (data not shown). Thus, during apoptin-induced apoptosis, Akt-mediated phosphorylation of p27Kip1 affects mainly the stability of p27Kip1 and thereby might relieve downstream effectors from p27Kip1-mediated inhibition.
One of the major findings of our study is the identification of CDK2 as the long-sought apoptin kinase. Phosphorylation at Thr-108 has previously been reported to be specific for tumor cells and required for apoptin's toxicity (55). Indeed, we demonstrated that CDK2 regulates apoptin's nuclear retention by direct phosphorylation. Interestingly, this phosphorylation was specifically mediated by cyclin A- but not cyclin E-associated CDK2. A functional role of CDK2 is further supported by our finding that CDK2 inactivation by pharmacological inhibitors, RNA interference, or targeted gene disruption severely impaired apoptin-induced cell death.
It was reported previously that phosphorylation of apoptin was strongly reduced by an apoptin mutant lacking residues 80 to 90, even if the Thr-108 phosphorylation site remained intact (55). Our results can explain this intriguing observation. Data presented in our previous work (42) and this study show that apoptin interacts with PI3-K via aa 80 to 90, which are also required for downstream CDK2 activation. Furthermore, we observed that the nuclear localization of Akt and apoptin occurs in parallel and requires CDK2-mediated Thr-108 phosphorylation, while the interaction of apoptin with Akt is transient. Based on these observations, we propose that Akt and apoptin interact and are transported to the nucleus, where Akt activates CDK2, which in turn phosphorylates apoptin (Fig. 9). This event finally results in nuclear sequestration of both proteins and, as a positive feedback loop, might thereby increase CDK2 activation. Apoptin has been reported to exert multiple effects in tumor cells, including nonspecific DNA binding, activation of p73, interaction with nuclear proteins such as DEDAF, PML, Nmi, and Hippi, ceramide production, and the activation of a Nur77/Bcl-2-controlled mitochondrial pathway (reviewed in references 3, 29, and 30). Triggering of these multiple events together might further contribute to the tumor-specific action of apoptin. It was also demonstrated that apoptin associates with subunit 1 of the anaphase-promoting complex/cyclosome (APC/C) and thereby inhibits APC/C function (60). Interestingly, among the substrates of the APC/C ubiquitin ligase are Skp2 and Cks1, which are required for the degradation of p27 (5, 66). Furthermore, not only cyclin B but also cyclin A is targeted for degradation by APC/C (67). Thus, although detailed spatiotemporal analyses are required to assess the outcome of such processes, all of these events might converge in increased CDK2 activation during apoptin-induced apoptosis.
FIG. 9.
Model for apoptin-activated signaling. Apoptin interacts with the SH3 domain of PI3-K, resulting in its constitutive activation, which leads to PDK1-dependent Akt activation and nuclear translocation of Akt. Nuclear Akt activates CDK2 by both direct and indirect mechanisms, including the proteasome-dependent degradation of p27Kip1. The activated CDK2 phosphorylates, among other substrates, apoptin at Thr-108 and thereby enforces its nuclear accumulation in cancer cells. The abnormal CDK2 activation, which might also involve apoptin-induced inhibition of APC/C, directly or indirectly influences the activity of regulators of the mitochondrial apoptotic pathway, including Nur77, Bcl-2, Bim, and others.
Whether Akt-mediated CDK2 activation is a unique feature of apoptin-induced apoptosis or a more general phenomenon is an intriguing question. In primary cells such as HMECs, stimulation of Akt by epidermal growth factor receptor signaling was unable to sensitize cells to apoptin-induced apoptosis. However, whereas in those nontransformed cells endogenous Akt remained cytoplasmic following apoptin exposure, expression of nucleus-targeted Akt could induce apoptosis, although to a weaker extent than that in transformed cells. We also observed with primary human B lymphocytes that a fraction of cells underwent apoptosis induced by apoptin when the cells were costimulated by IgM cross-linking, which is known to induce CDK2 activation but normally protects B cells against various apoptotic stimuli (data not shown). Thus, under certain conditions, a limited cytotoxicity of apoptin might be observed even in nontransformed cells.
The apoptosis-relevant targets of CDK2 are largely unknown. Though one report suggested that p53 is phosphorylated and activated by CDK2 during thymocyte apoptosis (26), apoptin-induced cell death is independent of p53. Nevertheless, CDK2 activation may not be restricted to apoptin-induced cell death. It was proposed, for instance, that starvation-induced apoptosis of endothelial cells is mediated through increased CDK2 activation (32). Very recently, deregulated CDK2 activation was also linked to increased Bim expression and apoptosis in response to actin damage (10). Furthermore, elevated activity of CDK2 has been found in certain forms of apoptosis, while overexpression of CDK2 accelerates thymocyte cell death (21). Interestingly, and consistent with our results, cyclin A expression can also induce apoptosis in fibroblasts upon serum withdrawal (27).
Various regulators of the PI3-K/Akt pathway are involved in tumorigenesis and are highly active in various types of cancers. PTEN, a phosphatase counteracting PI3-K action, is one of the most commonly mutated tumor suppressor genes (64). Both CDK2 and cyclin A are strongly overexpressed in several tumors (68). Hyperactivation of these pathways is associated with a poor clinical prognosis and contributes to drug resistance. Thus, targeting of these pathways by apoptin might be responsible for its tumor-specific effects. Our data strongly indicate that apoptin exploits survival pathways and redirects them from their survival function toward induction of cell death. Our results therefore establish a novel link between cell survival and cell death that may be important for the development of strategies to selectively kill tumor cells.
Acknowledgments
We thank M. B. Cohen, S. B. Gibson, A. Halayko, T. Hunter, D. Jans, P. Kaldis, E. Kardami, N. P. Malek, D. Morgan, M. A. Sussman, M. Tavassoli, and K. Walsh for sharing valuable reagents.
S.M. was funded by a studentship from MHRC and CCMF. S.W., U.F., and K.S.O. acknowledge support from the DFG (GK 1302, SFB 685, and SFB 773), the Deutsche Krebshilfe, the Comprehensive Cancer Center Tübingen, and the Wilhelm-Sander-Stiftung.
Footnotes
Published ahead of print on 22 December 2008.
REFERENCES
- 1.Ahn, J. Y., X. Liu, Z. Liu, L. Pereira, D. Cheng, J. Peng, P. A. Wade, A. W. Hamburger, and K. Ye. 2006. Nuclear Akt associates with PKC-phosphorylated Ebp1, preventing DNA fragmentation by inhibition of caspase-activated DNase. EMBO J. 252083-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aki, T., K. Yamaguchi, T. Fujimiya, and Y. Mizukami. 2003. Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene 228529-8535. [DOI] [PubMed] [Google Scholar]
- 3.Backendorf, C., A. E. Visser, A. G. de Boer, R. Zimmerman, M. Visser, P. Voskamp, Y. H. Zhang, and M. H. Noteborn. 2008. Apoptin: therapeutic potential of an early sensor of carcinogenic transformation. Annu. Rev. Pharmacol. Toxicol. 48143-169. [DOI] [PubMed] [Google Scholar]
- 4.Barkett, M., and T. D. Gilmore 1999. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 186910-6924. [DOI] [PubMed] [Google Scholar]
- 5.Bashir, T., N. V. Dorrello, V. Amador, D. Guardavaccaro, and M. Pagano. 2004. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428190-193. [DOI] [PubMed] [Google Scholar]
- 6.Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 131775-1785. [DOI] [PubMed] [Google Scholar]
- 7.Booy, E. P., D. Johar, S. Maddika, H. Pirzada, M. M. Sahib, I. Gehrke, S. D. Loewen, S. D. Louis, K. Kadkhoda, M. Mowat, and M. Los. 2006. Monoclonal and bispecific antibodies as novel therapeutics. Arch. Immunol. Ther. Exp. 541-17. [Google Scholar]
- 8.Burek, M., S. Maddika, C. J. Burek, P. T. Daniel, K. Schulze-Osthoff, and M. Los. 2006. Apoptin-induced cell death is modulated by Bcl-2 family members and is Apaf-1 dependent. Oncogene 252213-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1193-199. [DOI] [PubMed] [Google Scholar]
- 10.Chae, H. D., B. M. Kim, U. J. Yun, and D. Y. Shin. 2008. Deregulation of Cdk2 causes Bim-mediated apoptosis in p53-deficient tumors following actin damage. Oncogene 274115-4121. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, J. Q., C. W. Lindsley, G. Z. Cheng, H. Yang, and S. V. Nicosia. 2005. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene 247482-7492. [DOI] [PubMed] [Google Scholar]
- 12.Coffer, P. J., J. Jin, and J. R. Woodgett. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 3351-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danen-Van Oorschot, A. A., D. F. Fischer, J. M. Grimbergen, B. Klein, S. Zhuang, J. H. Falkenburg, C. Backendorf, P. H. Quax, A. J. Van der Eb, and M. H. Noteborn. 1997. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc. Natl. Acad. Sci. USA 945843-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danen-van Oorschot, A. A., A. J. van der Eb, and M. H. Noteborn. 2000. The chicken anemia virus-derived protein apoptin requires activation of caspases for induction of apoptosis in human tumor cells. J. Virol. 747072-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downward, J. 2004. PI 3-kinase, Akt and cell survival. Semin. Cell. Dev. Biol. 15177-182. [DOI] [PubMed] [Google Scholar]
- 16.Du, K., and P. N. Tsichlis. 2005. Regulation of the Akt kinase by interacting proteins. Oncogene 247401-7409. [DOI] [PubMed] [Google Scholar]
- 17.Freemantle, S. J., M. J. Spinella, and E. Dmitrovsky. 2003. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene 227305-7315. [DOI] [PubMed] [Google Scholar]
- 18.Fujio, Y., K. Guo, T. Mano, Y. Mitsuuchi, J. R. Testa, and K. Walsh. 1999. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol. Cell. Biol. 195073-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulda, S., and K. M. Debatin. 2004. Apoptosis signaling in tumor therapy. Ann. N. Y. Acad. Sci. 1028150-156. [DOI] [PubMed] [Google Scholar]
- 20.Ganoth, D., G. Bornstein, T. K. Ko, B. Larsen, M. Tyers, M. Pagano, and A. Hershko. 2001. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nat. Cell Biol. 3321-324. [DOI] [PubMed] [Google Scholar]
- 21.Gil-Gomez, G., A. Berns, and H. J. Brady. 1998. A link between cell cycle and cell death: Bax and Bcl-2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J. 177209-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golsteyn, R. M. 2005. Cdk1 and Cdk2 complexes (cyclin dependent kinases) in apoptosis: a role beyond the cell cycle. Cancer Lett. 217129-138. [DOI] [PubMed] [Google Scholar]
- 23.Gu, Y., J. Rosenblatt, and D. O. Morgan. 1992. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 113995-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guelen, L., H. Paterson, J. Gaken, M. Meyers, F. Farzaneh, and M. Tavassoli. 2004. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene 231153-1165. [DOI] [PubMed] [Google Scholar]
- 25.Guseva, N. V., A. F. Taghiyev, O. W. Rokhlin, and M. B. Cohen. 2002. Contribution of death receptor and mitochondrial pathways to Fas-mediated apoptosis in the prostatic carcinoma cell line PC3. Prostate 51231-240. [DOI] [PubMed] [Google Scholar]
- 26.Hakem, A., T. Sasaki, I. Kozieradzki, and J. M. Penninger. 1999. The cyclin-dependent kinase Cdk2 regulates thymocyte apoptosis. J. Exp. Med. 189957-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoang, A. T., K. J. Cohen, J. F. Barrett, D. A. Bergstrom, and C. V. Dang. 1994. Participation of cyclin A in Myc-induced apoptosis. Proc. Natl. Acad. Sci. USA 916875-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jänicke, R. U., D. Sohn, F. Essmann, and K. Schulze-Osthoff. 2007. The multiple battles fought by anti-apoptotic p21. Cell Cycle 6407-413. [DOI] [PubMed] [Google Scholar]
- 29.Janssen, K., T. G. Hofmann, D. A. Jans, R. T. Hay, K. Schulze-Osthoff, and U. Fischer. 2007. Apoptin is modified by SUMO conjugation and targeted to promyelocytic leukemia protein nuclear bodies. Oncogene 261557-1566. [DOI] [PubMed] [Google Scholar]
- 30.Klanrit, P., M. B. Flinterman, E. W. Odell, G. Melino, R. Killick, J. S. Norris, and M. Tavassoli. 2008. Specific isoforms of p73 control the induction of cell death induced by the viral proteins, E1A or apoptin. Cell Cycle 7205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunstle, G., J. Laine, G. Pierron, S. Kagami Si, H. Nakajima, F. Hoh, C. Roumestand, M. H. Stern, and M. Noguchi. 2002. Identification of Akt association and oligomerization domains of the Akt kinase coactivator TCL1. Mol. Cell. Biol. 221513-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levkau, B., H. Koyama, E. W. Raines, B. E. Clurman, B. Herren, K. Orth, J. M. Roberts, and R. Ross. 1998. Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol. Cell 1553-563. [DOI] [PubMed] [Google Scholar]
- 33.Liang, J., and J. M. Slingerland. 2003. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2339-345. [PubMed] [Google Scholar]
- 34.Liang, J., J. Zubovitz, T. Petrocelli, R. Kotchetkov, M. K. Connor, K. Han, J. H. Lee, S. Ciarallo, C. Catzavelos, R. Beniston, E. Franssen, and J. M. Slingerland. 2002. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 81153-1160. [DOI] [PubMed] [Google Scholar]
- 35.Lin, B., S. K. Kolluri, F. Lin, W. Liu, Y. H. Han, X. Cao, M. I. Dawson, J. C. Reed, and X. K. Zhang. 2004. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116527-540. [DOI] [PubMed] [Google Scholar]
- 36.Los, M., C. Stroh, R. U. Janicke, I. H. Engels, and K. Schulze-Osthoff. 2001. Caspases: more than just killers? Trends Immunol. 2231-34. [DOI] [PubMed] [Google Scholar]
- 37.Lu, B., L. Wang, C. Stehlik, D. Medan, C. Huang, S. Hu, F. Chen, X. Shi, and Y. Rojanasakul. 2006. Phosphatidylinositol 3-kinase/Akt positively regulates Fas (CD95)-mediated apoptosis in epidermal Cl41 cells. J. Immunol. 1766785-6793. [DOI] [PubMed] [Google Scholar]
- 38.Luciano, F., M. Krajewska, P. Ortiz-Rubio, S. Krajewski, D. Zhai, B. Faustin, J. M. Bruey, B. Bailly-Maitre, A. Lichtenstein, S. K. Kolluri, A. C. Satterthwait, X. K. Zhang, and J. C. Reed. 2007. Nur77 converts phenotype of Bcl-B, an antiapoptotic protein expressed in plasma cells and myeloma. Blood 1093849-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maddika, S., S. R. Ande, E. Wiechec, L. L. Hansen, S. Wesselborg, and M. Los. 2008. Akt mediated phosphorylation of CDK2 regulates its dual role in cell cycle progression and apoptosis. J. Cell Sci. 121979-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maddika, S., G. H. Bay, T. J. Kroczak, S. R. Ande, S. Maddika, E. Wiechec, S. B. Gibson, and M. Los. 2007. Akt is transferred to the nucleus of cells treated with apoptin, and it participates in apoptin-induced cell death. Cell. Prolif. 40835-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maddika, S., E. P. Booy, D. Johar, S. B. Gibson, S. Ghavami, and M. Los. 2005. Cancer-specific toxicity of apoptin is independent of death receptors but involves the loss of mitochondrial membrane potential and the release of mitochondrial cell-death mediators by a Nur77-dependent pathway. J. Cell Sci. 1184485-4493. [DOI] [PubMed] [Google Scholar]
- 42.Maddika, S., E. Wiechec, S. R. Ande, I. K. Poon, U. Fischer, S. Wesselborg, D. A. Jans, K. Schulze-Osthoff, and M. Los. 2008. Interaction with PI3-kinase contributes to the cytotoxic activity of apoptin. Oncogene 273060-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manning, B. D., and L. C. Cantley. 2007. AKT/PKB signaling: navigating downstream. Cell 1291261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404782-787. [DOI] [PubMed] [Google Scholar]
- 45.Nahta, R., D. Yu, M. C. Hung, G. N. Hortobagyi, and F. J. Esteva. 2006. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat. Clin. Pract. Oncol. 3269-280. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama, K., H. Nagahama, Y. A. Minamishima, M. Matsumoto, I. Nakamichi, K. Kitagawa, M. Shirane, R. Tsunematsu, T. Tsukiyama, N. Ishida, M. Kitagawa, K. Nakayama, and S. Hatakeyama. 2000. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 192069-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nimbalkar, D., M. K. Henry, and F. W. Quelle. 2003. Cytokine activation of phosphoinositide 3-kinase sensitizes hematopoietic cells to cisplatin-induced death. Cancer Res. 631034-1039. [PubMed] [Google Scholar]
- 48.Oro, C., and D. A. Jans. 2004. The tumour specific pro-apoptotic factor apoptin (Vp3) from chicken anaemia virus. Curr. Drug Targets 5179-190. [DOI] [PubMed] [Google Scholar]
- 49.Pekarsky, Y., A. Koval, C. Hallas, R. Bichi, M. Tresini, S. Malstrom, G. Russo, P. Tsichlis, and C. M. Croce. 2000. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc. Natl. Acad. Sci. USA 973028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piccaluga, P. P., M. Rondoni, S. Paolini, G. Rosti, G. Martinelli, and M. Baccarani. 2007. Imatinib mesylate in the treatment of hematologic malignancies. Expert Opin. Biol. Ther. 71597-1611. [DOI] [PubMed] [Google Scholar]
- 51.Pietersen, A. M., M. M. van der Eb, H. J. Rademaker, D. J. van den Wollenberg, M. J. Rabelink, P. J. Kuppen, J. H. van Dierendonck, H. van Ormondt, D. Masman, C. J. van de Velde, A. J. van der Eb, R. C. Hoeben, and M. H. Noteborn. 1999. Specific tumor-cell killing with adenovirus vectors containing the apoptin gene. Gene Ther. 6882-892. [DOI] [PubMed] [Google Scholar]
- 52.Poon, I. K., C. Oro, M. M. Dias, J. Zhang, and D. A. Jans. 2005. Apoptin nuclear accumulation is modulated by a CRM1-recognized nuclear export signal that is active in normal but not in tumor cells. Cancer Res. 657059-7064. [DOI] [PubMed] [Google Scholar]
- 53.Poon, R. Y., J. Lew, and T. Hunter. 1997. Identification of functional domains in the neuronal Cdk5 activator protein. J. Biol. Chem. 2725703-5708. [DOI] [PubMed] [Google Scholar]
- 54.Riccardi, C., and I. Nicoletti. 2006. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 11458-1461. [DOI] [PubMed] [Google Scholar]
- 55.Rohn, J. L., Y. H. Zhang, R. I. Aalbers, N. Otto, J. Den Hertog, N. V. Henriquez, C. J. Van De Velde, P. J. Kuppen, D. Mumberg, P. Donner, and M. J. Noteborn. 2002. A tumor-specific kinase activity regulates the viral death protein apoptin. J. Biol. Chem. 27750820-50827. [DOI] [PubMed] [Google Scholar]
- 56.Sekimoto, T., M. Fukumoto, and Y. Yoneda. 2004. 14-3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27(Kip1). EMBO J. 231934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shack, S., X. T. Wang, G. C. Kokkonen, M. Gorospe, D. L. Longo, and N. J. Holbrook. 2003. Caveolin-induced activation of the phosphatidylinositol 3-kinase/Akt pathway increases arsenite cytotoxicity. Mol. Cell. Biol. 232407-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spruck, C., H. Strohmaier, M. Watson, A. P. Smith, A. Ryan, T. W. Krek, and S. I. Reed. 2001. A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol. Cell 7639-650. [DOI] [PubMed] [Google Scholar]
- 59.Tavassoli, M., L. Guelen, B. A. Luxon, and J. Gaken. 2005. Apoptin: specific killer of tumor cells? Apoptosis 10717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teodoro, J. G., D. W. Heilman, A. E. Parker, and M. R. Green. 2004. The viral protein apoptin associates with the anaphase-promoting complex to induce G2/M arrest and apoptosis in the absence of p53. Genes Dev. 181952-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trotman, L. C., A. Alimonti, P. P. Scaglioni, J. A. Koutcher, C. Cordon-Cardo, and P. P. Pandolfi. 2006. Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsvetkov, L. M., K. H. Yeh, S. J. Lee, H. Sun, and H. Zhang. 1999. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9661-664. [DOI] [PubMed] [Google Scholar]
- 63.Van Gorp, A. G., K. M. Pomeranz, K. U. Birkenkamp, R. C. Hui, E. W. Lam, and P. J. Coffer. 2006. Chronic protein kinase B (PKB/c-akt) activation leads to apoptosis induced by oxidative stress-mediated Foxo3a transcriptional up-regulation. Cancer Res. 6610760-10769. [DOI] [PubMed] [Google Scholar]
- 64.Vivanco, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2489-501. [DOI] [PubMed] [Google Scholar]
- 65.Wada, T., and J. M. Penninger. 2004. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 232838-2849. [DOI] [PubMed] [Google Scholar]
- 66.Wei, W., N. G. Ayad, Y. Wan, G. J. Zhang, M. W. Kirschner, and W. G. Kaelin, Jr. 2004. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428194-198. [DOI] [PubMed] [Google Scholar]
- 67.Wolthuis, R., L. Clay-Farrace, W. van Zon, M. Yekezare, L. Koop, J. Ogink, R. Medema, and J. Pines. 2008. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell 30290-302. [DOI] [PubMed] [Google Scholar]
- 68.Yam, C. H., T. K. Fung, and R. Y. Poon. 2002. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 591317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]