Abstract
Mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) is a nuclear, dual-specificity phosphatase that has been shown to dephosphorylate MAP kinases. We used a “substrate-trap” technique involving a mutation in MKP-1 of the catalytically critical cysteine to a serine residue (“CS” mutant) to capture novel MKP-1 substrates. We transfected the MKP-1 (CS) mutant and control (wild-type, WT) constructs into phorbol 12-myristate 13-acetate (PMA)-activated COS-1 cells. MKP-1-substrate complexes were immunoprecipitated, which yielded four bands of 17, 15, 14, and 10 kDa with the CS MKP-1 mutant but not the WT MKP-1. The bands were identified by mass spectrometry as histones H3, H2B, H2A, and H4, respectively. Histone H3 was phosphorylated, and purified MKP-1 dephosphorylated histone H3 (phospho-Ser-10) in vitro; whereas, histone H3 (phospho-Thr-3) was unaffected. We have previously shown that thrombin and vascular endothelial growth factor (VEGF) upregulated MKP-1 in human endothelial cells (EC). We now show that both thrombin and VEGF caused dephosphorylation of histone H3 (phospho-Ser-10) and histone H3 (phospho-Thr-3) in EC with kinetics consistent with MKP-1 induction. Furthermore, MKP-1-specific small interfering RNA (siRNA) prevented VEGF- and thrombin-induced H3 (phospho-Ser-10) dephosphorylation but had no effect on H3 (phospho-Thr-3 or Thr-11) dephosphorylation. In summary, histone H3 is a novel substrate of MKP-1, and VEGF- and thrombin-induced H3 (phospho-Ser-10) dephosphorylation requires MKP-1. We propose that MKP-1-mediated H3 (phospho-Ser-10) dephosphorylation is a key regulatory step in EC activation by VEGF and thrombin.
Keywords: dephosphorylation; endothelial cells; serine-10, mitogen-activated protein kinase phosphatase-1; thrombin; vascular endothelial growth factor
mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) is a dual-specificity phosphatase that dephosphorylates MAP kinases (MAPKs) (12, 51). MKP-1 similar to other dual-specificity phosphatases has a shallow catalytic active site and efficiently dephosphorylates serine, threonine, and tyrosine residues (4, 39, 43, 52). MAPKs are characterized as conserved serine-threonine kinases that are involved in proliferation, differentiation, and stress response. The extracellular signal-related kinase (ERK), Jun NH2-terminal kinase (JNK), and p38 MAPK family members have been shown to be important in endothelial cell (EC) gene activation (26). MKP-1 is localized in the nucleus and dephosphorylates all MAPK family members in a cell type- and agonist-dependent manner (39, 53). MAPK is involved in numerous signaling pathways that are important in inflammation. Mkp-1-null mice exhibit enhanced JNK, p38, and ERK activity and resistance to diet-induced obesity (58). Four independent research groups reported a role for MKP-1 as a negative regulator of Toll-like receptors (TLRs), which are important in innate immunity (8, 18, 45, 59). Lipopolysacchride-induced death was higher in Mkp-1-null mice compared with death in wild-type (WT) mice (8, 18, 45, 59).
We have previously shown using antisense oligonucleotides that MKP-1 is critical in the modulation of thrombin-induced EC gene activation (7). Furthermore, we used Mkp-1-null mice and small interfering RNA (siRNA) in human EC to demonstrate that MKP-1 plays an important role in ex vivo aortic angiogenesis and EC migration, respectively (27). We now report that histone H3 is a novel MKP-1 substrate. We searched for novel substrates using the “substrate-trap” technique that has been used to identify novel protein substrates of other phosphatases (48). MKP-1 is critical for the regulation of inflammatory genes (7, 55), as is chromatin, which contains an octamer composed of a dimer-doublet histone H2A/H2B and tetramer of H3 and H4 core histone proteins. Histone modifications, such as phosphorylation, acetylation, methylation, ubiquitination, ADP-ribosylation, and glycosylation at the amino terminus of histone tails are important in regulating chromatin structure and thereby gene expression (25). Histone phosphorylation is involved in the regulation of chromosome condensation during mitosis, gene transcription, replication, and recombination (2, 37). Here we have investigated the role MKP-1 plays in the regulation of histone phosphorylation.
Site-specific histone H3 phosphorylation is critical in the regulation of chromatin reorganization (46, 57). The phosphorylation of the Ser-10 residue in the NH2-terminal tail of histone H3 is important in chromosome condensation and cell-cycle progression (10, 19, 57). In addition, phosphorylation of histone H3 Ser-10 facilitates methylation-acetylation of lysine-9 and lysine-14 (25, 30, 31). Several kinases have been reported to phosphorylate H3 Ser-10, such as ribosomal S6 serine-threonine kinase 2 (RSK2), mitogen- and stress-response kinases (MSK1 and MSK2), and tumor necrosis factor-α (TNF-α)-induced inhibitor κB kinase-α (IKK-α) (11, 13, 21, 37, 49); however, little is known about the enzymes responsible for histone dephosphorylation.
MATERIALS AND METHODS
Reagents and cell lines.
Vascular endothelial growth factor (VEGF)-A165 was purchased from R&D Systems (Minneapolis, MN). Thrombin receptor activating peptide (TRAP) with the peptide sequence SFLLRN-NH2 was custom synthesized by Peptide International (Louisville, KY), and purified phosphorylated histones were purchased form Millipore-Upstate (Billerica, MA). Other reagents and suppliers included Myc antibody (Millipore-Upstate), histone H3 antibody (Cell Signaling, Danvers, MA), histone H3 (Ser-10) antibody (Cell Signaling), histone H3 (Thr-3) antibody (Millipore-Upstate), histone H3 (Thr-11) antibody (Millipore-Upstate), phospho-serine antibody (Biodesign), phospho-tyrosine antibody PY20 (Transduction Laboratories, Lexington, KY), phospho-threonine antibody (Santa Cruz Biotechnology, Santa Cruz, CA), MKP-1 antibody (M-18AC, Santa Cruz Biotechnology), human embryonic kidney (HEK) 293, HEK 293T, and COS-1 cells. All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture and transfection.
Human EC were isolated by trypsin digestion of umbilical veins as previously described (47). EC were maintained on fibronectin-coated plates in MCDB/F12 medium (Sigma) containing 15% fetal bovine serum, 90 μg/ml heparin, and 150 μg/ml EC growth supplement. The cells were used between passages two and five.
COS-1 cells were transfected with the MKP-1 (WT) and MKP-1 (CS) mutant using Targefect F-2 plus the peptide enhancer from Targeting Systems (Santee, CA), according to the manufacturer's protocol as described before (27). Experiments were performed 24–36 h posttransfection.
Western blot analysis.
Cell extract from 105 EC was electrophoresed on a 12% SDS-PAGE gel. Proteins were transferred from the gel to nitrocellulose using a Trans-blot semidry transfer cell (Bio-Rad, Hercules, CA). The blot was blocked by TBST (150 mM NaCl, 50 mM Tris·HCl, pH 7.5, 0.1% Triton X-100) with 5% bovine serum albumin. Blots were incubated with target antibodies in TBST with 5% bovine serum albumin overnight at 4°C, washed three times with TBST, and incubated for 1 h with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody at room temperature. Protein signals were detected using an ECL Western blotting exposure kit, according to the manufacturer's instructions (Amersham Biosciences).
Construction and validation of MKP-1 siRNA.
siRNA was constructed that targeted the following mRNA sequences: MKP-1 600, AAG AGA CGT TGA TCA AGG CAG and MKP-1 838, AAG CTG GAC GAG GCC TTT GAG. MKP-2 siRNA were purchased from Ambion (s4372 ID:4392420, Austin, TX). The siRNAs were transfected as previously described (27). Real-time PCR was performed as previously described (27) using specific primers from SuperArray Bioscience (Frederick, MD).
Substrate trapping of MKP-1.
The substrate-trapping technique was performed as described by others (48), with minor modifications. To identify potential MKP-1 substrates, we mutated the catalytically critical cysteine residue of the phosphatase to serine, using site-directed mutagenesis. We transfected WT MKP-1 (provided by Dr. Jack Dixon) and CS mutant (constructed using site-directed mutagenesis kit) into 107 COS-1 cells. After 24 h, cells were stimulated with 80 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) for 10 min. The cell pellet was lysed with 2 ml of 1× RIPA buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 1 mM Na3VO4, 1 mM NaF, 0.1% SDS, 1× Complete protease inhibitor cocktail) from Roche (Indianapolis, IN) on ice and centrifuged to remove debris. The enzyme-substrate complex was immunoprecipitated from the total cell extract using an anti-MKP-1 antibody (M18AC, Santa Cruz Biotechnology) conjugated to agarose beads. The protein complex beads were pelleted and washed three times for 5 min with RIPA lysis buffer. Bound proteins were subjected to 1× SDS lamelli sample buffer, and proteins were separated by SDS-PAGE. The gel was stained with Coomassie blue (Bio-Rad), which is compatible with subsequent mass spectrometric peptide identification.
Mass spectrometry.
Protein bands were excised from the Coomassie blue-stained gel, destained, and dehydrated in acetonitrile. Gel pieces were digested with trypsin (20 μg; Promega, Madison, WI) overnight at room temperature. The eluted tryptic peptides were concentrated to 5 μl using a SpeedVac and reconstituted to 30 μl in 1% acetic acid. The tryptic peptides were analyzed by liquid chromatography/mass spectrometry using a Finnigan LTQ-Deca ion trap mass spectrometry system incorporating a 10 cm × 75 μM inner diameter Phenomenex Jupiter C18 reversed-phase capillary chromatography column (Phenomenex, Torrance, CA). Protein identification was performed by a database search using the Mascot search engine from Matrix Sciences (London, UK).
Cloning of human MKP-1 (WT) and (CS) in lentiviral vector.
MKP-1 (WT) and (CS) mutant cDNA were used as templates in polymerase chain reaction (PCR) amplification with primer sequences obtained from Integrated DNA Technologies (Coralville, IA): MKP-1 BamHI: sense, 5′-AATTTGGATCCGAGACCCAAGCTTGGTAC-3′; MKP-1 XhoI antisense, 5′-AATTTCTCGAGCTCTAGATCACAAGTCTTCTTCAG-3′. MKP-1 (WT) and (CS) amplification products were digested with BamHI-XhoI, and the product was cloned into the lentivirus cloning vector pLENTI-(XB/SC)6.9 (provided by Dr. Andrei Gudkov) using the Rapid Ligation Kit, according to the manufacturer's protocol (Roche). Packaging plasmids CMVΔ8.2 (4 μg), VSV-G (2 μg), and lentiviral plasmids MKP-1 (WT) or (CS) (4 μg) were transfected into HEK 293T cells in 100-mm plates using lipofectamine 2000, according to the manufacturers instructions. Lentiviral particles were prepared as previously described (17).
Immunoprecipitation of MKP-1 substrates in thrombin- and VEGF-activated EC.
For coimmunoprecipitation of MKP-1 with histones, lentiviral MKP-1 (WT) and (CS) mutant constructs were infected into 108 human umbilical vein endothelial cells. EC were treated with VEGF (10 ng/ml) and TRAP (100 μM) for 15 min. EC were harvested and lysed 48 h postinfection with 1× RIPA lysis buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 1 mM Na3VO4, 1 mM NaF, 0.1% SDS, 1× Complete protease inhibitor mixture) from Roche. Cell lysates for MKP-1 and histones were precleared using agarose and control IgG pulldowns, respectively. Immunoprecipitation was performed using an anti-myc-antibody for myc-tagged MKP-1 and an anti-histone H3 antibody for histones.
In vitro dephosphorylation of histones.
To determine phosphatase activity in cell extracts, myc-tagged MKP-1 (WT) and (CS) were transfected in two 150-mm dishes of COS-1 cells. Cells were harvested and lysed 48 h after transfection using lysis buffer [50 mM Tris·HCl, pH 7.6, 150 mM NaCl, 1.5 mM MgCl2, 0.1 mM EGTA, 1% Triton X-100 (vol/vol)] containing a protease inhibitor cocktail. COS-1 cells were treated with 10 μM MAPK inhibitors: PD-98059 (ERK inhibitor), SP-600125 (JNK inhibitor), and SB-203580 (p38 inhibitor) from (Calbiochem, San Diego, CA). MAPK inhibitors were added to prevent activated MAPK from binding to the active site and coprecipitating with MKP-1. Lysates were clarified by centrifugation to remove debris. The lysate was precleared using anti-mouse IgG and protein A/G agarose. Immunoprecipitation was performed using agarose-conjugated anti-myc antibody. The c-Myc Tag IP/Co-IP Kit from (Pierce) was used to isolate the immunoglobulin-bound proteins, according to the manufacturer's instructions. For the determination of protein concentration, bound proteins were eluted from the column using a 0.1 M glycine-HCl buffer (pH 2.6) and immediately neutralized with 1 M Tris (pH 9.5). Total protein was analyzed by the Bradford method. Dephosphorylation reactions were performed using 1 μg equivalent of agarose-bound total protein incubated with 5 μg of phosphorylated acid-extracted histones (Millipore-Upstate) for 30 min at 37°C. JNK-1 (2 μg) (Millipore-Upstate) was added to the reaction to enhance the activity of MKP-1 (48). Samples were analyzed by SDS-polyacrylamide gel electrophoresis. Detection of specific phosphorylated histone bands was determined using a phospho-histone H3 (Ser 10) antibody by immunoblot.
RESULTS
Coimmunoprecipitation of histones with catalytically inactive MKP-1.
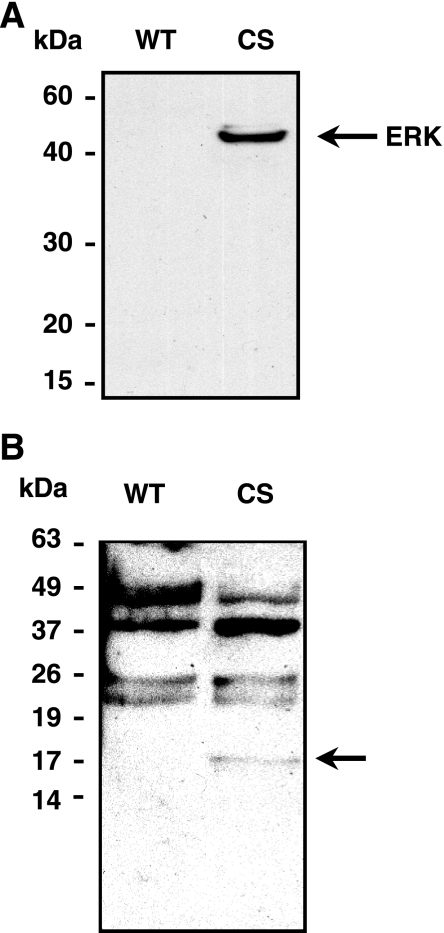
We used the substrate-trapping technique to identify novel phosphorylated substrates of MKP-1. The catalytically critical cysteine of MKP-1 was mutated to serine, denoted as (CS) mutant, allowing MKP-1 substrates to bind stably in the active site without dephosphorylation. We transfected the myc-tagged MKP-1 (CS) mutant or WT constructs into COS-1 cells and then stimulated with PMA to generate phosphorylated substrates. The enzyme-substrate complex was immunoprecipitated from the total cell extract using an anti-MKP-1 antibody. As shown in Fig. 1A, immuno-blot analysis showed the MKP-1 (CS) mutant, but not MKP-1 (WT), immunoprecipitated MAPK (ERK). We performed Western blot analysis using a combination of anti-phosphotyrosine, -threonine, and -serine antibodies. Immunoprecipitate from the MKP-1 (CS) mutant-transfected cell extract contained a unique ∼17-kDa protein band that was not present in the MKP-1 (WT) lane (Fig. 1B).
Fig. 1.
A: 17-kDa protein as a novel substrate of MAP kinase phosphatase-1 (MKP-1). Anti-MKP-1 antibody was used to immunoprecipitate the (cysteine to serine, CS) mutant-substrate complex from phorbol 12-myristate 13-acetate (PMA)-treated COS-1 cell extract. The (CS) mutant, but not (wild-type, WT) efficiently immunoprecipitated extracellular signal-related kinase (ERK), a known substrate of MKP-1. B: a specific 17-kDa band was detected in the CS lane when a combined anti-phospho-tyrosine, -threonine, and -serine antibody was used to immunoblot the immunoprecipitate.
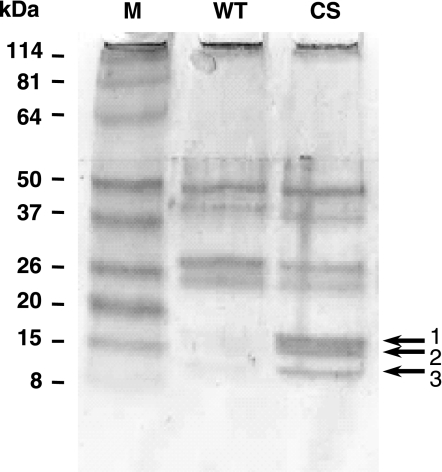
The immunoprecipitated protein complex from the COS-1 cells was subjected to SDS-PAGE analysis to separate the enzyme from the phosphorylated substrates. After Coomassie blue staining of the gel, we detected several specific low molecular mass bands of 17, 15, 14, and 10 kDa in the MKP-1 (CS) mutant-immunoprecipitate (IP) lane but not in the MKP-1 (WT)-IP lane (Fig. 2). The specific bands that were “trapped” using the MKP-1 (CS) mutant were excised from the Coomassie blue-stained gel, tryptic digestion was performed, and the peptide fragments were identified by mass spectrometry as histones H3, H2B, H2A, and H4.
Fig. 2.
Histones as potential substrates for MKP-1. Anti-MKP-1 antibody was used to immunoprecipitate (CS) mutant-substrate complex from PMA-treated COS-1 cell extract. Coomassie-stained SDS-PAGE analysis shows that the (CS) mutant, but not (WT) mutant, efficiently immuno-precipitated three specific bands labeled 1, 2, and 3.
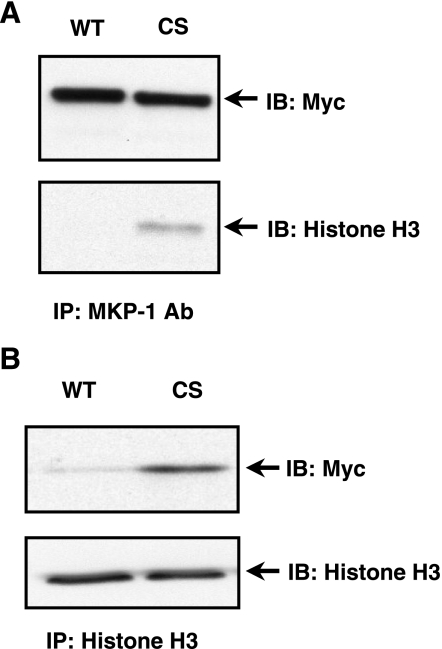
MKP-1 and histones are localized in the nucleus, and both are known to be involved in agonist-induced transcriptional gene regulation. We have previously shown that VEGF and thrombin transiently induce MKP-1 in cultured EC (27). The identification of the histone complex using the substrate trap and mass spectrometry experiments was confirmed in EC. Immuno-blot analysis using anti-histone H3 antibody showed that MKP-1 (CS) mutant pulled down histone H3 from VEGF-treated EC extract (Fig. 3A). In the reverse experiment, we immunoprecipitated histone H3 in VEGF-stimulated EC expressing either recombinant myc-tagged MKP-1 (WT) or (CS) mutant. As shown in Fig. 3B, histone H3 antibody immunoprecipitated MKP-1 (CS) mutant but not MKP-1 (WT). MKP-1 (WT) and MKP-1 (CS) were present at comparable protein levels (data not shown). These results indicate a potential role for MKP-1 in the modulation of histone phosphorylation.
Fig. 3.
Histone H3 is associated with MKP-1 in endothelial cells (EC). A: human EC were infected with myc-tagged, MKP-1 (WT) or (CS) expressing lentivirus and then stimulated with vascular endothelial growth factor (VEGF). The protein complex was precipitated with an agarose-conjugated anti-MKP-1 antibody and immunoblotted with an anti-histone H3 antibody. Myc-tagged MKP-1 protein was detected using a myc-specific antibody. B: in a reverse experiment, myc-tagged (CS) mutant, not (WT) was coprecipitated when histone H3 was immunoprecipitated using an anti-histone H3 antibody.
Dephosphorylation of histone H3 (Ser-10) by MKP-1 in vitro.
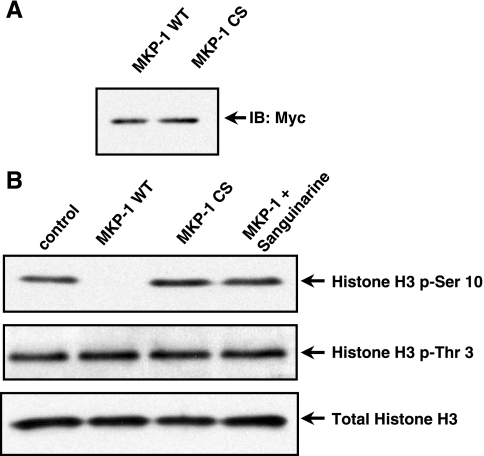
We confirmed a direct interaction between MKP-1 and histone substrates using an in vitro assay system. In this assay, we reconstituted the dephosphorylation reaction using immunoprecipitated recombinant MKP-1 (WT) or (CS) mutant and histone H3 isolated from EC. We transfected myc-tagged MKP-1 (WT) or MKP-1 (CS) mutant plasmid constructs into COS-1 cells and immunoprecipitated MKP-1 protein using sepharose-conjugated anti-myc antibody (Fig. 4A). The core histone substrate complex was prepared by acid extraction and acetone precipitation. Phosphorylation of histone H3 (Ser-10) and histone H3 (Thr-3) was detected by immunoblot in EC under basal conditions (Fig. 4B). Purified MKP-1 dephosphorylated histone H3 (Ser-10); however, histone H3 (Thr-3) was not dephosphorylated (Fig. 4B). The catalytically inactive MKP-1 (CS) mutant did not dephosphorylate histone H3 (Ser-10) or histone H3 (Thr-3) in vitro. Furthermore, the phosphatase inhibitor sanguinarine (SA) at 50 μM for 30 min (54) blocked MKP-1 dephosphorylation of histone H3 (Ser-10) (Fig. 4B).
Fig. 4.
MKP-1 dephosphorylates histone H3 at the Ser-10 position in vitro. A: immunoblot analysis of purified MKP-1 (WT) and (CS). B: phosphorylated histones (5 μg) were isolated by acid extraction then acetone precipitated. Histones were subsequently incubated with immunoprecipitated MKP-1 (WT) or (CS) for an in vitro dephosphorylation assay. Histone H3 (Ser-10) and histone H3 (Thr-3) phosphorylation were analyzed by immuno-blot. MKP-1 (WT) dephosphorylated the histone H3 (Ser-10), but not histone H3 (Thr-3). Sanguinarine (SA) blocked MKP-1 dephosphorylation of histone H3 (Ser-10) in vitro. Total histone H3 was used as a loading control.
VEGF and thrombin cause dephosphorylation of histone H3 (Ser-10) in EC.
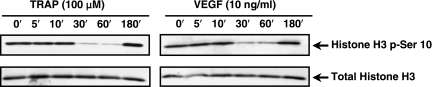
The impact of VEGF and thrombin on histone phosphorylation in EC has not been previously reported. VEGF- and thrombin-mediated EC activation have many similar signaling mediators, target genes, and phenotypic responses (33). Our previous studies showed that MKP-1 induction by VEGF and thrombin in EC begins as early as 10 min and peaked at 1 h (7, 27). Here, we treated EC with either VEGF or TRAP, the six-amino acid peptide that mimics the action of thrombin (SFLLRN), and examined histone phosphorylation state by immuno-blot analysis using specific histone antibodies. VEGF- and thrombin-stimulated EC extracts showed the dephosphorylation of histone H3 (Ser-10) at 30 min and 1 h and the phosphorylation reappeared at 3 h (Fig. 5). There was no change in the total histone H3 levels (Fig. 5).
Fig. 5.
Thrombin and VEGF stimulation decrease phosphorylation of histone H3 (Ser-10) in human EC. A: human EC treated with, VEGF (10 ng/ml), or thrombin receptor activating peptide (TRAP, 100 μM) over a 3-h time course. Immunoblot analysis was performed using a specific anti-histone H3 (phospho-Ser-10) antibody. Immunoblots of total histone H3 was used as loading controls. These blots represent 3 independent experiments.
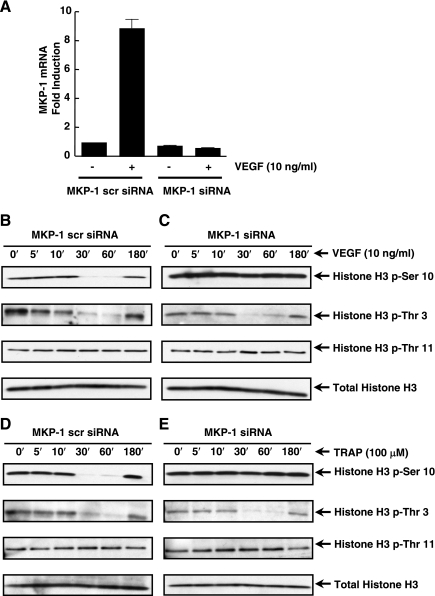
We next asked whether the depletion of MKP-1 activity would prevent VEGF- and thrombin-induced histone H3 (Ser-10) dephosphorylation. To deplete MKP-1, we synthesized MKP-1-specific siRNA and transiently transfected it into EC for 4 h. Thirty-two hours posttransfection the cells were treated with VEGF for 1 h. Figure 6A demonstrates that MKP-1-specific siRNA, not the scrambled siRNA, effectively depleted VEGF-induced MKP-1 mRNA. Furthermore, scrambled siRNA did not alter VEGF-induced dephosphorylation of histone H3 (Ser-10) (Fig. 6B). In addition, VEGF induced the dephosphorylation of histone H3 (Thr-3), while histone H3 (Thr-11) phosphorylation remained unaltered. MKP-1-specific siRNA prevented VEGF-induced histone H3 (Ser-10) dephosphorylation in EC but did not alter histone H3 (Thr-3) dephosphorylation (Fig. 6C).
Fig. 6.
MKP-1 is critical for the dephosphorylation of histone H3 (Ser-10) in human EC. A: EC were transfected with MKP-1-targeted small interfering RNA (siRNA) or scrambled siRNA. Real-time PCR analysis showed a reduction in basal and VEGF-induced MKP-1 mRNA. B–E: human EC were transfected with MKP-1-targeted siRNA or scrambled siRNA for 24 h. EC were then stimulated with either VEGF or TRAP. Protein lysates were separated using SDS-PAGE and immunoblotted with specific histone H3 (phospho-Ser-10, -Thr-3 or -Thr-11) antibodies. Total histone H3 antibody was used for a loading control.
We also investigated the role of thrombin-induced MKP-1 dephosphorylation of histone H3 (Ser-10) in EC. Similar to VEGF, MKP-1-specific siRNA blocked thrombin-stimulated histone H3 (Ser-10) dephosphorylation (Fig. 6, D and E). However, there was no effect on histone H3 (-Thr-3 and -Thr-11) phosphorylation profiles (Fig. 6, D and E). Therefore, MKP-1 specifically dephosphorylated phospho-histone H3 (Ser-10), but not phospho-histone H3 (-Thr-3 or -Thr-11), in VEGF- or thrombin-activated EC.
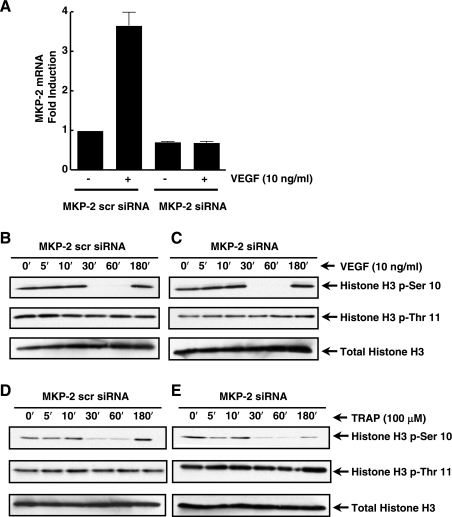
We recently observed that VEGF and thrombin also induced MAPK phosphatase-2 (MKP-2) with similar temporal kinetics as MKP-1 in EC (data not shown). MKP-2 is localized in the nucleus and participates in cellular responses to inflammatory agonists (1, 20, 22). MKP-2 siRNA or MKP-2 scrambled siRNA was transfected into human EC, which were then stimulated with VEGF. MKP-2 mRNA levels were significantly reduced in VEGF-stimulated EC (Fig. 7A). Furthermore, VEGF- and thrombin-treated EC were transfected with MKP-2 siRNA and analyzed for phosphorylation of histone H3 (Ser-10). No change was observed in agonist-induced dephosphorylation of histone H3 (Ser-10) in EC depleted of MKP-2, compared with EC transfected with MKP-2-scrambled siRNA (Fig. 7, B–E). We propose that thrombin and VEGF induce MKP-1 through the ERK and JNK pathways, respectively (27), leading to the specific dephosphorylation of histone H3 (Ser-10).
Fig. 7.
VEGF and TRAP-induced histone H3 (Ser-10) dephosphorylation is MKP-2 independent. A: MKP-2 siRNA (Ambion) and scrambled siRNA were transfected into EC. RNA was isolated from VEGF-stimulated EC and real-time PCR was performed using specific primers for MKP-2. B–E: phosphorylation states of histones H3 (-Ser-10 and -Thr-11) were analyzed by immunoblotting protein lysates from VEGF- or thrombin-stimulated EC transfected with MKP-2 siRNA and MKP-2 scrambled siRNA. Total histone H3 was used for a loading control.
DISCUSSION
VEGF and thrombin via the activation of their respective receptors trigger multiple signaling cascades in EC that are critical in inflammation and blood vessel growth. In this study, we used the substrate-trap technique (15, 51) to identify histone H3 as a novel MKP-1 substrate. Furthermore, using multiple biochemical approaches, we have demonstrated for the first time that MKP-1 induced by VEGF and thrombin efficiently dephosphorylated the Ser-10 residue of histone H3 in EC.
We have showed that histone H3 (Ser-10) is transiently dephosphorylated between 30 and 60 min in EC treated with VEGF and thrombin. Though many reports have described the action of histone kinases, little is known about histone phosphatases. In this study, we also demonstrated both VEGF and thrombin cause dephosphorylation of histone H3 (Thr-3). Unlike histone H3 (Ser-10) dephosphorylation, depletion of MKP-1 did not prevent the dephosphorylation of histone H3 (Thr-3), suggesting the involvement of alternate phosphatases in the posttranslational modification of histones in EC upon VEGF and thrombin stimulation.
The role of histone posttranslational modifications in agonist-mediated gene-induction is well documented (2, 37). Epidermal growth factor (EGF)-induced CREB and histone H3 (Ser-10) phosphorylation were activated via the Ras-MAPK-ERK pathway and have been proposed to modulate aberrant gene expression in oncogene-transformed mouse fibroblast. MSK1 has also been reported to phosphorylate histone H3 (Ser-10), leading to immediate early gene induction. In addition, reports show histone H3 (Ser-10) phosphorylation correlates with immediate early gene induction of c-fos, c-jun, and MKP-1 (9, 24, 29). Phosphatases are key regulatory modulators in maintaining a tight balance over the phosphorylation status of these signaling molecules. However, only a few nonmammalian phosphatases have been identified to dephosphorylate histone H3 (Ser-10). In Drosophila maelanogaster, protein phosphatase 2A (PP2A) has been linked to genome-wide dephosphorylation of histone H3 (Ser-10) and the transcriptional regulation of heat shock genes (38). Furthermore, dephosphorylation of histone H3 (Ser-10) during mitotic chromosome condensation (57) correlates with MKP-1 upregulation during the first growth phase (G1) of the cell cycle (34). These studies were limited by their reliance on pharmacological phosphatase inhibitors to identify histone phosphatases. Their limited “target” proteins focused only on protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A), which were subsequently confirmed using Drosophila mutants. Our substrate-trap approach enabled us to directly identify a potential pool of substrates for MKP-1 by mass spectrometry. Although, substrate-trapping mutants can vary in their efficiencies, the MKP-1 (CS) mutant has been shown to effectively bind to substrates with low catalytic turnover (40). Our data were confirmed in EC treated with MKP-1 siRNA by immunoblot analysis of phosphorylated histones.
In eukaryote models, Ipl1/aurora family kinases, Ipl1p in Saccharomyces cerevisiae and AIR-2 in Caenorhabditis elegans regulate mitotic histone H3 (Ser-10) phosphorylation (23, 35, 42). Furthermore, two related type 1 phosphatases Glc7p in S. cerevisiae and CeGLC-7 in C. elegans are required for regulation of mitotic histone H3 dephosphorylation (23, 35, 42). Our results show agonist-mediated dephosphorylation of histone H3 (Ser-10) in human EC by MKP-1. Interestingly, MKP-1 dephosphorylated histone H3 in a site-specific manner that is consistent with previous mitosis-focused studies. Histone H3 (Ser-10) phosphorylation is critical in numerous cellular functions, but it appears that this protein site may be controlled by a cohort of function-specific kinases and phosphatases (41).
Histone H3 phosphorylation and subsequent acetylation are important in the regulation of gene transcription. Histone H3 phosphorylation has been linked to the regulation of numerous genes including VEGF- and thrombin-induced genes, such as urokinase plasminogen activator, VCAM-1, and E selection (14, 24, 28, 50). We have previously reported that MKP-1 activity is critically important in the negative regulation of some thrombin-induced EC genes, such as E-selectin and VCAM-1 (7). We also demonstrated an important role for MKP-1 induction in VEGF-stimulated EC migration (27). VEGF- and thrombin-stimulated dephosphorylation of histone H3 (Ser-10) may represent a causative link in the modulation of E-selectin gene induction in EC. In agreement with our results, TNF-α-mediated MKP-1 induction via the JNK/p38 signaling pathway has been shown to be a negative regulator of E-selectin expression in EC (55). Equally, TNF-α-induced histone H3 (Ser-10) dephosphorylation correlates with MKP-1 induction in EC (55). Our results suggest that histone H3 (Ser-10) dephosphorylation by MKP-1 in VEGF- and thrombin-stimulated EC may act as a temporal repressor or regulator, to control access of the transcriptional machinery to the promoters of inflammatory genes.
Using Mkp-1-null mice and cells, numerous reports have shown that MKP-1 is physiologically important in the regulation of innate/adaptive immunity and metabolic homeostasis. Recent studies revealed that Mkp-1-deficient mice have inducible nitric oxide synthase-mediated hypotension when challenged with low doses of endotoxin (3). In correlative studies, MKP-1 was shown to be important in switching arginine metabolism from nitric oxide synthase to arginase following LPS challenge of peritoneal macrophages derived from WT or Mkp-1 null mice (36). Other investigators reported increased mortality in Mkp-1 null mice challenged with Gram-positive bacterial infection, through the inactivation of JNK and p38 (56). Mkp-1 null mice have shown enhanced susceptibility to anaphylaxis, while maintaining sensitivity to glucocorticoids in bone marrow-derived mast cells from the same mice (32). However, MKP-1 in EC was presented as a novel and crucial mediator of the anti-inflammatory action of glucocorticoids (16). These findings support the case that MKP-1 is a key inflammatory modulating switch that is finely balanced in physiological homeostasis depending on the cell-type and disease state.
Posttranslational modifications of proteins are critical for regulating VEGF and thrombin-mediated gene induction in EC. Multiple studies have shown histone H3 acetylation by p300 is critical for gene induction (6, 44). More recent reports have shown acetylation of MKP-1 by p300 inhibits innate immune signaling by blocking LPS-activated Toll-like receptor signaling (5). These results correlate with our data and support the case for the “histone code hypothesis” in regulating VEGF- and thrombin-induced genes in EC (25). The dephosphorylation of histone H3 (Ser-10) in the nucleus by the dual specificity phosphatase MKP-1 led us to consider the potential role of this mechanism in the induction of specific inflammatory genes. Chromatin immunoprecipitation-based approaches will be required to address which VEGF and thrombin-regulated EC genes are modulated by MKP-1. Our studies should help to identify new mechanisms and therapies related to MKP-1 in this newly discovered MKP-1-histone pathway.
GRANTS
A grant from the National Heart, Lung, and Blood Institute (NHLBI) (HL-29582 awarded to P. E. DiCorleto) supported this study. C. M. Kinney was supported in part with grants from the NHLBI (T32-HL-07653), and by the Bill and Melinda Gates Foundation (Gates Millennium Scholarship).
Acknowledgments
We thank Dr. Andrei Gudkov for kindly providing lentiviral packaging vectors. We also thank Drs. Smarajit Bandyopadhyay and Ratan Maitra for advice with the lentiviral cloning. We thank Dr. Jack Dixon (University of California, San Diego) for kindly providing the myc-tagged human MKP-1 cDNA. We also thank Lori Mavrakis, Angela Money, and Sara Bundy for cell culture and technical assistance. Human umbilical vein endothelial cells were harvested through Birthing Services Department at the Cleveland Clinic Foundation and the Perinatal Clinical Research Center (National Institutes of Health Research Center Award RR-00080) at the Cleveland Metrohealth Hospital.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Berasi SP, Huard C, Li D, Shih HH, Sun Y, Zhong W, Paulsen JE, Brown EL, Gimeno RE, Martinez RV. Inhibition of gluconeogenesis through transcriptional activation of EGR1 and DUSP4 by AMP-activated kinase. J Biol Chem 281: 27167–27177, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Berger SL Histone modifications in transcriptional regulation. Curr Opin Genet Dev 12: 142–148, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Calvert TJ, Chicoine LG, Liu Y, Nelin LD. Deficiency of mitogen-activated protein kinase phosphatase-1 results in iNOS-mediated hypotension in response to low-dose endotoxin. Am J Physiol Heart Circ Physiol 294: H1621–H1629, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 14: 6–16, 2000. [PubMed] [Google Scholar]
- 5.Cao W, Bao C, Padalko E, Lowenstein CJ. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med 205: 1491–1503, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci 114: 2363–2373, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekharan UM, Yang L, Walters A, Howe P, DiCorleto PE. Role of CL-100, a dual specificity phosphatase, in thrombin-induced endothelial cell activation. J Biol Chem 279: 46678–46685, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci USA 103: 2274–2279, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clayton AL, Rose S, Barratt MJ, Mahadevan LC. Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J 19: 3714–3726, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, and Sassone-Corsi P. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol 22: 874–885, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davie JR MSK1 and MSK2 mediate mitogen- and stress-induced phosphorylation of histone H3: a controversy resolved. Sci STKE 2003: PE33, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci 119: 4607–4615, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Duncan EA, Anest V, Cogswell P, Baldwin AS. The kinases MSK1 and MSK2 are required for epidermal growth factor-induced, but not tumor necrosis factor-induced, histone H3 Ser10 phosphorylation. J Biol Chem 281: 12521–12525, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Edelstein LC, Pan A, Collins T. Chromatin modification and the endothelial-specific activation of the E-selectin gene. J Biol Chem 280: 11192–11202, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flint AJ, Tiganis T, Barford D, Tonks NK. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci USA 94: 1680–1685, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furst R, Schroeder T, Eilken HM, Bubik MF, Kiemer AK, Zahler S, Vollmar AM. MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. FASEB J 21: 74–80, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Gurova KV, Hill JE, Razorenova OV, Chumakov PM, Gudkov AV. p53 pathway in renal cell carcinoma is repressed by a dominant mechanism. Cancer Res 64: 1951–1958, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med 203: 15–20, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene 20: 3021–3027, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hardy K, Hunt NH. Effects of a redox-active agent on lymphocyte activation and early gene expression patterns. Free Radic Biol Med 37: 1550–1563, 2004. [DOI] [PubMed] [Google Scholar]
- 21.He Z, Ma WY, Liu G, Zhang Y, Bode AM, Dong Z. Arsenite-induced phosphorylation of histone H3 at serine 10 is mediated by Akt1, extracellular signal-regulated kinase 2, and p90 ribosomal S6 kinase 2 but not mitogen- and stress-activated protein kinase 1. J Biol Chem 278: 10588–10593, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch DD, Stork PJ. Mitogen-activated protein kinase phosphatases inactivate stress-activated protein kinase pathways in vivo. J Biol Chem 272: 4568–4575, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF, Lin R, Smith MM, Allis CD. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102: 279–291, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Illi B, Nanni S, Scopece A, Farsetti A, Biglioli P, Capogrossi MC, Gaetano C. Shear stress-mediated chromatin remodeling provides molecular basis for flow-dependent regulation of gene expression. Circ Res 93: 155–161, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Jenuwein T, Allis CD. Translating the histone code. Science 293: 1074–1080, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kinney CM, Chandrasekharan UM, Mavrakis L, DiCorleto PE. VEGF and thrombin induce MKP-1 through distinct signaling pathways: role for MKP-1 in endothelial cell migration. Am J Physiol Cell Physiol 294: C241–C250, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lee CW, Lin WN, Lin CC, Luo SF, Wang JS, Pouyssegur J, Yang CM. Transcriptional regulation of VCAM-1 expression by tumor necrosis factor-alpha in human tracheal smooth muscle cells: involvement of MAPKs, NF-kappaB, p300, and histone acetylation. J Cell Physiol 207: 174–186, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Gorospe M, Hutter D, Barnes J, Keyse SM, Liu Y. Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. Mol Cell Biol 21: 8213–8224, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo WS, Duggan L, Emre NC, Belotserkovskya R, Lane WS, Shiekhattar R, Berger SL. Snf1–a histone kinase that works in concert with the histone acetyltransferase Gcn5 to regulate transcription. Science 293: 1142–1146, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu JY, Allis CD, Marmorstein R, Berger SL. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol Cell 5: 917–926, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Maier JV, Brema S, Tuckermann J, Herzer U, Klein M, Stassen M, Moorthy A, Cato AC. Dual specificity phosphatase 1 knockout mice show enhanced susceptibility to anaphylaxis but are sensitive to glucocorticoids. Mol Endocrinol 21: 2663–2671, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, Kohro T, Ge X, Aburatani H, Hamakubo T, Kodama T, Aird WC. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J Biol Chem 279: 50537–50554, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopus egg extracts. Cell 79: 475–486, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Murnion ME, Adams RR, Callister DM, Allis CD, Earnshaw WC, Swedlow JR. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J Biol Chem 276: 26656–26665, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, English BK, Liu Y. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol 293: C632–C640, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Nowak SJ, Corces VG. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet 20: 214–220, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Nowak SJ, Pai CY, Corces VG. Protein phosphatase 2A activity affects histone H3 phosphorylation and transcription in Drosophila melanogaster. Mol Cell Biol 23: 6129–6138, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26: 3203–3213, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Pratt PF, Bokemeyer D, Foschi M, Sorokin A, Dunn MJ. Alterations in subcellular localization of p38 MAPK potentiates endothelin-stimulated COX-2 expression in glomerular mesangial cells. J Biol Chem 278: 51928–51936, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci 116: 3677–3685, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Rogers E, Bishop JD, Waddle JA, Schumacher JM, Lin R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J Cell Biol 157: 219–229, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudolph J Cdc25 phosphatases: structure, specificity, mechanism. Biochemistry 46: 3595–3604, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Sadoul K, Boyault C, Pabion M, Khochbin S. Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie 90: 306–312, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol 176: 1899–1907, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Sauve DM, Anderson HJ, Ray JM, James WM, Roberge M. Phosphorylation-induced rearrangement of the histone H3 NH2-terminal domain during mitotic chromosome condensation. J Cell Biol 145: 225–235, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shankar R, de la Motte CA, Poptic EJ, DiCorleto PE. Thrombin receptor-activating peptides differentially stimulate platelet-derived growth factor production, monocytic cell adhesion, and E-selectin expression in human umbilical vein endothelial cells. J Biol Chem 269: 13936–13941, 1994. [PubMed] [Google Scholar]
- 48.Slack DN, Seternes OM, Gabrielsen M, Keyse SM. Distinct binding determinants for ERK2/p38alpha and JNK map kinases mediate catalytic activation and substrate selectivity of map kinase phosphatase-1. J Biol Chem 276: 16491–16500, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J 22: 2788–2797, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strelkov IS, Davie JR. Ser-10 phosphorylation of histone H3 and immediate early gene expression in oncogene-transformed mouse fibroblasts. Cancer Res 62: 75–78, 2002. [PubMed] [Google Scholar]
- 51.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75: 487–493, 1993. [DOI] [PubMed] [Google Scholar]
- 52.Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol 3: S3009, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tonks NK Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7: 833–846, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Vogt A, Tamewitz A, Skoko J, Sikorski RP, Giuliano KA, Lazo JS. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J Biol Chem 280: 19078–19086, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Wadgaonkar R, Pierce JW, Somnay K, Damico RL, Crow MT, Collins T, Garcia JG. Regulation of c-Jun N-terminal kinase and p38 kinase pathways in endothelial cells. Am J Respir Cell Mol Biol 31: 423–431, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Meng X, Kuhlman JR, Nelin LD, Nicol KK, English BK, Liu Y. Knockout of Mkp-1 enhances the host inflammatory responses to gram-positive bacteria. J Immunol 178: 5312–5320, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci USA 95: 7480–7484, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu JJ, Roth RJ, Anderson EJ, Hong EG, Lee MK, Choi CS, Neufer PD, Shulman GI, Kim JK, Bennett AM. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab 4: 61–73, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, Chang CH, Liu Y. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 203: 131–140, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]