how atp and other nucleotides are released from intact cells is a fundamental question, given the existence of multiple purinergic receptor signaling cascades operative in most vertebrate tissues (25). It is well-established that neurons and neuroendocrine cells release ATP via classical mechanisms involving Ca2+-dependent exocytotic release of nucleotides copackaged with other neurotransmitters within specialized secretory vesicles or granules. However, many, indeed most, nonexcitable cell types locally release ATP via nonlytic mechanisms that do not involve obvious or readily measured exocytosis of nucleotide-containing vesicles or granules. An alternative mechanism for nonlytic ATP release is its facilitated efflux from the cytosolic compartment through plasma membrane transport proteins. Various membrane transport proteins or functionally characterized permeability pathways have been suggested as “ATP channels,” including some ATP-binding cassette (ABC)-family transporters, volume-regulated anion channels, plasma membrane variants of the mitochondrial voltage-dependent anion channel (VDAC) porins, and maxianion channels (31). Additionally, a strong and growing body of data indicates that ATP release from many cell types is mediated by so-called hemichannels composed of protein subunits from the well-characterized connexin (Cx) family or the recently described pannexin (Panx) family (19, 23, 26). Hemichannels can act as low-resistance conduits for the efflux of ATP and other cytosolic metabolites (59). The ubiquitous expression of the Panx1 gene in most tissues and cell types suggests that Panx1 hemichannels may comprise one of the most widely used efflux pathways for ATP release in different paracrine and autocrine signaling responses (4, 30). Notably, extracellular ATP itself, acting via certain P2Y or P2X receptors, can elicit intracellular signals that favor the gating of hemichannels to the open state. This facilitates a pathway of what can be termed “ATP-induced ATP release” linked to paracrine signaling “waves” that allow multiple cells within a tissue to respond proactively to environmental stresses (e.g., metabolic inhibition, mechanical shear, and microbial invasion) sensed by only a few cells at the immediate locus of environmental insult or stimulation (51). This sort of paracrine signaling can play a positive role in adaptive responses, such as ischemic preconditioning, relief of mechanical stress, or killing of invading pathogens, with clear physiological benefit to the whole animal. However, a cascade of P2 receptor activation coupled to the gating of ATP-permeable hemichannels also comprises a positive feedback loop that, if unrestrained, could lead to maladaptive and malignant depletion of intracellular ATP stores, collapse of ionic gradients, and cell death. Thus considerable attention has been directed toward the identification of endogenous factors that can inhibit or restrict the gating/conductance of hemichannels. In a paper by Qui and Dahl (40a), they describe a highly novel mechanism based on a direct inhibitory action of extracellular ATP on the gating and/or activity of pannexin-1 hemichannels.
Cell Biology of Pannexins and Connexins
Pannexin-1 belongs to a family of three membrane proteins (Panx 1, 2, and 3) that exhibit similar topology, but minor sequence homology, to the larger (20 unique members) family of connexin membrane proteins (10). In contrast to Panx2, which is restricted to the brain, and Panx3, which is predominantly a skin and connective tissue gene product, Panx-1 is expressed in most cells and tissues examined thus far. Given its ubiquitous distribution and unusual functional characteristics as a channel, Panx1 has become the focus of recent studies in a broad range of tissues and physiological responses. [See Shestapolov and Panchin (56) for a recent review of this rapidly developing area]. For example, Panx1 has recently been linked to the transmission of gustatory receptor signals in taste buds (43), the activation of inflammatory and immune signaling responses in macrophages (38) and T lymphocytes (54), pressure overload-induced fibrosis in the heart (36), and the generation of N-methyl-d-aspartate (NMDA) receptor-dependent epileptiform electrical activity in the hippocampus (63).
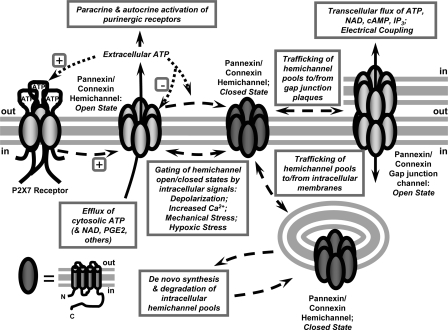
All pannexins and connexins share a similar structure of four transmembrane domains, intracellular amino and carboxy termini, and two extracellular loops (56). Six subunits coassemble to form a hexameric hemichannel during synthesis and trafficking through the ER/Golgi network before delivery at the plasma membrane (Fig. 1). Hemichannel assemblies composed of connexin subunits are known as connexons, whereas those composed of pannexins are pannexons. Both homomeric connexons composed of a single connexin subtype and heteromeric connexons containing different subunits can be expressed in different tissues. Depending on both subunit composition and cell type, connexons or pannexons may be predominantly trafficked to the plasma membrane or retained within intracellular membrane pools (39).
Fig. 1.
Trafficking and regulated gating of connexin or pannexin hemichannels. The biosynthesis and trafficking of connexons or pannexons can result in nonjunctional hemichannels in the plasma membrane or further assembly into gap junctional complexes at sites of cell-to-cell contact. Multiple extracellular and intracellular stimuli have been implicated in the gating of nonjunctional hemichannels to the open state. These channels are permeable to both ions and small organic molecules, such as intracellular ATP or extracellular fluorescent dyes. The gating of hemichannels can be triggered by extracellular nucleotides that target various P2X or P2Y receptor subtypes. This can initiate a positive-feedback cycle of ATP-induced ATP release. However, the studies described by Qui and Dahl in this issue of AJP-Cell suggest that the released extracellular ATP can exert a negative allosteric action on pannexin-1-based hemichannels and thus dampen this positive-feedback cycle.
A major difference between connexons versus pannexons is their fate and function following delivery to the plasma membrane (Fig. 1). At sites of direct cell-cell contact, the apposed connexon hemichannels from two cells readily dock together (via extracellular loop domains) to form a transcellular gap junction channel. Clusters of such gap junction channels comprise morphologically identifiable plaques when analyzed by immunohistochemistry. Consistent with their role in metabolic and electrical coupling, gap junction channels are permeable to both ions and cytosolic metabolites, including ATP. In contrast to connexons, pannexons in the plasma membrane do not readily assemble into the plaque-like ensembles that typify gap junctions (39, 71). Notably, and in contrast to most connexins, both Panx1 and Panx3 are exported to the cell surface as glycosylated proteins, and this glycosylation may impede the docking between pannexons on adjacent cells (7, 39). Although early studies indicated that functional gap junctions could be formed by Panx1 heterologously overexpressed in Xenopus oocytes or human prostate carcinoma cells, no data has as yet been presented that support an in vivo role for Panx1 as a component of physiologically relevant gap junction channels. Panx1 is also highly expressed in cells, such as mammalian erythrocytes, that do not form gap junctions as part of their physiological repertoire. Thus nonjunctional pannexon hemichannels comprise the predominant structural state, and presumed functional state, of Panx1 in the plasma membrane of mammalian cells (Fig. 1).
Connexin Hemichannels: Roles and Regulation
The proposed role for Panx1 hemichannels as conduits for ATP release needs to be considered within the context of the many previous (and current) studies describing the regulation and functions of nonjunctional connexin hemichannels. Connexon-based gap junction channels represent known transport proteins that readily faciliate the flux of ATP between cells. Gap junctions composed of different connexin subtypes exhibit graded degrees of permeability to ATP and ADP with connexin 43 (Cx43) channels being 50 times more permeable to these nucleotides than Cx32 channels (26). The flux of metabolites and ions through gap junctions can be rapidly attenuated by various intracellular signals, such as acidification or sustained increases in cytosolic Ca2+. However, many studies of both native and heterologous connexins have indicated that connexon hemichannels at nonjunctional membrane sites can also be gated to the open state (15, 46, 47, 51). Under normal conditions, the gating of nonjunctional hemichannels in healthy cells is, and must be, a low-frequency event to prevent the collapse of ionic gradients and loss of ATP and other intracellular metabolites that would result in cell death. In response to various cell stresses, such as reduction in extracellular Ca2+ or metabolic inhibition, cells that express native or recombinant connexins become transiently permeable to extracellular fluorescent dyes such as lucifer yellow (Mr 522 Da) and calcein (Mr 660 Da) (22, 28, 41). Electrophysiological analyses using human embryonic kidney (HEK)293 cells transfected with Cx43 cDNA (28) or single Xenopus oocytes injected with Cx50 or Cx46 cRNAs (66) have also demonstrated that reduced extracellular Ca2+ reversibly triggers activation of nonselective ionic currents carried by connexin hemichannels.
That gap junctions are highly permeable to ATP and that connexin hemichannels are permeable to dyes with molecular masses similar to that of ATP supported the speculation that connexin hemichannels provide a major pathway for facilitated efflux of ATP. Early reports by the Nedergaard group (12) addressed this as part of studies showing that nonjunctional connexon hemichannels were involved in intercellular Ca2+ wave propagation in astrocytes. “Ca2+ wave propagation” is the process whereby acute changes in cytosolic Ca2+ levels within one cell can rapidly be propagated to nearby cells. Connexins and gap junctions play critical roles in Ca2+ wave propagation in astrocytes and other cell types (9, 26, 27, 45, 49). One mechanism is that gap junction channels between adjacent cells directly facilitate the movement of Ca2+ per se and Ca2+-mobilizing inositol 1,4,5-trisphosphate (IP3) from one cell to another (9). The alternative mechanism for wave propagation, as demonstrated by the Nedergaard group, is that one cell releases an extracellular signaling molecule, such as ATP, which then activates Ca2+-mobilizing receptors in nearby cells (Fig. 1). The particular roles of released ATP and Ca2+-mobilizing P2 receptors in wave propagation have been demonstrated in multiple cell models (18, 42, 48, 55, 60, 67, 70). In most cases, these analyses of ATP-dependent Ca2+-wave propagation involve nonlytic mechanical stimulation of a single cell within an optical field to trigger Ca2+ mobilization in that cell. However, rapid Ca2+ transients are also observed in surrounding cells after variable delays that are directly proportional to the distance from the mechanically stimulated cell. The seminal findings of Nedergaard and colleagues set the stage for additional investigations (by the same group and others) for further analyzing the possible role of connexin hemichannels in stimulus-induced nucleotide release in astrocytes, endothelial cells, and osteocytes (2, 23, 60).
Thus a wide range of experimental evidence supports a role for connexin hemichannels in stimulus-induced ATP release in several types of cells. However, other data have highlighted the difficulty of establishing direct cause-and-effect relationships between connexin expression/activity and ATP release. Spray and colleagues (50, 52, 61, 62) compared the relative contributions of gap junctional communication versus the released ATP/paracrine P2 receptor activation pathway to Ca2+ wave propagation in cultures of neonatal cardiac myocytes or neonatal spinal cord astrocytes obtained from wild-type versus Cx43 knockout mice. Cx43 compromises more than 90% of the connexins expressed in wild-type myocytes and astrocytes. Consistent with this, myocytes from Cx43-null mice exhibited major deficits in gap junction-mediated Ca2+ wave propagation (62). However, Ca2+ wave propagation mediated by the paracrine purinergic pathway was similar in both wild-type and Cx43-deficient cells. This indicated that ATP release from the myocytes was not attenuated by the absence of the major connexin component of these cells. Similarly, the absence of Cx43 in spinal cord astrocytes reduced gap-junctional coupling by more than 65% but with little effect on mechanically induced Ca2+ wave propagation via the paracrine purinergic route (52). These various findings with connexin knockout cells clearly supported the existence of mechanisms that reciprocally coordinate Ca2+ wave intercellular signaling via the connexin-based and purinergic pathways. However, the observation that purinergic paracrine pathways are not significantly disrupted by deletion of the major connexin subtype within these astrocytes and myocytes raised significant questions as to whether other channels, with pharmacological and gating properties similar to those of connexin hemichannels, act as the actual conduits for ATP release. The identification of Panx1 as a ubiquitous, and predominantly nonjunctional, plasma membrane channel complex sets the stage for a recent and rapidly developing series of studies by several groups aimed at establishing the role of Panx1 as an ATP-release channel.
Pannexin-1 Hemichannels: Roles and Regulation
Most of the same tools and experimental approaches used to analyze the role of connexin hemichannels in ATP release have been adapted to the study of Panx1 hemichannels. These include analysis of: 1) natively expressed Panx1 channels in cells treated with various channel hemichannel inhibitors (e.g., carbenoxolone) or small interfering RNAs (siRNAs) that suppress Panx1; and 2) analysis of Panx1 function in various heterologous expression systems. Using the latter approach, Dahl and colleagues (3, 32, 33, 57, 68) have extensively characterized the properties of mammalian Panx1 channels expressed in single Xenopus oocytes. At the electrophysiological level, these studies have revealed that: 1) whole cell currents carried by Panx1 hemichannels in intact oocytes can be gated in response to plasma membrane potentials >10 mV; 2) the conductance of single channel currents carried by Panx1 in cell-free membrane patches is markedly increased at membrane potentials greater than −20 mV; 3) Panx1 single channel currents can also be activated by direct mechanical stress applied to membrane patches held at hyperpolarized (−50 mV) potentials; 4) Panx1 single channel currents can exhibit very large unitary conductance (∼500 pS) and complex subconductance states; 5) Panx1 currents can also be gated in oocytes held at normal resting potentials (apporximately −50 mV) in response to either Ca2+ ionophore or extracellular ATP when Panx1 is coexpressed with Ca2+-mobilizing P2Y1 and P2Y2 receptors. Recent analyses by Surprenant and colleagues (34) have indicated similar, but not identical, electrophysiological characteristics of Panx1 hemichannels (native or recombinant) expressed in mammalian cell backgrounds. A notable difference is that Panx1 channel activity in these mammalian cells is largely insensitive to perturbations in cytosolic Ca2+ concentration.
As with connexons, the hemichannel activity of Panx1 has also been assayed by changes in the influx or efflux of fluorescent dyes, which are similar in size to ATP (20, 37, 38, 40, 57, 63). In contrast to connexin-based dye fluxes (13, 21, 60), the hemichannel activity of Panx1 is not activated by reduction by low extracellular divalent cation concentration (34, 40a). However, Panx1-mediated dyes fluxes can be triggered by either depolarization or mechanical stress. Notably, these same stimuli elicit increased ATP release from single Xenopus oocytes expressing recombinant human Panx1 (3).
Pannexin-1 Hemichannels as Components of P2X7 Receptor Signaling Pathways
A major development in Panx1 biology was the finding by Pelegrin and Surprenant (38) that Panx1 hemichannels are involved in the nonselective permeability pathway, typically assayed by influx of ethidium or propidium dyes, which is a secondary response to activation of ionotropic P2X7 receptors by extracellular ATP (Fig. 1). The binding of ATP to this receptor gates its intrinsic nonselective cation channel activity, leading to rapid influx of extracellular Na+ and Ca2+ and efflux of cytosolic K+. However, sustained (for more than several seconds) or repeated activation of these ATP-gated cation channels elicits an increased permeability to organic anions and cations as large as 900 Da in mass. This unusual permeability response has been linked to the physiological role of the P2X7 receptor in the regulation of immune and inflammatory responses that culminate in the death of invading microbes, activation and possible death of the host leukocytes that kill these microbes, and clearance of infected or damaged “bystander” cells at local sites of microbial invasion or tissue damage (17). For example, P2X7 receptors are highly expressed in monocytes, macrophages, and microglia wherein they regulate caspase-based signaling pathways coupled to the production and release of potent proinflammatory cytokines (IL-1β and IL-18). Activation of P2X7 receptors also initiates pathways of regulated death in macrophages and T cells; this limits the accumulation of activated leukocytes that might otherwise contribute to autoimmune or autoinflammatory diseases (14). Activation of the Panx1-dependent permeability pathway modulates these death-related inflammatory and immune responses in leukocytes by poorly understood mechanisms involving collapse of the normal ionic and metabolite gradients across both the plasma membrane and (secondarily) intracellular membranous organelles, such as lysosomes and endosomes (24, 35).
Thus the gating of Panx1 hemichannels by P2X7 receptors at the single cell level has two distinct but related consequences. The first is the initiation of intracellular signaling pathways that allow the responding leukocyte to contribute to innate or acquired immune responses protective to the organism, albeit at the possible cost of the leukocyte's own survival. The second consequence is that Panx hemichannels facilitate release of the leukocyte's own ATP pool for extracellular signaling responses; these include both autocrine actions, which sustain P2X7 signaling within that leukocyte, and paracrine actions, which trigger P2X7 receptors in nearby leukocytes and other P2 receptor subtypes in nonleukocyte cell types (6). These other P2 responses can include Ca2+ wave propagation (67).
Multiple Roles for ATP in Panx1 Hemichannel Function
The interplay between P2X7 receptor signaling and Panx1 hemichannel activity must involve precisely coordinated roles for ATP as both an upstream extracellular regulator (via its agonist action on P2X7) of Panx1 gating and a downstream intracellular substrate for efflux through Panx1 channels. The timing and magnitude of Panx1 hemichannel opening must be regulated to minimize, or at least sufficiently delay, excessive loss of cytosolic ATP and other metabolites (e.g., NAD) required for the kinase, protease, and redox signaling reactions that underlie responses to P2X7 receptor activation. In this regard, the experiments of Qui and Dahl describe one novel and unexpected regulatory mechanism that involves a direct inhibitory action of extracellular ATP on the gating and/or activity of Panx1 hemichannels.
The authors demonstrated that submillimolar levels of extracellular ATP rapidly and reversibly decreased the ionic currents activated by depolarization of Xenopus oocytes expressing recombinant murine Panx1. Extracellular ATP also suppressed depolarization-induced influx of ethidium into Xenopus erythrocytes that presumably express the amphibian orthologue of Panx1. Studies aimed at characterizing the selectivity of this inhibitory action of ATP revealed that ADP, AMP, and other nonadenine nucleotide triphosphates did not mimic the effect of ATP when tested at concentrations as high as 1 mM. However, the analog 3-O-benzoylbenzoic-ATP (BzATP) was effective and more potent than ATP itself (IC50 ∼ 100 μM vs. IC50 ∼ 1,000 μM for ATP). Notably, this selectivity and rank order of potency for inhibition of Panx1 currents is similar to that characterizing the activation of the P2X7 receptor. This suggested that an analogous pharmacology characterized the ATP binding sites on Panx1 and P2X7. Supporting this possibility were the surprising findings that Panx1-mediated currents or dye uptake were also inhibited by a broad range of pharmacological agents that antagonize or block P2X7 receptor activation. These included nonselective reagents, such as suramin and brilliant blue G (BBG), which target multiple P2 receptor subtypes in addition to P2X7. However, A438079, a selective P2X7 antagonist, also suppressed Panx1 hemichannel currents albeit at higher concentrations than those that antagonize ATP activation of P2X7 receptor function. As an initial foray into a molecular analysis of the interaction site(s) on Panx1 channels, Qiu and Dahl identified arginine-75 in the first extracellular loop of pannexin-1 as key site for interaction with ATP, BzATP, or BBG. The authors also observed that treatment of Xenopus erythrocytes with BBG suppressed the release of ATP induced by depolarization.
Thus domains within the extracellular loops and/or membrane-spanning segments of hexameric Panx1channels comprise allosteric binding sites for agonistic and antagonistic compounds that also are recognized by trimeric P2X7 channels. These findings indicate that ATP may play yet another role in the interaction of P2X7 and Panx1: as an extracellular allosteric modulator of Panx1 hemichannel activity. Although Qiu and Dahl exclusively analyzed the effects of ATP on Panx1 expressed in an amphibian cell background, Ma et al. (34) have recently described a similar inhibitory action of ATP on depolarization-activated hemichannel current carried by human or murine Panx1 expressed in HEK293 cells. However, some differences were noted in the effects of nucleotides on Panx1 currents in this mammalian cell background: 1) the nonadenine nucleotide triphosphates UTP and GTP mimicked the action of ATP; and 2) BzATP was less potent than ATP. This nucleotide pharmacology is distinct from that of the P2X7 receptor and suggests that the conformation of the nucleotide interaction site(s) may be different in Panx1 hemichannels expressed in amphibian versus mammalian backgrounds. Possible interactions of the pannexons with other proteins expressed only in mammalian (or amphibian) tissues might underlie such differences. Alternatively, different intracellular ionic environments, native cytosol for the Xenopus oocytes studied by two-microelectrode voltage-clamp versus a defined, minimal ionic buffer for HEK293 cells studied by whole cell patch clamp, might also affect the extracellular conformational state of the Panx1 hemichannels.
Implications and Additional Questions
Feedback regulation of ATP release via Panx1 hemichannels.
Regardless of the mechanistic details, the strong inhibitory action of exogenously added extracellular ATP on Panx1 hemichannels indicates that a similar inhibition may occur if endogenous ATP, which effluxes through open-state hemichannels, transiently accumulates at the outer vestibule of such channels. The resulting allosteric inhibition by this ATP within the pannexon ectodomain has the potential to limit the loss of intracellular ATP and thus act as a classical negative feedback regulator. Although Qiu and Dahl framed their analysis within the specific context of P2X7 → Panx1 signaling loops, it is important to emphasize that Panx1 channels have been linked to ATP release in cell types and signaling pathways in which P2X7 receptors do not play a significant or obvious role. Whether other environmental stresses and upstream signals induce Panx1-dependent ATP release is as-yet poorly understood. For example, Nishida et al. (36) have recently reported that mechanical stress triggers ATP and UDP release via Panx1 hemichannels in mouse and rat cardiomyocytes and that these released nucleotides induce an autocrine cascade involving P2Y6 receptors → G12/13 → Rho → induction of fibrosis gene expression. This cascade was upregulated in an in vivo model of pressure overload-induced cardiac fibrosis and could be dissociated from the parallel pathways that lead to hypertrophic gene expression. Excessive loss of ATP via mechanically gated Panx1 channels in cardiac myocytes would be catastrophic for the normal cardiac cycle that involves repetitive mechanical perturbation. A feedback effect of released ATP on hemichannel activity would clearly be beneficial in this context. Indeed, an important goal for future studies will be to determine whether extracellular ATP (and perhaps other nucleotides) can allosterically inhibit the gating/conductance of Panx1 hemichannels that are natively expressed in critical cell types such as cardiomyocytes and neurons.
Feedback regulation of nonselective ionic currents carried by Panx1 hemichannels.
Although ATP-dependent suppression of ATP efflux through hemichannels is one important consequence of this regulatory effect, this allosteric action of extracellular nucleotides may also be important in preventing excessive ionic currents via Panx1 channels, particularly in electrically excitable cells such as neurons and myocytes. In this regard, MacVicar and colleagues (65) previously described the opening of hemichannel-type currents in hippocampal neurons subjected to ischemic stresses. These authors concluded that hemichannel opening may lead to the significant dysregulation of ionic homeostasis during stroke and may be a major component of ischemic neuronal death. A recent study by the same group (63) found that transient activation of NMDA receptors in these neurons can induce a delayed and long-lasting gating of Panx1 hemichannels that could be correlated with aberrant ionic currents and dysregulated neuronal firing patterns that contribute to epileptiform seizure activity. Again, a suppressive action of released ATP on these gated Panx1 channels would act to attenuate such disruptive ionic currents. The authors suggested the very intriguing possibility that intracellular ATP may also exert a negative allosteric effect on Panx1 hemichannels and that depletion of this ATP pool, during ischemia or excitotoxic NMDA neurotransmission, may relieve this suppression, thus leading to the aberrant gating of Panx1 currents. It will be interesting to further test this hypothesis but it should be noted that the Surprenant group did not observe obvious effects of intracellular ATP on the depolarization-induced gating of recombinant Panx1 hemichannels expressed in HEK293 cells (34).
Reevaluation of the biological responses regulated by pharmacological agents that target P2X7 receptors.
That depolarization- and mechanical stress gating of Panx1-mediated ionic currents is inhibited by a variety of small molecule reagents used as P2X7 receptor antagonist has significant implications for P2X7 receptor pharmacology. These findings indicate that additional caution needs to be exercised in assuming an involvement of P2X7 receptors in complex biological responses, particularly in vivo responses, that are suppressed by these reagents. Most of these inhibitors (e.g., suramin) are known as nonselective reagents and are seldom used in in vivo studies. However, others, such as A438079, have been developed as highly selective P2X7 antagonists for use in whole animal studies and as possible therapeutic agents in human disease. As noted above, Panx1 hemichannels contribute to important autocrine and paracrine signaling responses that do not directly involve P2X7 receptors. Thus it will be important to test newly developed P2X7 antagonists for possible off-target effects on Panx1 hemichannels. In addition, it will be important to identify molecular entities that can block Panx1 hemichannels but that lack P2X7 antagonism. A number of compounds have recently been suggested as Panx1 inhibitors (57). Potentially preferable to the use of pharmacological agents will be the use of mice with knockout of pannexin channels, in particular, to determine the in vivo physiological and pharmacological significance of allosteric effects of ATP and P2X7 antagonists on Panx1-dependent functions. The first use of such Panx1-null animals, for studies of mechanically stimulated ATP release in cochlear cells, has been recently described by Anselmi et al. (1). Interestingly, these authors found that connexins 26 and 32, rather than Panx1, comprised the ATP-permeable hemichannels that mediated neurotransmission in this pathway. Given the roles for these and other connexin hemichannels (23) as ATP release conduits in particular tissues, it will be important to test whether extracellular ATP might also allosterically inhibit these nonpannexin based, but ATP-permeable, hemichannels.
Hemichannels as ATP release conduits: pannexins versus connexins.
Recent studies on hemichannel-mediated ATP release have emphasized the technical difficulty of distinguishing whether pannexons or connexons act as the predominant ATP conduit in cells that coexpress members of both protein families (51, 53, 59, 64). Most studies have utilized pharmacological blockers of hemichannel gating or conductance to attenuate stimulus-induced ATP release. Some commonly used hemichannel inhibitors include 18 β-glycyrrhetinic acid (18-GA), carbenoxolone (an 18-GA derivative), flufenamic acid derivatives, lanthanides, and probenecid (5, 45, 57, 58). A major limitation with this approach is that these reagents often lack selectivity for pannexons versus connexins and can also interact with channels or transporters other than hemichannels. A more specific group of hemichannel blockers are the so-called mimetic peptides (also known as gap peptides) that are short peptides that correspond to the sequences of extracellular (or intracellular) loops of various connexins.(8, 11, 16, 26) or pannexins (38, 63). By interacting with these loop domains, the gap peptides act to disrupt the normal conformation of connexon or pannexon complexes with consequent inhibition of either gap junctional or hemichannel function. However, some mimetic peptides can cross-inhibit both pannexin and connexin hemichannels (68). Thus the use of animals with global or tissue-specific knockout of particular pannexins (1) or connexins (1, 29, 69) will be essential for unequivocal determination of how particular hemichannels mediate ATP release in different tissues or in response to different stimuli within the same tissue.
GRANTS
This work was supported in part by National Institutes of Health Grants P01-HL-18708 and RO1-GM-36387.
REFERENCES
- 1.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA 105: 18770–18775, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA 99: 9840–9845, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 21: 103–114, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bennett MV, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Saez. Gap junctions: new tools JC, new answers, new questions. Neuron 6: 305–320, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1β release from microglia. J Immunol 174: 7268–7277, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Boassa D, Qiu F, Dahl G, Sosinsky G. Trafficking dynamics of glycosylated pannexin 1 proteins. Cell Commun Adhes 15: 119–132, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca2+ signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol 279: L623–L630, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Braet K, Paemeleire K, D'Herde K, Sanderson MJ, Leybaert L. Astrocyte-endothelial cell calcium signals conveyed by two signalling pathways. Eur J Neurosci 13: 79–91, 2001. [PubMed] [Google Scholar]
- 10.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA 100: 13644–13649, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaytor AT, Bakker LM, Edwards DH, Griffith TM. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br J Pharmacol 144: 108–114, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J 25: 34–44, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97: 587–600, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Ebihara L New roles for connexons. News Physiol Sci 18: 100–103, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans 29: 606–612, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, and Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176: 3877–3883, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 1208–1218, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104: 6436–6441, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 295: C752–C760, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John S, Cesario D, Weiss JN. Gap junctional hemichannels in the heart. Acta Physiol Scand 179: 23–31, 2003. [DOI] [PubMed] [Google Scholar]
- 22.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem 274: 236–240, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci 28: 4702–4711, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26: 433–443, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785–795, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes 10: 251–257, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Leybaert L, Cabooter L, Braet K. Calcium signal communication between glial and vascular brain cells. Acta Neurol Belg 104: 51–56, 2004. [PubMed] [Google Scholar]
- 28.Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol 134: 1019–1030, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci 28: 681–695, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litvin O, Tiunova A, Connell-Alberts Y, Panchin Y, Baranova A. What is hidden in the pannexin treasure trove: the sneak peek and the guesswork. J Cell Mol Med 10: 613–634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res 18: 558–565, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103: 7655–7659, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580: 239–244, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Ma W, Hui H, Pelegrin P, Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J Pharmacol Exp Ther, Nov 20, 2008. [DOI] [PMC free article] [PubMed]
- 35.Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, Nunez G. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via Cryopyrin/NLRP3 independently of Nod2. J Immunol 180: 4050–4057, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, Ide T, Suzuki K, Inoue K, Nagao T, Kurose H. P2Y(6) receptor-Galpha(12/13) signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J 27: 3104–3115, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem 282: 2386–2394, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci 120: 3772–3783, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Penuela S, Celetti SJ, Bhalla R, Shao Q, Laird DW. Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell Commun Adhes 15: 133–142, 2008. [DOI] [PubMed] [Google Scholar]
- 40a.Qiu F, Gadhl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol (October 22, 2008). doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed]
- 41.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol 148: 1063–1074, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romanello M, Pani B, Bicego M, D'Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun 289: 1275–1281, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J 26: 657–667, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol 132: 731–744, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83: 1359–1400, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Saez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MV. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand 179: 9–22, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 1711: 215–224, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauer H, Hescheler J, Wartenberg M. Mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation. Am J Physiol Cell Physiol 279: C295–C307, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Scemes E Components of astrocytic intercellular calcium signaling. Mol Neurobiol 22: 167–179, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Scemes E, Dermietzel R, Spray DC. Calcium waves between astrocytes from Cx43 knockout mice. Glia 24: 65–73, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol 3: 199–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci 20: 1435–1445, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schalper KA, Palacios-Prado N, Orellana JA, Saez JC. Currently used methods for identification and characterization of hemichannels. Cell Commun Adhes 15: 207–218, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 1: ra6, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Schwiebert EM Extracellular ATP-mediated propagation of Ca2+ waves. Focus on “Mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation”. Am J Physiol Cell Physiol 279: C281–C283, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Shestopalov VI, Panchin Y. Pannexins and gap junction protein diversity. Cell Mol Life Sci 65: 376–394, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295: C761–C767, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spray DC Molecular physiology of gap junction channels. Clin Exp Pharmacol Physiol 23: 1038–1040, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia 54: 758–773, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277: 10482–10488, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Suadicani SO, De Pina-Benabou MH, Urban-Maldonado M, Spray DC, Scemes E. Acute downregulation of Cx43 alters P2Y receptor expression levels in mouse spinal cord astrocytes. Glia 42: 160–171, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suadicani SO, Vink MJ, Spray DC. Slow intercellular Ca2+ signaling in wild-type and Cx43-null neonatal mouse cardiac myocytes. Am J Physiol Heart Circ Physiol 279: H3076–H3088, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322: 1555–1559, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Thompson RJ, Macvicar BA. Connexin and pannexin hemichannels of neurons and astrocytes. Channels (Austin) 2008 Mar 29; 2, 2008. [DOI] [PubMed]
- 65.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science 312: 924–927, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci USA 93: 5836–5841, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol 166: 6383–6391, 2001. [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol 293: C1112–C1119, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, Chanson M, Goodenough DA, Kwak BR. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med 12: 950–954, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Yao J, Suwa M, Li B, Kawamura K, Morioka T, Oite T. ATP-dependent mechanism for coordination of intercellular Ca2+ signaling and renin secretion in rat juxtaglomerular cells. Circ Res 93: 338–345, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 146: 9–16, 2007. [DOI] [PubMed] [Google Scholar]