Abstract
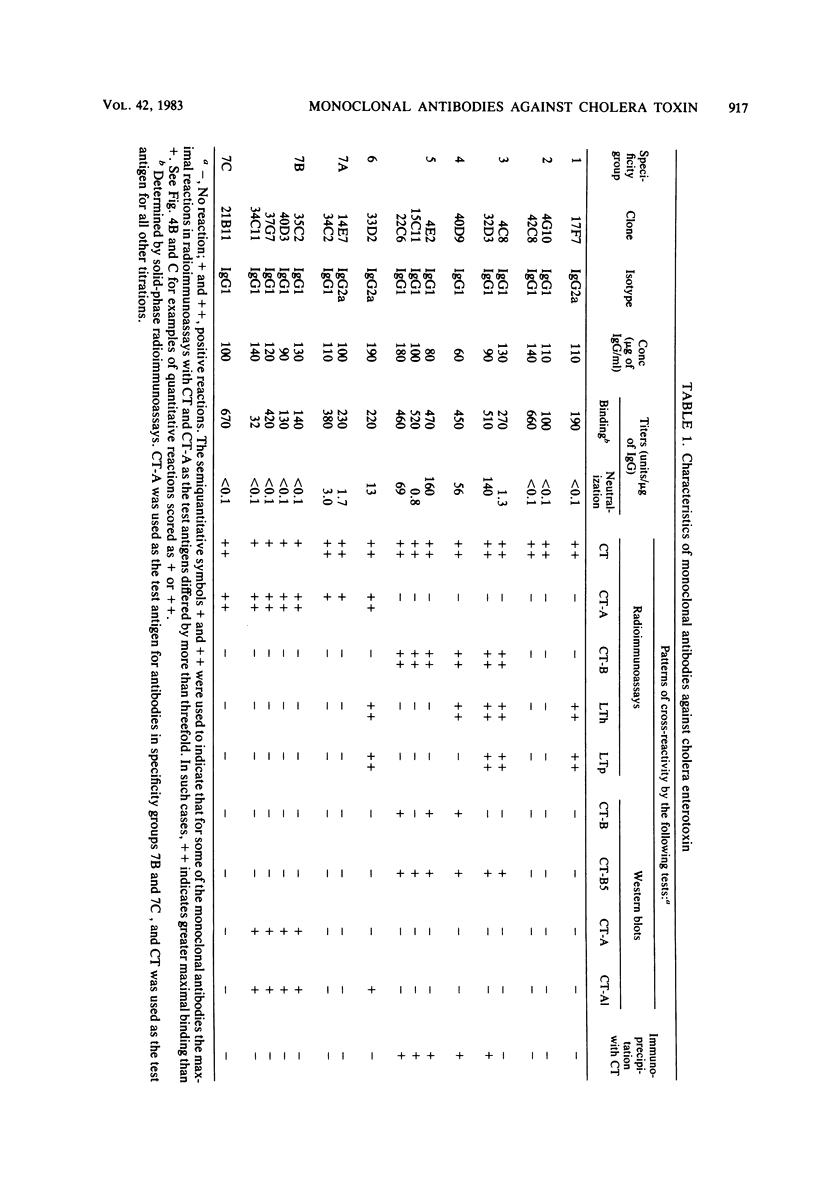
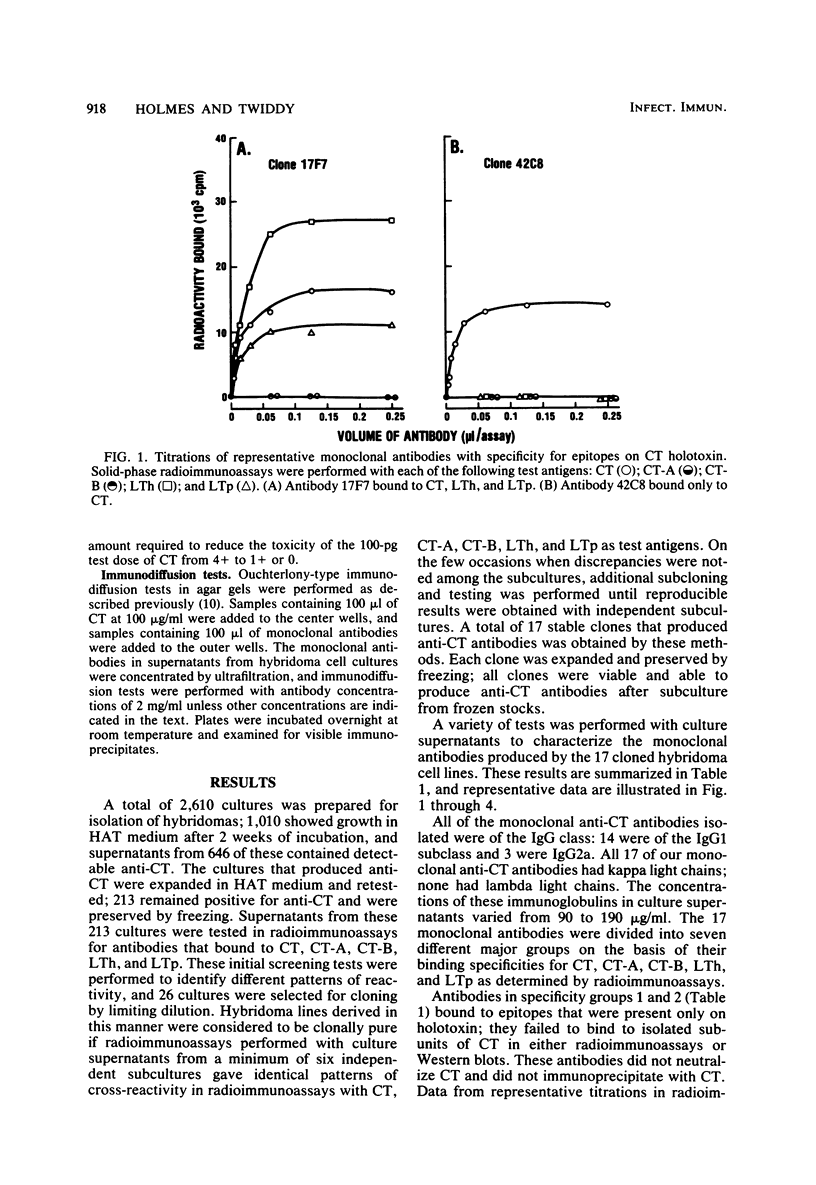
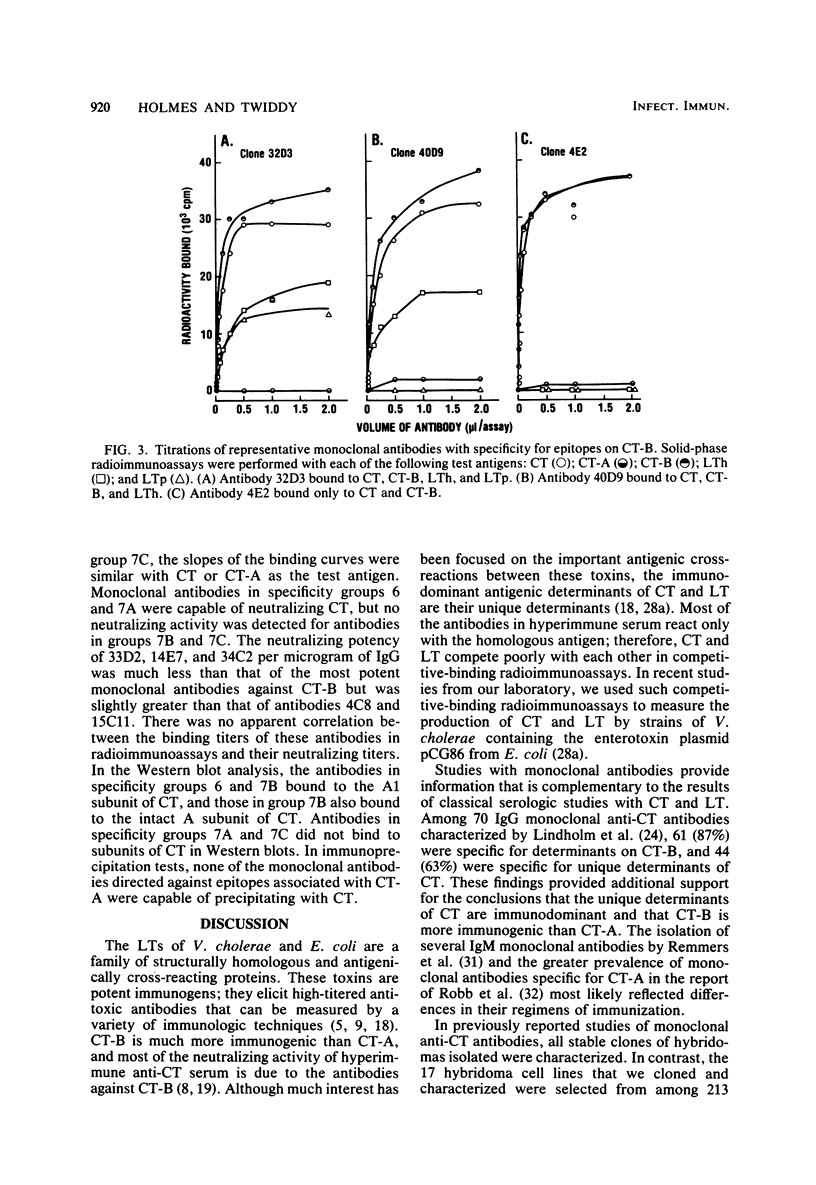
Seventeen selected hybridoma cell lines that produced monoclonal antibodies against cholera enterotoxin (CT) were isolated and characterized. All of the monoclonal antibodies contained the kappa light chain; 14 were of the immunoglobulin G1 (IgG1) isotype and 3 were IgG2a. The 17 monoclonal antibodies were divided into a minimum of seven different specificity groups based on their abilities to bind to the following purified test antigens in solid-phase radioimmunoassays: CT, the A and B polypeptides of CT (CT-A and CT-B, respectively), and the heat-labile enterotoxins designated LTh and LTp from Escherichia coli. The binding of these antibodies to the following subunits and fragments of CT was also determined in Western blots: pentameric CT-B, monomeric CT-B, intact CT-A, and the A1 fragment of CT-A. Each of the monoclonal antibodies was tested for neutralization of CT and for precipitation with CT in immunodiffusion tests. Antigenic determinants were identified on CT that were not present either on CT-A or CT-B. One class was unique for CT and another was shared with LTh and LTp. Antibodies directed against these holotoxin-specific determinants had no neutralizing activity. Most of the monoclonal antibodies that reacted strongly with CT-A or CT-B also reacted strongly with CT holotoxin; however, one class of antibody reacted strongly with CT-A but weakly with CT. Among the monoclonal antibodies against CT-A or CT-B, some were specific for CT and others cross-reacted with LTh and LTp or with LTh only. The most potent neutralizing antibodies were against CT-B, and all of our monoclonal antibodies against CT-B had some neutralizing activity. In contrast, only some of the monoclonal antibodies against CT-A had neutralizing activity, and their specific activities were low. We found no direct correlation between the ability of monoclonal antibodies to neutralize CT and to cross-react with LTh or LTp. None of the epitopes recognized by our monoclonal anti-CT antibodies was present on CT-A and CT-B.
Full text
PDF



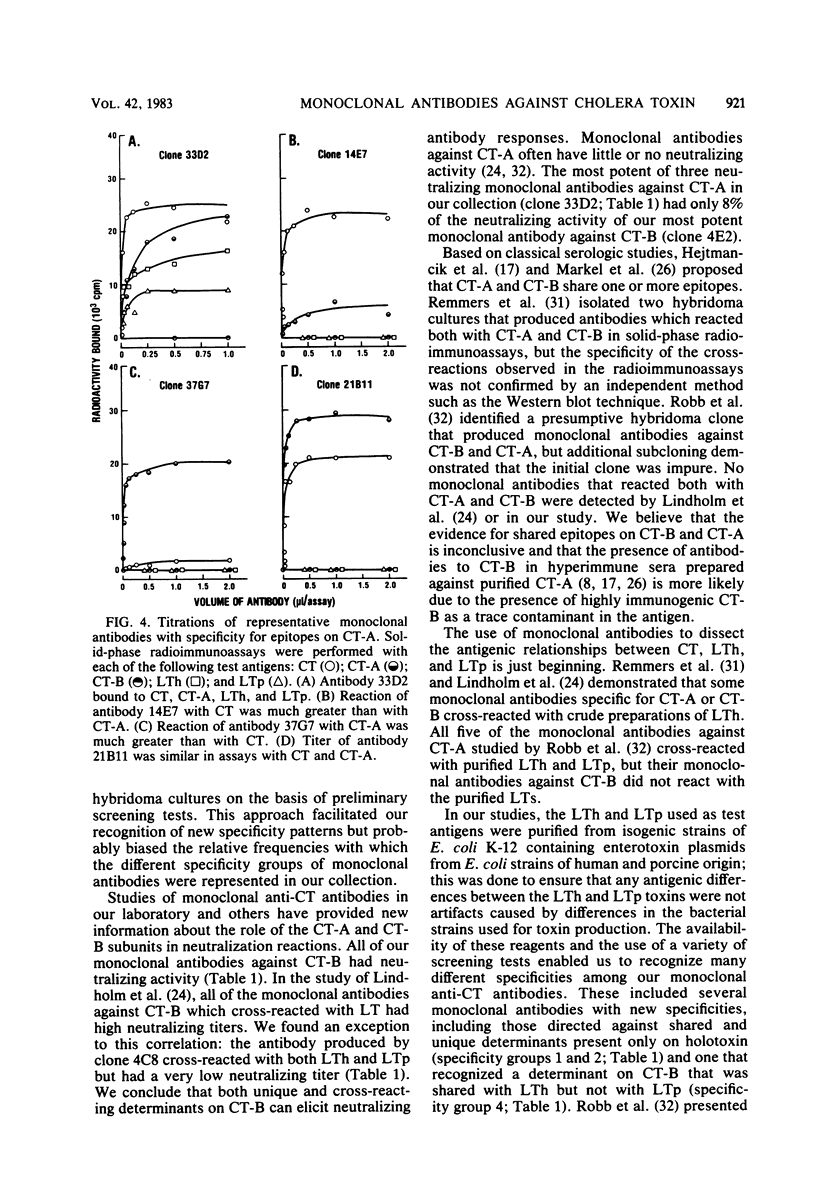


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohn W. A fixation method for improved antibody penetration in electron microscopical immunoperoxidase studies. J Histochem Cytochem. 1978 Apr;26(4):293–297. doi: 10.1177/26.4.77869. [DOI] [PubMed] [Google Scholar]
- Bramucci M. G., Twiddy E. M., Baine W. B., Holmes R. K. Isolation and characterization of hypertoxinogenic (htx) mutants of Escherichia coli KL320(pCG86). Infect Immun. 1981 Jun;32(3):1034–1044. doi: 10.1128/iai.32.3.1034-1044.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Flint D. C., Klipstein F. A. Immunological and physicochemical characterization of heat-labile enterotoxins isolated from two strains of Escherichia coli. Infect Immun. 1982 Nov;38(2):806–809. doi: 10.1128/iai.38.2.806-809.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Yancey R. J., Finkelstein R. A. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980 Jul;29(1):91–97. doi: 10.1128/iai.29.1.91-97.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Geary S. J., Marchlewicz B. A., Finkelstein R. A. Comparison of heat-labile enterotoxins from porcine and human strains of Escherichia coli. Infect Immun. 1982 Apr;36(1):215–220. doi: 10.1128/iai.36.1.215-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A. Pathogenesis of acute bacterial diarrheal disorders. Annu Rev Med. 1981;32:341–357. doi: 10.1146/annurev.me.32.020181.002013. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Green B. A., Neill R. J., Ruyechan W. T., Holmes R. K. Evidence that a new enterotoxin of Escherichia coli which activates adenylate cyclase in eucaryotic target cells is not plasmid mediated. Infect Immun. 1983 Jul;41(1):383–390. doi: 10.1128/iai.41.1.383-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik K. E., Peterson J. W., Markel D. E., Kurosky A. Radioimmunoassay for the antigenic determinants of cholera toxin and its components. Infect Immun. 1977 Sep;17(3):621–628. doi: 10.1128/iai.17.3.621-628.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Mechanisms of disease and immunity in cholera: a review. J Infect Dis. 1977 Aug;136 (Suppl):S105–S112. doi: 10.1093/infdis/136.supplement.s105. [DOI] [PubMed] [Google Scholar]
- Honda T., Tsuji T., Takeda Y., Miwatani T. Immunological nonidentity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1981 Nov;34(2):337–340. doi: 10.1128/iai.34.2.337-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Robertson D. C. Purification and chemical characterization of the heat-labile enterotoxin produced by enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):586–596. doi: 10.1128/iai.25.2.586-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindholm L., Holmgren J., Wikström M., Karlsson U., Andersson K., Lycke N. Monoclonal antibodies to cholera toxin with special reference to cross-reactions with Escherichia coli heat-labile enterotoxin. Infect Immun. 1983 May;40(2):570–576. doi: 10.1128/iai.40.2.570-576.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markel D. E., Hejtmancik K. E., Peterson J. W., Martin F. B., Kurosky A. Characterization of the antigenic determinants of cholera toxin subunits. Infect Immun. 1979 Aug;25(2):615–626. doi: 10.1128/iai.25.2.615-626.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Purification of cholera toxin and its subunits: new methods of preparation and the use of hypertoxinogenic mutants. Infect Immun. 1978 May;20(2):552–558. doi: 10.1128/iai.20.2.552-558.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill R. J., Ivins B. E., Holmes R. K. Synthesis and secretion of the plasmid-coded heat-labile enterotoxin of Escherichia coli in Vibrio cholerae. Science. 1983 Jul 15;221(4607):289–291. doi: 10.1126/science.6857285. [DOI] [PubMed] [Google Scholar]
- Neill R. J., Twiddy E. M., Holmes R. K. Synthesis of plasmid-coded heat-labile enterotoxin in wild-type and hypertoxinogenic strains of Escherichia coli and in other genera of Enterobacteriaceae. Infect Immun. 1983 Sep;41(3):1056–1061. doi: 10.1128/iai.41.3.1056-1061.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Mekalanos J. J. Molecular cloning of Vibrio cholerae enterotoxin genes in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 May;79(9):2976–2980. doi: 10.1073/pnas.79.9.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers E. F., Colwell R. R., Goldsby R. A. Production and characterization of monoclonal antibodies to cholera toxin. Infect Immun. 1982 Jul;37(1):70–76. doi: 10.1128/iai.37.1.70-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb M., Nichols J. C., Whoriskey S. K., Murphy J. R. Isolation of hybridoma cell lines and characterization of monoclonal antibodies against cholera enterotoxin and its subunits. Infect Immun. 1982 Oct;38(1):267–272. doi: 10.1128/iai.38.1.267-272.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Gyles C. L. The relationship between two apparently different enterotoxins produced by enteropathogenic strains of Escherichia coli of porcine origin. J Med Microbiol. 1970 Aug;3(3):387–401. doi: 10.1099/00222615-3-3-387. [DOI] [PubMed] [Google Scholar]
- So M., Dallas W. S., Falkow S. Characterization of an Escherichia coli plasmid encoding for synthesis of heat-labile toxin: molecular cloning of the toxin determinant. Infect Immun. 1978 Aug;21(2):405–411. doi: 10.1128/iai.21.2.405-411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E. K., Noble J. A. Escherichia coli heat-labile enterotoxin. Nucleotide sequence of the A subunit gene. J Biol Chem. 1982 May 25;257(10):5716–5721. [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Cloning of deoxyribonucleic acid regions encoding a heat-labile and heat-stable enterotoxin originating from an enterotoxigenic Escherichia coli strain of human origin. J Bacteriol. 1980 Aug;143(2):652–660. doi: 10.1128/jb.143.2.652-660.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]