Abstract
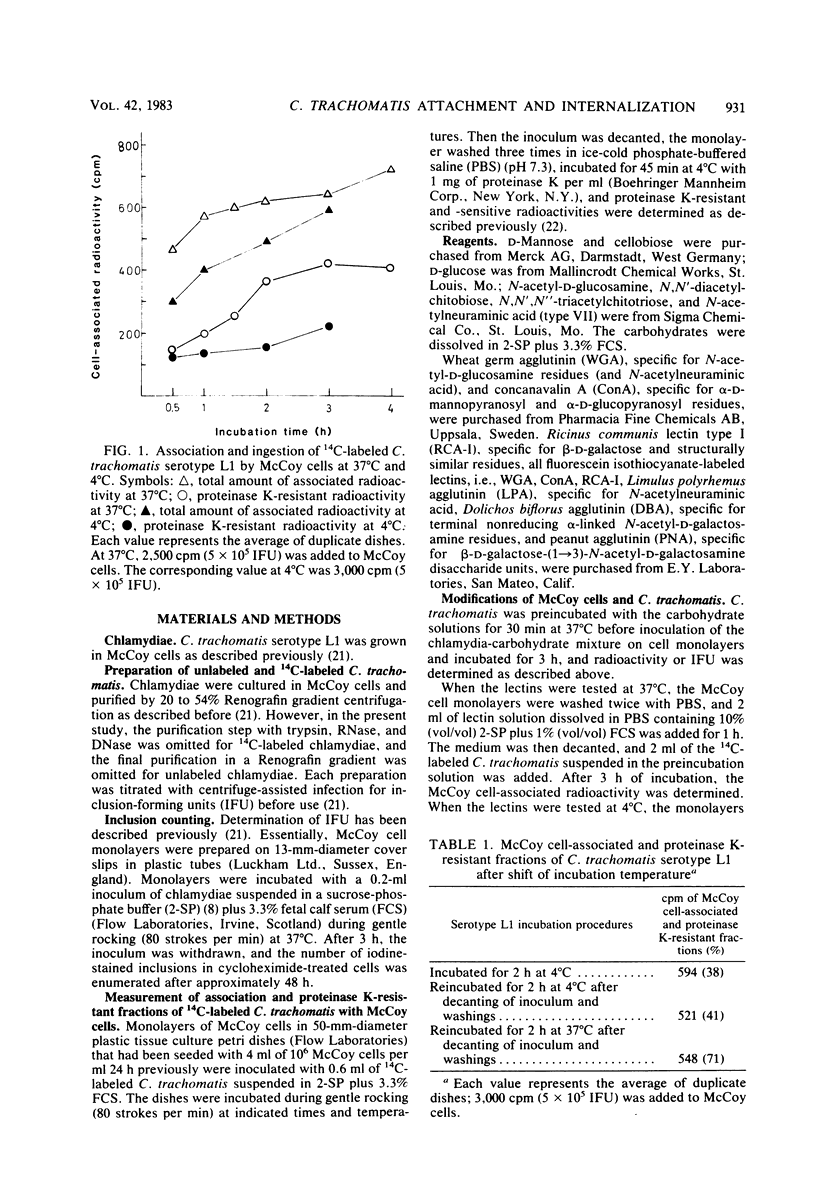
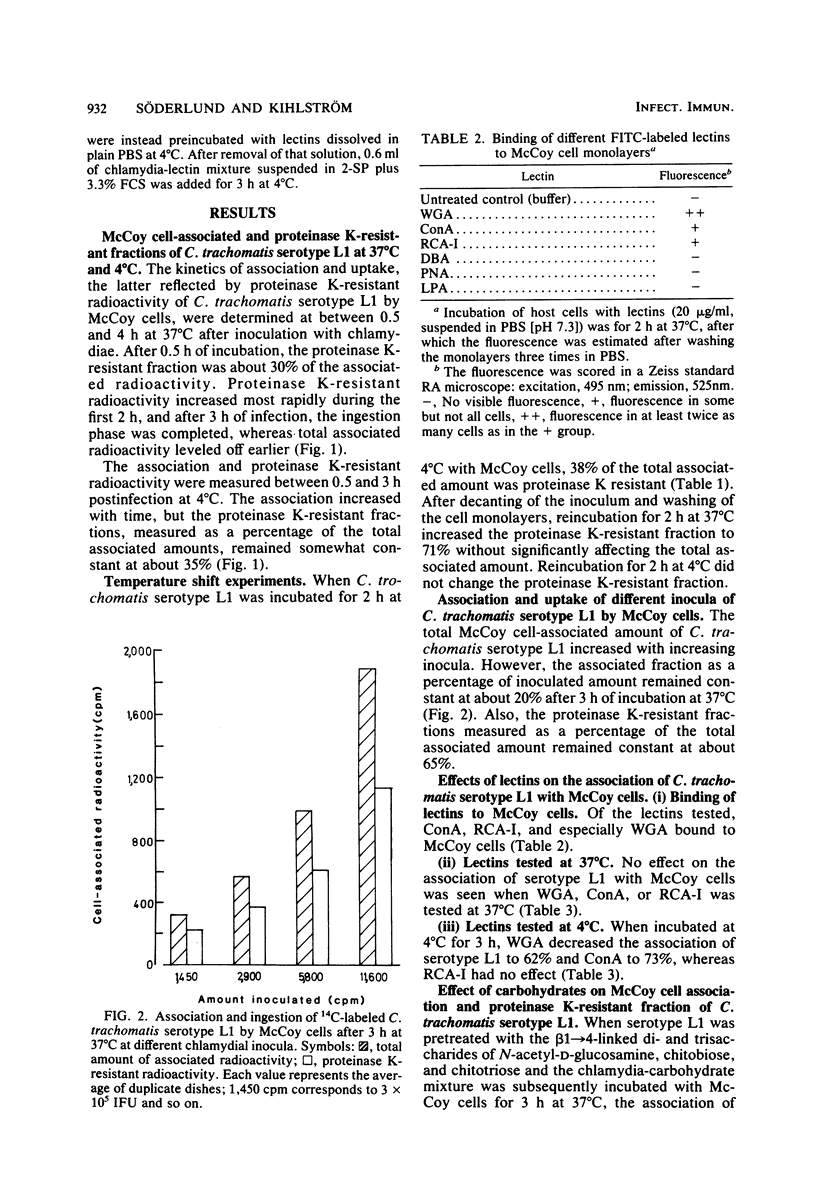
The kinetics of attachment and ingestion of Chlamydia trachomatis serotype L1 by monolayers of McCoy cells were studied by using a method that discriminated between attachment and uptake. When about 1% of the McCoy cells was infected, the proteinase K-resistant chlamydial fraction, regarded as ingested chlamydiae, reached a constant value after about 3 h of incubation at 37 degrees C. Uptake of chlamydiae at 4 degrees C could not be demonstrated. The attached and ingested chlamydial fractions were constant over an eightfold increase in chlamydial inoculum. Chitobiose and chitotriose, the di- and trisaccharides of N-acetyl-D-glucosamine, reduced the association of C. trachomatis serotype L1 with McCoy cells. Higher concentrations of chitobiose also selectively inhibited ingestion of chlamydiae. A corresponding effect of chitobiose was also observed on the number of chlamydial inclusions. Wheat germ agglutinin, specific for N-acetyl-D-glucosamine residues, reduced the association of chlamydiae when incubated at 4 degrees C, but not at 37 degrees C. A small inhibiting effect of concanavalin A on association of chlamydiae, but no effect of the corresponding carbohydrates, indicates a nonspecific effect on chlamydial attachment of this lectin. These results suggest that beta 1 leads to 4-linked oligomers of N-acetyl-D-glucosamine are important in the specificity of attachment of C. trachomatis to McCoy cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Bose S. K., Paul R. G. Purification of Chlamydia trachomatis lymphogranuloma venereum elementary bodies and their interaction with HeLa cells. J Gen Microbiol. 1982 Jun;128(6):1371–1379. doi: 10.1099/00221287-128-6-1371. [DOI] [PubMed] [Google Scholar]
- Bose S. K., Smith G. B., Paul R. G. Influence of lectins, hexoses, and neuraminidase on the association of purified elementary bodies of Chlamydia trachomatis UW-31 with HeLa cells. Infect Immun. 1983 Jun;40(3):1060–1067. doi: 10.1128/iai.40.3.1060-1067.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Kinetics of phagocytosis of Chlamydia psittaci by mouse fibroblasts (L cells): separation of the attachment and ingestion stages. Infect Immun. 1978 Feb;19(2):607–612. doi: 10.1128/iai.19.2.607-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Gregory W. W., Byrne G. I., Gardner M., Moulder J. W. Cytochalasin B does not inhibit ingestion of Chlamydia psittaci by mouse fibroblasts (L cells) and mouse peritoneal macrophages. Infect Immun. 1979 Jul;25(1):463–466. doi: 10.1128/iai.25.1.463-466.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Effect of polycations, polyanions and neuraminidase on the infectivity of trachoma-inclusin conjunctivitis and lymphogranuloma venereum organisms HeLa cells: sialic acid residues as possible receptors for trachoma-inclusion conjunction. Infect Immun. 1973 Jul;8(1):74–79. doi: 10.1128/iai.8.1.74-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Grayston J. T. Differentiation of TRIC and LGV organisms based on enhancement of infectivity by DEAE-dextran in cell culture. J Infect Dis. 1972 Mar;125(3):313–317. doi: 10.1093/infdis/125.3.313. [DOI] [PubMed] [Google Scholar]
- Lee C. K. Interaction between a trachoma strain of Chlamydia trachomatis and mouse fibroblasts (McCoy cells) in the absence of centrifugation. Infect Immun. 1981 Feb;31(2):584–591. doi: 10.1128/iai.31.2.584-591.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. J. Wheat germ agglutinin blockage of chlamydial attachment sites: antagonism by N-acetyl-D-glucosamine. Infect Immun. 1979 Sep;25(3):946–953. doi: 10.1128/iai.25.3.946-953.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUI Y., KAJIMA M., NISHIMURA A., KONISHI K. Morphology and developmental cycle of the trachoma agent. Morphology of trachoma agent in conjunctiva and chick embryo. Ann N Y Acad Sci. 1962 Mar 5;98:131–144. doi: 10.1111/j.1749-6632.1962.tb30538.x. [DOI] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Effect of methylamine and monodansylcadaverine on the susceptibility of McCoy cells to Chlamydia trachomatis infection. Infect Immun. 1983 May;40(2):534–541. doi: 10.1128/iai.40.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Physicochemical surface properties of elementary bodies from different serotypes of chlamydia trachomatis and their interaction with mouse fibroblasts. Infect Immun. 1982 Jun;36(3):893–899. doi: 10.1128/iai.36.3.893-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Dobberstein B. Protein transfer across microsomal membranes reassembled from separated membrane components. Nature. 1978 Jun 15;273(5663):569–571. doi: 10.1038/273569a0. [DOI] [PubMed] [Google Scholar]


