Abstract
Non-nutrient-dependent salt absorption across the brush-border membrane of intestinal epithelial cells is primarily mediated by coupled apical Na+/H+ (aNHE) and anion exchange transport, with the latter suspected of being mediated by DRA (downregulated in adenoma; SLC26A3) that is defective in congenital chloridorrhea. To investigate DRA in greater detail and determine whether DRA and NHE activities can be coupled, we measured 22Na+ and 36Cl− uptake in Caco2BBE colon cells infected with the tet-off-inducible DRA transgene. Under basal conditions, DRA activity was low in normal and infected Caco2BBE cells in the presence of tetracycline, whereas NHE activities could be easily detected. When apical NHE activity was increased by transfection or serum-induced expression of the aNHE isoforms NHE2 and NHE3, increased 36Cl− uptake was observed. Inhibition of DRA activity by niflumic acid was greater than that by DIDS as well as by the NHE inhibitor dimethylamiloride and the carbonic anhydrase inhibitor methazolamide. DRA activity was largely aNHE-dependent, whereas a component of DRA-independent aNHE uptake continued to be observed. Coupled aNHE and DRA activities were inhibited by increased cellular cAMP and calcium and were associated with synaptotagmin I-dependent, clathrin-mediated endocytosis. In summary, these data support the role of DRA in electroneutral NaCl absorption involving functional coupling of Cl−/base exchange and apical NHE.
Keywords: anion exchange, electroneutral NaCl transport, intestinal transport, endocytosis, diarrheal diseases, sodium absorption, congenital chloridorrhea, SLC26A3, SLC26, synaptotagmin, clathrin, membrane biotinylation
absorption of sodium and chloride by intestinal epithelial cells occurs through a number of pathways and varies along the longitudinal axis of the intestinal tract (15). In the small intestine, one of the major pathways for Na+ absorption is Na+/H+ exchange (NHE). At least nine NHE isoforms exist, and two of these, NHE2 and NHE3, are expressed as intestinal brush-border membrane proteins (7, 22, 44). In a canine model, the participation of these two pathways was elucidated through the use of amiloride analogs, demonstrating that NHE3 appears to be the pathway with higher capacity (45). The greater role for NHE3 as the apical intestinal Na+ transporter is also supported by the NHE3 and NHE2 knockout mice, which demonstrate that NHE3 contributes a much greater percentage of the mucosal-to-serosal Na+ flux (17, 18). Both clinical and experimental evidence show that most non-nutrient-dependent electroneutral NaCl absorption in the gastrointestinal (GI) tract is mediated by NHE activity coupled with apical Cl−/base exchange (6, 14, 27, 40, 42). This coupling has been hypothesized to involve brush-border membrane carbonic anhydrase activity, which may provide H+ and HCO3− for exchange with Na+ and Cl−, respectively (20), but also may assist trafficking of apical Na+ and Cl− transporters (8, 9).
Although the information about apical NHEs is more complete, the identity of the anion exchangers coupled to apical NHEs is less clear. Members of two anion exchanger gene families have been proposed to mediate brush-border anion exchange, SLC4 and SLC26. The erythrocyte anion exchanger (SLC4A1, also called band 3) has been found in intestinal (colonic) mucosa (9, 36). In addition, the related exchangers AE2 (SLC4A2) and AE3 (SLC4A3) also have been detected in the intestine (1, 2, 11). The localization of the AE2 protein is not clearly established, with some reports indicating an apical, and others a basolateral, location (2, 11). The SLC26 anion transporter family includes at least 11 genes; however, the most likely candidates for intestinal apical anion exchange are DRA (SLC26A3) and PAT-1 (SLC26A6). DRA was originally identified as a gene that is downregulated in colonic adenomas and adenocarcinomas (38). PAT-1 was discovered through a database search for SLC26 gene family transporters (29). DRA and PAT-1 are expressed in many types of epithelial cells (3, 16, 30, 32, 41, 43) and may transport a number of anions, including Cl−, HCO3−, SO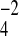 , oxalate, and formate (10, 16, 25, 26, 28, 43). Of the many candidates, DRA is most likely to be a major mediator for electroneutral Cl− absorption, because mutations of this protein underlie congenital chloridorrhea (21, 32, 33) and a similar condition develops in DRA knockout mice (39). PAT-1-deficient mice, on the other hand, have not been reported to demonstrate a Cl−-rich watery diarrhea (43), suggesting that it is less important in mediating bulk intestinal Cl− absorption.
, oxalate, and formate (10, 16, 25, 26, 28, 43). Of the many candidates, DRA is most likely to be a major mediator for electroneutral Cl− absorption, because mutations of this protein underlie congenital chloridorrhea (21, 32, 33) and a similar condition develops in DRA knockout mice (39). PAT-1-deficient mice, on the other hand, have not been reported to demonstrate a Cl−-rich watery diarrhea (43), suggesting that it is less important in mediating bulk intestinal Cl− absorption.
In the present study, we have shown that DRA, expressed in human Caco2BBE colon cells, significantly increases Cl− uptake that is dependent on both carbonic anhydrase activity and apical NHE activity. The regulation of expressed DRA and NHE activity is analogous to that seen for electroneutral NaCl absorption in normal intestinal epithelium. Thus these data strongly suggest that apical NHE and DRA, when coexpressed, mediate coupled electroneutral NaCl absorption in the gut.
MATERIALS AND METHODS
Cell culture.
The Caco2BBE derivative of the Caco2 cell line was a gift of Dr. M. Mooseker (Yale University, New Haven, CT) and was selected based on its relatively differentiated, highly polarized state, well-developed microvillus membrane, villus-like phenotype, and ability to form tight junctions as confluent monolayers (35). Cells were used between passages 48 and 65. Caco2BBE cells express a small amount of NHE2 and NHE3 activity and protein under normal culture conditions but express more if cultured with 30% (vol/vol) fetal bovine serum (37).
DRA adenoviral construction and infection.
Full-length SLC26A3 (GenBank accession no. NM_000111), or DRA, was blunt ended and cloned into the pTre-Shuttle vector (Clontech, Palo Alto, CA), excised in a SceI-CeuI cassette, and cloned into the TRE (tetracycline response element)-regulated adenovirus. PCR analysis confirmed the orientation and sequence, and human embryonic kidney (HEK-293) cells were transformed with this plasmid by using the polyamine-derived LT-1 reagent. HEK-293 cells were obtained from Clontech and used within four passages to produce viral stocks. HEK cells were plated at 20% confluence and then transfected 1 day later when cells were 60–70% confluent. Cells were kept alive for 3–5 additional days whenever the appearance of the cells had changed (rounded but still attached to the plastic culture dishes) due to viral production. At the point where a majority of the cells were well rounded, the medium was carefully removed and the cells were scraped and lysed in phosphate-buffered saline. To concentrate the virus, the viral homogenate was placed in an 11-ml polyallomer tube on top of a solution of cesium chloride. The viral samples were spun at 37,000 rpm for 18 hr at 20°C, and a band of virus appeared as a cloudy layer in the tube. The band was pulled in a volume <1 ml, and the cesium was removed by dialysis against saline at 4°C. Virus was frozen in aliquots at −80°C. To maximize viral infection of Caco2BBE cells, we employed a calcium-switch procedure, since the Coxsackie adenoviral receptor is part of the tight junctions (5, 13). Tight junctions were transiently opened by brief removal of magnesium and calcium by addition of EDTA, enhancing the efficiency of adenoviral infection. Caco2BBE cells grown on collagen-coated permeable filters were incubated on both apical and basolateral sides in a solution of 140 mM NaCl, 5 mM KCl, 2 mM EDTA, 2 mM NaH2PO4, 25 mM NaHCO3, pH 7.4, and 5 mM glucose with appropriate dilutions of viral stocks for 15 min at 37°C. Complete medium was then added, and monolayers were generally used 2 days later. Different dilutions of the DRA-encoding adenovirus were added to Caco2BBE cells, starting at 1:100 and diluting to as much as 1:300,000. The Caco2BBE cells were observed for 48 h for cytopathic changes associated with viral infection, such as rounding and lifting. A viral dilution was selected for experiments that did not cause rounding and lifting and was slightly lower than the lowest dilution that caused these effects, generally 1:3,000. The tetracycline (tet)-operator virus was propagated and packaged in HEK cells, and a 1:10,000 dilution of this viral stock was used. To keep the adenovirus turned off after infection, we included 10 μg/ml tetracycline in the medium. After 2 days, the medium was changed, and monolayers were incubated for 10 min each in two incubations of medium without tetracycline and then either incubated in medium without tetracycline to turn on adenoviral DRA expression or left with tetracycline to keep the virally encoded DRA turned off. Cells were used 2 days later for flux measurement or harvested for protein analysis by Western blotting.
Influx measurements.
Unidirectional apical medium-to-cell uptakes (influxes) were performed under non-acid-loaded conditions, as previously described (31). This was done to avoid the use of artificial pH gradients that can potentially confound measurements of physiological relevance. Briefly, cells were grown on Transwells and treated as appropriate for each set of experiments. Immediately before flux, Transwells were washed once at room temperature with isotonic choline-Cl solution (composition in mM: 5 KCl, 10 HEPES, pH 7.4, 1 MgCl2, 2 CaCl2, and 150 choline-Cl) by dipping wells into buffer and immediately removing. Transwells were placed on top of a 2-ml room temperature flux solution without radioactivity, [composition in mM: 20 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, and 10 HEPES, pH 7.4, or when designated, 10 mM MES, pH 5.5, 30 choline Cl, an 100 N-methyl-d-glucamine (NMDG)-gluconate]. One milliliter of room temperature flux solution containing 1 μCi/ml 22NaCl or H36Cl was then placed on the apical (top) side of the Transwell. The concentrations of Na+ and Cl− were chosen so that both should be above the Km value. In previous studies we have demonstrated that the Km values of NHE2 and NHE3 to mediate apical 22Na+ fluxes are around 12–14 mM (31). The concentration of Cl− used was chosen on the basis of studies of DRA expressed in heterologous cell systems as well as intestinal brush-border vesicle uptake studies (10, 25, 26, 28). After 10 min, the flux solution was removed and the Transwell was washed rapidly (<7 s) in ice-cold saline (composition in mM: 5 KCl, 150 NaCl, and 10 HEPES, pH 7.4) four times. Transwells were placed upside down and allowed to air dry for 10–30 min. Filters containing the cells were cut from the support and placed into vials with scintillation fluid (Budget Solve; RPI, Mt. Prospect, IL). Samples were solubilized overnight, and 22Na+ or 36Cl− was quantified by liquid scintillation spectroscopy. When appropriate, inhibitors were added simultaneously with flux solutions except for inhibition of carbonic anhydrase, when methazolamide (0.2 mM) was added 2 h before flux and kept in the influx solution. For studies on the role of basolateral HCO3−, the basolateral flux solution contained 20 mM NaHCO3, 5 mM KHCO3, 1 MgCl2, 2 CaCl2, 10 HEPES, pH 7.4, 30 mM choline Cl, 25 mM NMDG-Cl, and 75 mM NMDG-gluconate to maintain the concentrations of 20 mM Na+ and 58 mM Cl− used in the standard apical flux buffer in the present studies.
Analysis of DRA and apical NHE protein expression.
For Caco2BBE on Transwells, cells were scraped off the wells after a treatment if appropriate and pelleted in ice-cold saline (14,000 g for 30 s at 4°C). Cell pellets were homogenized in lysis buffer [composition: 10 mM Tris, pH 7.1, and 5 mM MgCl2 with 50 U/ml DNase and RNase and the Complete protease inhibitor cocktail (Roche Molecular Biochemicals, Indianapolis, IN)]. An aliquot was removed for protein determination using the bicinchoninic acid procedure (Pierce Chemical, Rockford, IL). Laemmli stop solution [composition: 1% (wt/vol) SDS, 11 mM Tris, pH 6.8, 16% (vol/vol) glycerol, 3% (vol/vol) 2-mercaptoethanol, and 3 mg/ml bromphenol blue] was added to the remainder of the sample and heated to 65°C for 10 min. Cellular protein (10 μg) was resolved on 12.5% SDS-PAGE and immediately transferred to polyvinylidene difluoride membranes (Polyscreen PVDF; Perkin Elmer Biosciences, Boston, MA) in 1× Towbin's buffer [composition: 25 mM Tris and 192 mM glycine, pH 8.8, with 15% (vol/vol) methanol]. Blots were blocked for 1 h in 5% (wt/vol) nonfat dry milk (Carnation, Solon, OH) in Tris-buffered saline with Tween 20 [T-TBS; composition in mM: 150 NaCl, 5 KCl, and 10 Tris, pH 7.4, with 0.05% (vol/vol) Tween 20]. Membranes were incubated overnight with primary antibodies in T-TBS, washed five times (10 min at room temperature), incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoBiologicals, West Grove, PA) in T-TBS for 1 h at room temperature, and washed four times (10 min each) with T-TBS, with a final wash (10 min) in TBS. Blots were visualized using an enhanced chemiluminescence system (SuperSignal; Pierce Biochemical, Rockford, IL).
DRA, NHE2, or NHE3 present on the luminal membrane of confluent cell monolayers grown on Transwells or mouse small intestine were labeled using surface biotinylation. Caco2BBE monolayers or loops of mouse small intestinal (10 cm filled with serum-free DMEM) were stimulated with 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate (8-CPT-cAMP; 100 μM, 15 min) or thapsigargin (100 ng/ml, 15 min). Transwells or ligated loops of mouse intestine were washed once and then placed in ice-cold HEPES-buffered saline (HBS; composition in mM: 150 NaCl, 4 KCl, and 10 HEPES, pH 7.4). Proteins in the apical membrane were labeled using the cell-impermeant biotin Sulfo-NHS-biotin (1 mg/ml; Pierce Chemical) for 30 min in the cold. Biotinylation was terminated by the addition of 1 M Tris (1:100 dilution), a free amine that reacts with the free biotin and was added to the apical medium of the Transwell or the intestinal loop opened and placed in HBS with the added Tris. From the intestinal loops, a segment of 5 cm was scraped off using glass slides. A mucosal homogenate was made in 5 ml of lysis buffer (10 mM HEPES, pH 7.4, and 2 mM EDTA, with the Complete protease inhibitor cocktail) and homogenized for 20 strokes with a Teflon pestle homogenizer. Intact cells, nuclei, and mitochondria were removed by centrifugation (10,000 g at 4°C for 10 min), and the microsomal membrane was pelleted from the supernatant (100,000 g at 4°C for 40 min). Membrane pellets were resuspended in 500 μl of RIPA immunoprecipitation buffer [composition in mM: 150 NaCl, 2 EDTA, 0.1% (wt/vol) SDS, 0.5% (wt/vol) Na-deoxycholate, and 1% (vol/vol) Triton X-100]. Twenty microliters were removed to determine protein concentration. Five hundred micrograms of protein were diluted to 450 μl in RIPA buffer, and 50 μl of a 50% (wt/vol) slurry of immobilized streptavidin (Pierce Chemical) were added and rotated in the cold for 120 min. The beads were pelleted (14,000 g for 10 s at 4°C) and washed five times with RIPA buffer. Samples were eluted from the beads by addition of 50 μl Laemmli stop buffer and heating to 65°C. Samples were immediately analyzed by Western blotting for DRA, NHE2, or NHE3 with polyclonal antisera developed in our laboratory using the Western blotting protocol described above. For Caco2BBE surface biotinylation, a sample of the total cell homogenate equivalent to 500 μg of protein was used. This aliquot was diluted into RIPA buffer, and biotinylated proteins were isolated and analyzed for DRA, NHE2, and NHE3 protein expression as described for mouse intestinal samples.
Small interfering RNA silencing of synaptotagmin I, adaptor protein 2 μ-subunit, and the heavy chain of clathrin in Caco2BBE cells.
Stealth oligonucleotides specific for human synaptotagmin I (Syt I; GenBank NM_005639, bases 613–637), the μ-subunit of adaptor protein 2 (AP2μ; NM_004068, bases 79–103), or the heavy chain of clathrin (NM_004859, bases 3307–3331), or altered base controls for each, were used to silence these proteins involved in apical membrane protein trafficking as previously described (34). Two treatments of Stealth oligos were used, with the last at 12 h before experiments. The level of knockdown was confirmed by immunoblotting of total cell lysates. Monolayers were stimulated by 8-CPT-cAMP or thapsigargin and harvested after 15 min. Cell homogenates were prepared as described above, and an aliquot of 500 μg of protein was diluted in immunoprecipitation buffer with only Triton X-100 as detergent to preserve protein interactions [composition in mM: 150 NaCl, 10 HEPES, pH 7.4, 2 EDTA, 1 PMSF, 0.1 vanadate, 1 mM NaF, and 1% (vol/vol) Triton X-100, with the Complete protease inhibitor cocktail]. Immunoprecipitation samples were cleaned using Pansorbin cells and then incubated with antibodies to Syt I (rabbit polyclonal; Stressgen, Victoria, BC, Canada), the α-subunits of AP2 (goat polyclonal; Rockland Immunologics, Gaithersburg, MD), or heavy chain clathrin (Transduction Labs/Pharmingen, Lexington, KY) that were conjugated to agarose beads using the Seize primary kit (Pierce Chemical). After an overnight incubation, reactions were washed four times with immunoprecipitation buffer, and then samples were eluted from the beads with IgG elution buffer provided with the Seize kit. Samples were mixed with Laemmli stop solution, heated, and analyzed by SDS-PAGE, and Western blots were generated and analyzed for DRA, Syt I, AP2μ, or the heavy chain of clathrin.
RESULTS
Infection with virus containing DRA increases apical Na+ and Cl− influxes.
To investigate which anion exchangers are expressed in the human colonic Caco2BBE cell line, we measured mRNA, protein, and 36Cl− uptake activities. RNA was extracted from cells grown in 10 and 30% (vol/vol) FBS and reverse transcribed, and primers specific to SLC26A3 (DRA; NM_000111, bases 1157–1319), SLC26A4 (pendrin; NM_000441, bases 1962–2127), and SLC26A6 (PAT-1; AF279265, bases 1218–1379) were analyzed using real-time PCR. Caco2BBE cells express mRNA for both DRA (A3) and PAT-1 (A6), but not for pendrin (Fig. 1A). To confirm that the primers amplified the correct product, PCR products were sequenced and confirmed to be correct. Neither DRA nor PAT-1 expression was affected by increased serum concentration (Fig. 1A). To determine whether protein expression corresponded to the mRNA, we used Western blot analysis. Caco2BBE cells express DRA protein that does not increase when serum is increased. Using a commercially available anti-SLC26A6 antibody (Novus, Boulder, CO), we could detect no SLC26A6 protein in Caco2BBE cells (data not shown). The lack of PAT-1 and presence of DRA were supported by using apical 36Cl− uptakes and determining the sensitivity to the stilbene DIDS. Whereas PAT-1 is well inhibited by micromolar concentrations of DIDS, DRA requires higher concentrations and may not demonstrate complete inhibition. Although the basal flux of apical 36Cl− uptake was very small, the concentration dependence resembled that of DRA and not PAT-1 (Fig. 1C). In later studies where DRA activity was increased by adenoviral infection, a similar pattern of DIDS sensitivity was observed (Fig. 1D), supporting the pharmacological identification of the small basal apical 36Cl− flux as being DRA mediated. It should be noted that our influx studies were performed in the absence of an artificial pH gradient to limit variables that can confound physiological interpretation of data. Thus NHE and DRA function can be studied in their natural states of activation, similar to conditions found in the native intestine.
Fig. 1.
Caco2BBE cells express an apical Cl− uptake system similar to DRA (downregulated in adenoma). Caco2BBE cells were grown on Transwells for 10–14 days postconfluence and then used directly or infected with a tetracycline (tet)-off adenovirus containing the DRA cDNA. A: Caco2BBE cell monolayers were treated with increased medium serum [increased from 10 to 30% (vol/vol)], RNA was harvested and reverse transcribed, and cDNA was analyzed for DRA (SLC26A3), pendrin (SLC26A4), and PAT-1 (SLC26A6), members of the SLC26 anion exchanger family. B: 22Na+ or 36Cl− apical uptakes were measured as described in materials and methods in uninfected cells (No DRA) or DRA-infected cells under 4 conditions: with normal medium (10% FBS), with increased medium serum (30% FBS), or as C2 cells stably transfected with rat Na+/H+ exchanger 2 (NHE2; C2N2) or rat NHE3 (C2N3). A representative Western blot of total cell DRA expression for the 5 conditions is presented below the flux data. C and D: concentration-dependent inhibition of 36Cl− uptake by niflumic acid or DIDS. Monolayers either without adenoviral infection of DRA (endogenous; C) or with DRA adenovirus infection (D) and DRA increased by omission of medium tetracycline were treated with varying concentrations of the 2 Cl− transporter inhibitors for 10 min before 36Cl− uptake. The blot in A is representative of 3 separate experiments. In B–D, data are means ± SE for 4 separate experiments, and the blot in B is representative of 1 of 4 experiments.
Apical DRA activity increases when apical NHE activity is increased.
To determine whether DRA mediates apical Cl− uptake in Caco2BBE cells, we used an inducible adenoviral system. This inducible system was used because stable transfectants of Cl− exchanger proteins in eukaryotic cells proved to be challenging to establish and maintain. Upon infection with DRA adenovirus, induced expression only occurs in the absence of tetracycline (tet). After infection of Caco2BBE cells and removal of tet, apical 36Cl− as well as protein expression increased (Fig. 2, A and B). It should be noted that endogenous DRA expression occurs for all conditions. The endogenous DRA expressed is, however, small in amount compared with the large increase that occurs due to expression from the tet-sensitive “transgene.”
Fig. 2.
DRA may mediate apical Cl− uptake, and DRA activity is functionally coupled to apical Na+/H+ exchange activity. A: caco2BBE monolayers were infected with DRA and tetracycline (+Tet) was added to the medium and removed after 2 days when appropriate (−Tet). Monolayers were treated for 10 min before fluxes with inhibitors DIDS (500 μM) and DMA (500 μM) or for 2 h with methazolamide (Methazol; 0.2 mM). Flux data are means ± SE for 4 separate experiments. *P < 0.05; +P < 0.01 compared with control 36Cl− or 22Na+ fluxes, by analysis of variance using a Bonferroni correction. B: Western blot of DRA, NHE2, and NHE3 protein expression in Caco2BBE monolayers treated with 10 vs. 30% (vol/vol) serum and with or without medium Tet. All monolayers used were DRA adenovirus infected; however, only one-half had Tet removed to turn on transcription. Images are representative of 4 separate experiments. Densitometry was performed using NIH Image 1.54, and means ± SE (X ± SE) of densitometric values are presented using 10% FBS medium and Tet inclusion as control (X ± SE = 100% for all cases). +P < 0.01; ++P < 0.001 compared with 10% FBS + Tet control, by analysis of variance using a Bonferroni correction.
To determine whether DRA activity might be dependent on NHE function, we used two approaches. First, Caco2BBE cells were infected with the DRA adenovirus, and the serum concentration was increased to 30% (vol/vol) to induce apical NHE activity. With the use of this approach, increases in apical 22Na+ transport were observed in cells where DRA expression was not induced (+tet), but this was enhanced further when DRA expression was induced (−tet) (Fig. 2A). To determine the contribution of apical 22Na+ influx mediated by apical NHE, we used the pharmacological inhibitor dimethylamiloride (DMA), and to determine the component of apical 36Cl− uptake mediated by DRA, we employed DIDS inhibition. It is notable that the pharmacological inhibition of apical NHE activity also inhibits apical DRA activity, and vice versa, DIDS inhibition of DRA was associated with decreases in NHE activity (Fig. 2A). The interdependency of these activity suggested functional coupling of apical DRA and NHE. Because functional coupling of apical NHE and Cl−/base exchange has been shown in native intestine (19), cells were treated with the carbonic anhydrase inhibitor methazolamide (0.2 mM) for 2 h before apical uptake experiments. Methazolamide decreased apical NHE and apical DRA activity (Fig. 2A), indicating that carbonic anhydrase plays a role in the activities of apical NHEs and DRA. Western blots confirmed that increased serum induced both NHE2 and NHE3 protein expression (Fig. 2B). Increasing serum did increase DRA protein expression, which accounted for the small increase in 36Cl− influx (compare controls, Fig. 2B, top left and top right) upon increasing medium serum.
Serum induced both apical NHE2 and NHE3 expression and activity (Fig. 2, A and B), making it difficult to determine which apical NHE isoform might be coupled to DRA. Therefore, we performed studies on Caco2BBE cells stably transfected to express NHE2 or NHE3, where expression is driven by the CMV promoter (31). In the absence of DRA expression (+tet), the NHE2-transfected (C2N2) and NHE3-transfected (C2N3) Caco2BBE cells exhibited only a small increase of apical 36Cl− flux, probably due to endogenous DRA activity (Fig. 3A). However, when DRA expression was increased (−tet; Fig. 3B), apical 36Cl− flux increased greatly in both the NHE2- and NHE3-expressing cells (Fig. 3A). In all instances, inhibition by DIDS resulted in a significant reduction in basal and DRA transgene-dependent 36Cl influx. However, NHE2- and NHE3-mediated 22Na+ influx in the absence of DRA transgene expression appeared to be significantly affected. In contrast, when the DRA transgene was induced (+tet), there was clearly a component of NHE2 and NHE3 influx that was dependent on Cl−/HCO3− anion exchange. With the NHE inhibitor DMA, there was significant inhibition of basal and DRA transgene-associated NHE2 and NHE3 influxes. However, there also was significant inhibition of basal (+tet) and DRA transgene-associated (−tet) 36Cl− influx, as well. These findings again suggest that the functions of apical intestinal DRA and NHEs are coupled. Western blot confirmations of specific NHE2, NHE3, and induced DRA expression are shown in Fig. 3B.
Fig. 3.
Increasing apical NHE2 or NHE3 activity increases activity of DRA expressed from transgene. Caco2BBE cells that stably express rat NHE2 or rat NHE3 were maintained in the presence of 200 μg/ml G418 antibiotic. They were grown on Transwells as nontransfected Caco2BBE and infected with DRA encoding Tet-inducible adenovirus as for regular cells. A: monolayers were treated for 10 min before fluxes with inhibitors DIDS (500 μM) or DMA (500 μM), and 22Na+ and 36Cl− fluxes were measured as described in materials and methods. Flux data are means ± SE for 4 separate experiments. *P < 0.05; +P < 0.01 compared with control Cl− or Na+ flux, by analysis of variance using a Bonferroni correction. B: Western blot of DRA, NHE2, and NHE3 protein expression in Caco2BBE monolayers treated with 10 vs. 30% (vol/vol) serum and with or without Tet. All monolayers used were DRA adenovirus infected; however, only one-half had Tet removed to turn on transcription. Images shown are representative of 4 separate experiments. Densitometry was performed using NIH Image 1.54, and means ± SE of densitometric values are presented comparing results with and without Tet for each antiserum used. C2N2 expressed 581 ± 102% NHE2 compared with regular Caco2BBE, and C2N3 the percentage increase was 473 ± 88; n = 4 for both comparisons.
DRA may transport a number of intracellular anions, including HCO3−, OH−, and Cl−. We reduced the apical flux buffer pH to 5.5 to provide a cell-to-apical medium OH− gradient. A set of wells from the same passage was used to compare fluxes with a normal apical flux buffer pH of 7.4. All monolayers were infected with the DRA adenovirus and had tetracycline removed from the medium to induce DRA expression. Apical 36Cl− influxes were not increased but, instead, decreased when apical flux buffer was decreased to pH 5.5 (Fig. 4A). Decreased apical 22Na+ influxes also were observed that might be anticipated due to decreased cell-to-apical medium H+ gradient when the apical medium pH is lowered. These results add support to a functional coupling between apical NHEs and DRA and to the suggestion that DRA activity is NHE dependent.
Fig. 4.
DRA-mediated Cl− influx is decreased at acidic apical flux pH and does not require basolateral (BL) HCO3− and Na+/HCO3− transport to supply HCO3−. Monolayers were infected with DRA as described in materials and methods, and Tet was removed from all to induce DRA expression. A: influxes were performed as usual except that the pH of the apical flux buffer was decreased to 5.5 using a MES buffer. Data are means ± SE for 3 separate experiments. B: monolayers were treated with BL DIDS (300 μM) for 30 min before flux, as designated. For 1 set, HCO3− was added to the BL flux solution (right), and Na+ and Cl− concentrations were maintained at 20 and 60 mM by alterations of buffer salts as detailed in materials and methods. Fluxes were measured with standard apical pH 7.4 flux medium for both conditions (±HCO3− BL). Values are means ± SE for 3 separate experiments. *P < 0.05; +P < 0.01 compared with control in each set (for 22Na+ or 36Cl− flux, respectively), by analysis of variance using a Bonferroni correction.
Additional experiments were designed to test the contribution of HCO3− brought into the cell by the basolateral Na+-HCO3− cotransporter (NBC). All monolayers were infected with the DRA adenovirus and had tetracycline removed from the medium to induce DRA expression. Cell monolayers were treated for 30 min before flux with basolateral DIDS (300 μM) to inhibit basolateral NBC activity. Apical 36Cl− and 22Na+ influxes were measured in these monolayers in both the absence and presence of basolateral 25 mM HCO3− during the flux period. In the basolateral medium, Na+ and Cl− were kept at their normal levels of 20 and 58 mM, respectively, by using 20 mM NaHCO3, 5 mM KHCO3, 1 mM MgCl2, 2 mM CaCl2, 10 HEPES, 30 mM choline-Cl, 25 NMDG-Cl, and 75 mM NMDG-gluconate. The addition of DIDS to the basolateral medium did not alter the apical 36Cl− or 22Na+ influxes compared with fluxes obtained in other sets of experiments (Fig. 4B, left, compared with apical medium at pH 7.4 in Fig. 4A). The inclusion of HCO3− in the basolateral flux buffer, to which the monolayers were exposed for only the 10-min flux period, did not increase apical 36Cl− or 22Na+ influxes, and this was not effected by preincubation with basolateral DIDS. It is important to note that the cell monolayers were grown in a medium with 25 mM HCO3− and in a 5% CO2 environment and were used within a short time after removal from the incubator so that the intracellular HCO3− concentration should be sufficient to support apical Cl−/HCO3− exchange.
Regulation of DRA.
To determine whether DRA activity is regulated by second messenger cAMP or intracellular calcium, we treated DRA transgene-infected Caco2BBE monolayers with either the permeable 8-CPT-cAMP (100 μM, 15 min) or the Ca2+-ATPase inhibitor thapsigargin (100 ng/ml, 15 min) to increase intracellular free Ca2+. These agents inhibit a large percentage of the apical Na+ influx mediated by NHE2 and NHE3 (34). Both of these second messenger pathway regulators also inhibited DRA activity (Fig. 5A). The functional changes in NHE3 activity induced by cAMP or thapsigargin are associated with its endocytosis from the apical membrane. These events involve a multistep process that includes the adaptor proteins Syt 1 (34), the AP2 complex, and clathrin (12, 23, 46). Cell stimulation with cAMP or thapsigargin also decreased the apical surface abundance of DRA (Fig. 5B). To determine whether its membrane trafficking was similar to that of apical NHE3, we focused on the association of DRA with proteins involved in the induced endocytosis of NHE3, namely, Syt I, AP2, and clathrin. Under nonstimulated conditions, little DRA coimmunoprecipitated with any of these three proteins (Fig. 5C); however, upon stimulation with cAMP or thapsigargin, DRA abundance increased in membrane compartments containing Syt I, the AP2 complex (using the AP2α protein to immunoprecipitate), and the clathrin complex (using antibody to the heavy chain of clathrin to immunoprecipitate) (Fig. 5C). Silencing RNA of individual proteins (Syt I, AP2, or the heavy chain of clathrin) impaired DRA coassociation (Fig. 5C). Silencing Syt I blocked DRA appearance in the Syt I-, AP2-, and clathrin-containing membrane compartments, suggesting that DRA first appears in an Syt I-containing membrane compartment. Silencing AP2μ blocked DRA appearance in the AP2- and clathrin-containing compartments, whereas clathrin only blocked DRA appearance in the clathrin-containing compartment, supporting the temporal order of DRA to first be passed to an Syt I-containing membrane complex, then to an AP2-containing complex, and later to a clathrin-containing complex. Endocytosis may involve movement through many compartments that contain a large number of proteins. Appearance in these compartments does not necessarily imply that DRA would bind directly to any of these proteins.
Fig. 5.
DRA is endocytosed after cAMP stimulation or increases of cell Ca2+, a process that involves synaptotagmin 1 (Syt I), adaptor protein 2 (AP2), and clathrin complexes. Caco2BBE cells were used after DRA-encoding adenovirus infection, and all monolayers had Tet removed to induce DRA expression. A: total apical 36Cl− influx was inhibited by cAMP or thapsigargin stimulation of cell monolayers. Data are means ± SE for 4 separate experiments. *P < 0.05 compared with unstimulated (No Stim), by analysis of variance using a Bonferroni correction. B: apical surface-expressed DRA was biotinylated, and the abundance of apical surface-expressed DRA decreased after cAMP or thapsigargin stimulation of the monolayers. cA, cAMP; Th, thapsigargin; U, unstimulated. C: during apical endocytosis stimulated by cAMP or thapsigargin, DRA associated with membrane compartments containing Syt I, the AP2 complex, and a clathrin-containing complex. Individually silencing Syt I, AP2, or the heavy chain (HC) of clathrin demonstrated that DRA associated with Syt I first, then with the AP2 complex, and third with the clathrin compartment. Silencing Syt I prevented association with all compartments; silencing the AP2 μ-subunit prevented association with the AP2 and clathrin compartments but not Syt I; and silencing the clathrin HC only inhibited DRA appearance in the clathrin-containing compartment while association of DRA with Syt I- and AP2-containing compartments was not blocked. D: DRA was endocytosed in the small intestine and occurred in both NHE2 and NHE3 knockout mice. Loops of small intestinal were made ex vivo and filled with medium with no stimulus, cAMP, or thapsigargin. Loops were rapidly chilled, and apical surface proteins were biotinylated followed by isolation of biotinylated proteins and their analysis by Western blotting. DRA was expressed at the brush border, and the abundance decreased at the brush border by >70% for both cAMP- and calcium-mediated (thapsigargin) stimulation. Images for Western blots in C and D are representative of those of 3 separate occasions. For all coimmunoprecipitations, a Western blot for DRA was analyzed to determine that equivalent amounts of DRA were used for immunoprecipitations (IP).
To determine the physiological relevance of these findings and to determine whether DRA endocytosis is dependent on NHE2 or NHE3 membrane trafficking, we stimulated ex vivo closed loops of mouse small intestine with cAMP or thapsigargin and biotinylated apical surface proteins. Loops were made from jejunum of three types of mice: NHE2 knockout, NHE3 knockout, and heterozygote NHE2 or NHE3 mice (only 1 allele knocked out). Both agents stimulated decreases in the apical surface abundance of DRA, which was seen in both NHE2 and NHE3 knockout mice (Fig. 5D). These data demonstrate that induced DRA endocytosis does occur in native intestine. Because NHE2 and NHE3 double-knockout mice were unavailable, we cannot exclude the possibility that induced endocytosis of DRA apical Na+/H+ is completely independent of apical NHE exchangers.
DISCUSSION
Non-nutrient-dependent electroneutral absorption of NaCl is found throughout the small and large intestines and is mediated by coupled Na+/H+ and Cl−/HCO3− exchange (6, 14, 27, 42). Although DRA has been suspected of being the primary anion exchanger coupled to apical NHEs, this issue still remains unresolved, and the nature of this coupling process is poorly understood. In support of its role in mediating intestinal Na-Cl absorption is the clinical observation that defects in DRA function are the basis of congenital chloridorrhea (21) and that DRA knockout mice develop a similar diarrheal disease (39). In addition, DRA expression is primarily seen in villus enterocytes and colonic surface absorptive cells, a pattern similar to that seen with apical membrane NHE2 and NHE3 (3, 7, 21, 43). The expression of PAT-1, on the other hand, may be more restricted. It is highly expressed by upper villus cells of the proximal small intestine (41); however, expression in other segments of the intestinal tract has not been extensively studied, although PAT-1 mediates oxalate transport in murine ileum (16, 43) as it has been demonstrated to do in the kidney (25, 43). It is important to note, however, that SLC26A6 knockout mice fail to demonstrate diarrhea (43).
The present studies demonstrate that activity of the anion exchanger DRA does mediate Cl− uptake at the brush border of Caco2BBE cells, and, importantly, this activity is coupled and dependent on apical NHE2 and NHE3 function. The coupling of DRA and apical NHEs is also regulated by carbonic anhydrase in Caco2BBE cells. Inhibition of carbonic anhydrase in intact intestinal mucosa also prevents electroneutral NaCl absorption (19). The role of carbonic anhydrase is complex, because it, as well as Pco2, appears to modulate intestinal Na+ and Cl− transport through effects on vesicular trafficking in certain regions of the bowel (8, 9). In addition, carbonic anhydrase has been found to bind PAT-1 (SLC26A6) and regulate the movement of this anion exchanger in a complex termed a metabolon (4).
The present studies do not exclude the ability of DRA to mediate Cl−/OH−, Cl−/Cl−, or exchange with other anions. Studies on DRA expressed in heterologous systems (HEK cells, Xenopus oocytes), as well as studies in renal epithelia, suggest that DRA is capable of transporting a number of anions (25, 28). In the kidney, DRA has been shown to transport organic anions such as formate or oxalate. With respect to intestinal NaCl absorption, however, Cl− is likely to be the principle anion transported from the luminal solution to the cell, especially in the small intestine, where the luminal fluid is chloride rich and at neutral pH. In exchange for Cl−, several anions could be candidates, including HCO3−, Cl−, or OH−. However, Cl−/Cl− exchange is not likely, because this would not lead to vectorial absorption of chloride.
Although we do not know the basis of the NHE dependence of DRA function, similar observations have been made in intact intestinal mucosa of NHE2 and NHE3 knockout mice (17, 18). In both cases, a significant reduction of electroneutral Cl− absorption was observed when the NHE3 isoform, in particular, was absent. In contrast, apical NHE2 and NHE3 activities are not completely dependent on DRA function. In fact, the DRA-deficient mice seem to have increased expression and activity of the NHE3 apical exchanger (39). Our studies also have shown that in almost all instances, a DRA-independent NHE activity can be demonstrated, either under basal conditions (where there is little DRA activity or expression) or when induced DRA transgene function is inhibited by DIDS (Figs. 1B, 2, and 3). This is not surprising, since luminal perfusion studies of human small intestine (6, 42) have shown that electroneutral Na+ absorption in the proximal human small intestine is associated with luminal acidification. This observation strongly suggests that apical NHE activity in this region of the bowel occurs independently of anion exchange.
We also report that second messenger regulation of DRA function and membrane trafficking is analogous to that observed for NHE2 and NHE3 (31). Upon stimulation by agents that increase intracellular cAMP or calcium, inhibition of apical NHE and DRA function is observed, analogous to that observed in intact tissues and in vitro studies. These changes are associated with rapid membrane endocytosis of apical DRA and NHE, although studies in the NHE2 and NHE3 knockout tissue would suggest that DRA membrane trafficking is not dependent on or coupled to apical NHE trafficking. However, we cannot rule out these possibilities because we were unable to perform studies in the double NHE2/NHE3 knockout mice. Second messenger-induced DRA endocytosis is dependent on association with the adaptor protein Syt I with subsequent recruitment of AP2 and clathrin. The process therefore appears to be common with that observed for apical NHEs (34).
In conclusion, we have demonstrated that DRA, expressed in polarized intestinal epithelial cells, mediates apical membrane Cl−/base exchange through functional coupling to apical NHE2 and/or NHE3. However, there is clearly a component of NHE2 and NHE3 function that is independent of DRA activity. Second messenger-induced inhibition of DRA function is associated with its membrane endocytosis, a process that requires association with a preendocytic pathway that includes Syt I, AP2, and clathrin. These studies support the role of DRA as an important anion exchanger that can couple with apical NHE function to mediate electroneutral NaCl absorption by intestinal epithelium. They also provide new insights into the functional and membrane trafficking interactions between these transporters following second messenger regulation.
GRANTS
This work was supported by National Institutes of Health Grants DK-38510 (to E. B. Chang), Digestive Disease Center Grant DK-42086 (to University of Chicago), and the Gastro-Intestinal Research Foundation of Chicago.
Acknowledgments
We acknowledge the excellent technical assistance of Ken Drabik.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am J Physiol Gastrointest Liver Physiol 277: G321–G332, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Alrefai WA, Tyagi S, Nazir TM, Barakat J, Anwar SS, Hadjiagapiou C, Bavishi D, Sahi J, Malik P, Goldstein J, Layden TJ, Ramaswamy K, Dudeja PK. Human intestinal anion exchanger isoforms: expression, distribution, and membrane localization. Biochim Biophys Acta 1511: 17–27, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IF, Dudeja PK, Wu GD. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am J Physiol Gastrointest Liver Physiol 293: G923–G934, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez BV, Vilas GL, Casey JR. Metabolom disruption: a mechanism that regulates bicarbonate transport. EMBO J 24: 2499–2511, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anders M, Hansen R, Dina RX, Rauen KA, Bissell MJ, Korn M. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc Natl Acad Sci USA 100: 1943–1948, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieberdorf FA, Gordon P, Fordtran JS. Pathogenesis of congenital alkalosis with diarrhea: implications for the physiology of normal ileal electrolyte absorption and secretion. J Clin Invest 51: 1958–1968, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bookstein C, DePaoli AM, Xie Y, Niu P, Musch MW, Rao MC, Chang EB. Na/H exchangers NHE1 and NHE3, of rat intestine. J Clin Invest 93: 106–113, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charney AN, Alexander-Chacko J, Gummaconda R, Egnor RW. Non-catalytic role of carbonic anhydrase in rat intestinal absorption. Biochim Biophys Acta 1573: 141–148, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Charney AN, Egnor RW, Henner D, Rashid H, Cassai N, Sidhu GS. Acid base effects on intestinal Cl absorption and vesicular trafficking. Am J Physiol Gastrointest Liver Physiol 286: G1062–G1070, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL. Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549: 3–19, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow A, Zhou W, Jacobson R. Regulation of AE2 Cl/HCO3 exchanger during intestinal development. Am J Physiol Gastrointest Liver Physiol 271: G330–G337, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Chow CW, Khurana S, Woodside M, Grinstein S, Orlowski J. The epithelial Na+/H+ exchanger, NHE3, is internalized through a clathrin-mediated pathway. J Biol Chem 274:37551–37558, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Cohen CJ, Shieh JTC, Pickels RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackie and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA 98: 15191–15196, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field M, Fromm M, McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol 220: 1388–1396, 1971. [DOI] [PubMed] [Google Scholar]
- 15.Field M Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate transport and urinary excretion and enhanced in Slc26a6 mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Gawenis LR, Hut H, Bot AG, Shull GE, de Jonge HR, Stien X, Miller ML, Clarke LL. Electroneutral sodium absorption and electrogenic anion secretion across murine small intestine are regulated in parallel. Am J Physiol Gastrointest Liver Physiol 287: G1140–G1149, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Gawenis LR, Stien X, Shull GE, Schultheis PJ, Woo AL, Walker NM, Clarke LL. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol 282: G776–G784, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Goldfard DS, Chan AJ, Hernandez D, Charney AN. Effect of thiazides on colonic NaCl absorption: role of carbonic anhydrase. Am J Physiol Renal Fluid Electrolyte Physiol 261: F452–F458, 1991. [DOI] [PubMed] [Google Scholar]
- 20.Goldfarb DS, Sly WS, Waheed A, Charney AN. Acid base effect on electrolyte transport in CA II-deficient mouse colon. Am J Physiol Gastrointest Liver Physiol 278: G409–G415, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere J. Mutations of the down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14: 316–319, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Hoogerwerf WA, Tsao SC, Devuyst O, Levine SA, Yun CH, Yip JW, Cohen ME, Wilson PD, Lazenby AJ, Tse CM, Donowitz M. NHE2 and NHE3 are human and rabbit intestinal brush-border proteins. Am J Physiol Gastrointest Liver Physiol 270: G29–G41, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl−/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediate by mouse Slc26A6. J Biol Chem 277: 33963–33967, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Knauf F, Yang CL, Thompson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA 98: 9425–9430, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knickelbein R, Aronson PS, Schron CM, Seifter J, Dobbins JW. Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol Gastrointest Liver Physiol 249: G236–G245, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Lamprecht G, Baisch S, Schoenleber E, Gregor M. Transport properties of the human intestinal anion exchanger DRA (down-regulated in adenoma) in transfected HEK293 cells. Pflügers Arch 44: 479–490, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Lohi H, Kujala M, Kerkela E, Saarialho-Kere U, Kestila M, Kere J. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics 70: 102–112, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol 284: C769–C779, 2003. [DOI] [PubMed] [Google Scholar]
- 31.McSwine RL, Musch MW, Bookstein C, Xie Y, Rao MC, Chang EB. Regulation of apical membrane Na/H exchangers NHE2 and NHE3 in intestinal epithelial cell line CACO-2BBE/bbe. Am J Physiol Cell Physiol 275: C693–C701, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl−/HCO3− exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem 274: 22855–22861, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 276: G185–G192, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Musch MW, Arvans DL, Walsh-Reitz MM, Uchiyama K, Fukuda M, Chang EB. Synaptotagmin I binds intestinal epithelial NHE3 and mediates cAMP and Ca2+-induced endocytosis by recruitment of AP2 and clathrin. Am J Physiol Gastrointest Liver Physiol 292: G1549–G1588, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Peterson MD, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci 105: 445–460, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Ranjendran VM, Black J, Ardito TA, Sangan P, Alper SA, Schweinfest C, Kashgarian M, Binder HJ. Regulation of DRA and AE1 in rat colon by dietary depletion. Am J Physiol Gastrointest Liver Physiol 279: G931–G942, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Rocha F, Musch MW, Lishansky L, Bookstein C, Sugi K, Xie Y, Chang EB. IFN-γ downregulates expression of Na+/H+ exchangers NHE2 and NHE3 in rat intestine and human Caco2/bbe cells. Am J Physiol Gastrointest Liver Physiol 280: G1224–G1232, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Schweinfest CW, Henderson KW, Suster S, Kondoh N, Papas TS. Identification of a colon mucosa gene that is down-regulated in colon adenomas and adenocarcinomas. Proc Natl Acad Sci USA 90: 4166–4170, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Sellin JS, Desoignie R. Rabbit proximal colon: a distinct transport epithelium. Am J Physiol Gastrointest Liver Physiol 246: G603–G610, 1984. [DOI] [PubMed] [Google Scholar]
- 41.Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Turnberg LA, Bieberdorf FA, Morawski SG, Fordtran J. Interrelationship of chloride, bicarbonate, sodium and hydrogen transport in human ileum. J Clin Invest 49: 557–567, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Wormmeester L, Sanchez de Medina F, Kokke F, Tse CM, Khurana S, Bowser J, Cohen ME, Donowitz M. Quantitative contribution of NHE2 and NHE3 to rabbit ileal brush-border Na+/H+ exchange. Am J Physiol Cell Physiol 274: C1261–C1272, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Yeo C, Barry K, Gontarek D, Donowitz M. Na+-H+ exchange mediates meal-stimulated ileal absorption. Surgery 116: 338–394, 1994. [PubMed] [Google Scholar]
- 46.Zhao M, Lamprecht G, Steplock D, Tariq N, Shenolikar S, Donowitz M, Yun CH, Weinman EJ. cAMP-induced phosphorylation and inhibition of Na+/H+ exchanger 3 (NHE3) are dependent on the presence but not the phosphorylation of the NHE regulatory factor. J Biol Chem 274: 24753–24758, 1999. [DOI] [PubMed] [Google Scholar]







