Abstract
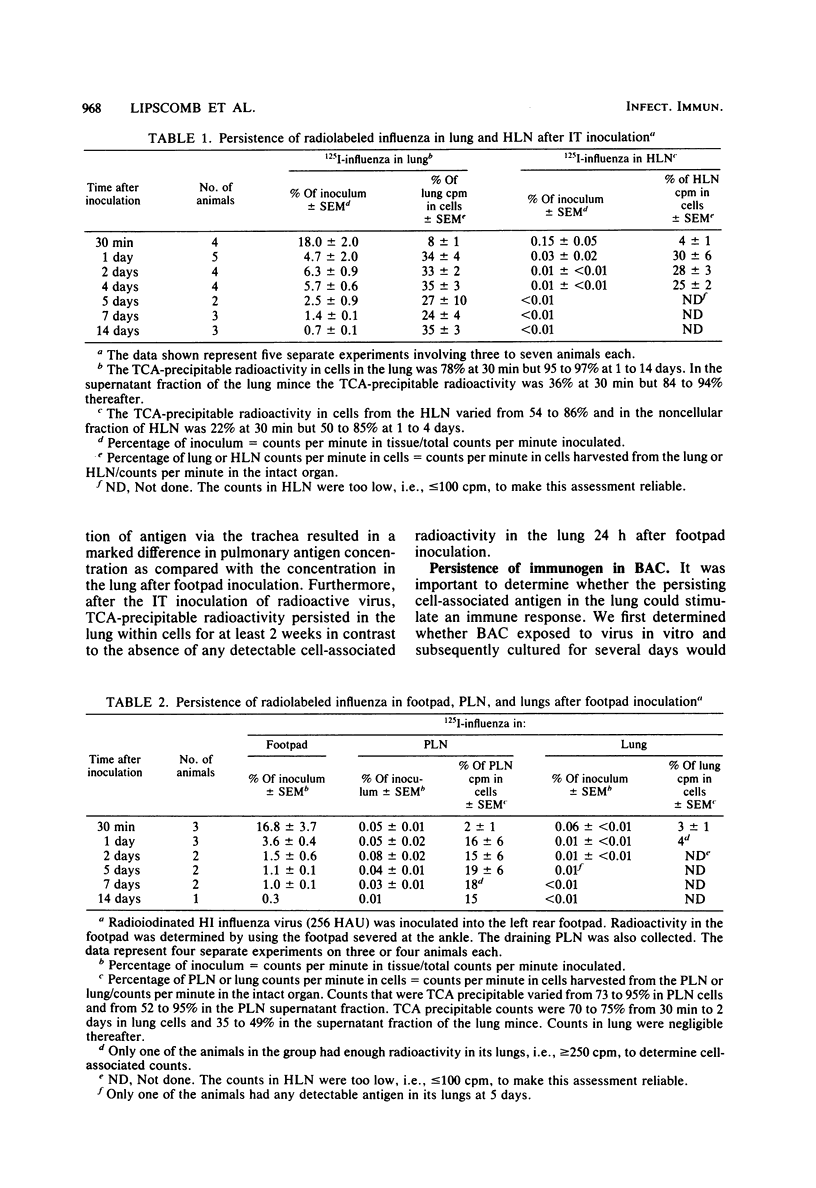
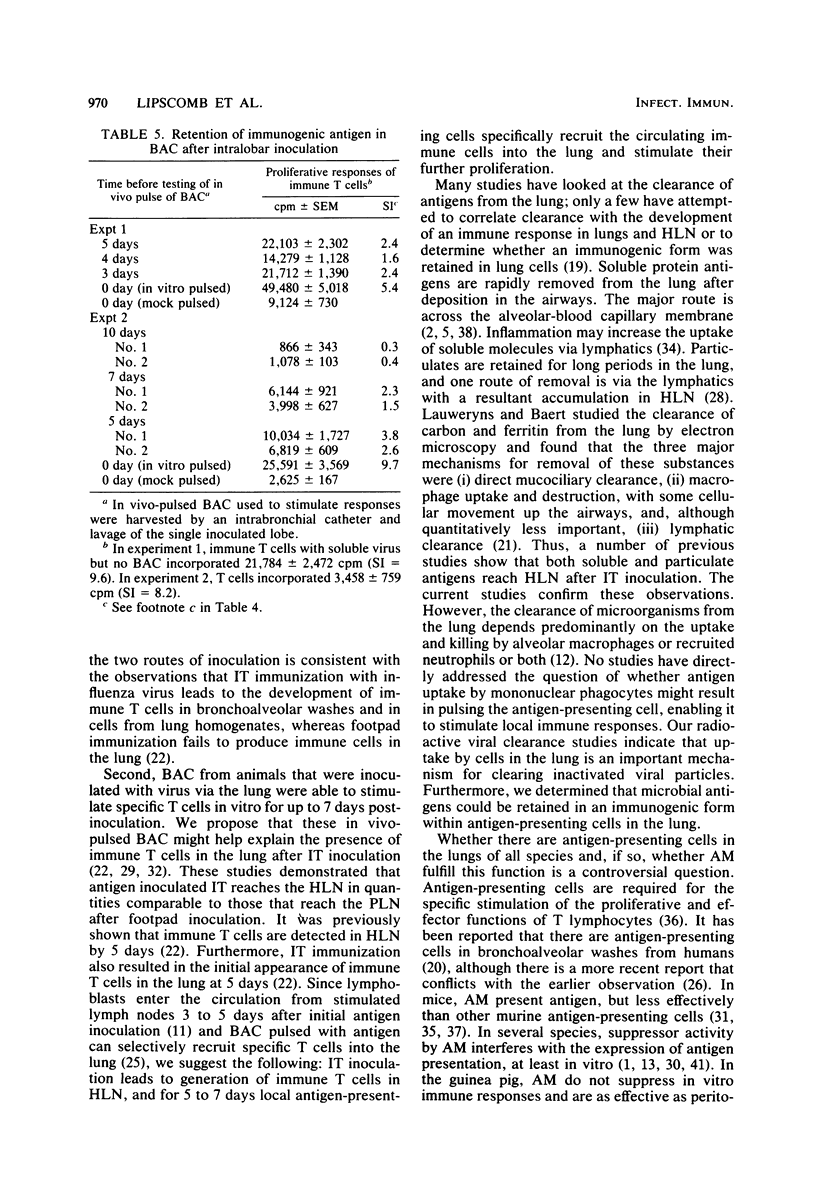
Influenza antigens inoculated into the lung induce local immune responses. It has been proposed that this induction might be partly regulated by local antigen-presenting cells. The purpose of the current study was to inoculate heat-inactivated influenza virus into the tracheae of guinea pigs and determine the quantity of antigens that became cell-associated. Second, we determined how long antigen-presenting bronchoalveolar cells that had taken up virus in vivo retained their ability to specifically stimulate virus-immune T lymphocytes. Radioiodinated heat-inactivated influenza virus was inoculated into the tracheae of guinea pigs. The animals were killed from 30 min to 14 days after intratracheal inoculation, and radioactivity was determined in cells isolated from lung tissue. At least one-third of the radioactivity in the lungs was cell-associated from 1 to 14 days post-inoculation. In separate studies, heat-inactivated virus was inoculated into the airways of guinea pigs, and animals were killed at various times thereafter. Bronchoalveolar cells from these animals were compared with those from uninoculated controls in their ability to specifically stimulate virus-immune T cells to proliferate in vitro. Bronchoalveolar cells from virus-inoculated animals specifically stimulated T lymphocytes for up to 7 days after virus inoculation. These studies suggest that immunogenic virus persists in the lung within antigen-presenting cells for at least 1 week and possibly for up to 2 weeks. The persisting immunogenic stimulus after the termination of viral infections might be critical in ensuring the development of a local protective immune response.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansfield M. J., Kaltreider H. B., Caldwell J. L., Herskowitz F. N. Hyporesponsiveness of canine bronchoalveolar lymphocytes to mitogens: inhibition of lymphocyte proliferation by alveolar macrophages. J Immunol. 1979 Feb;122(2):542–548. [PubMed] [Google Scholar]
- Bensch K. G., Dominguez E. A. Studies on the pulmonary air-tissue barrier. IV. Cytochemical tracing of macromolecules during absorption. Yale J Biol Med. 1971 Feb-Apr;43(4-5):236–241. [PMC free article] [PubMed] [Google Scholar]
- Bice D. E., Degen M. A., Harris D. L., Muggenburg B. A. Recruitment of antibody-forming cells in the lung after local immunization is nonspecific. Am Rev Respir Dis. 1982 Oct;126(4):635–639. doi: 10.1164/arrd.1982.126.4.635. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Harris D. L., Hill J. O., Muggenburg B. A., Wolff R. K. Immune responses after localized lung immunization in the dog. Am Rev Respir Dis. 1980 Nov;122(5):755–760. doi: 10.1164/arrd.1980.122.5.755. [DOI] [PubMed] [Google Scholar]
- Braley J. F., Dawson C. A., Moore V. L., Cozzini B. O. Absorption of inhaled antigen into the circulation of isolated lungs from normal and immunized rabbits. J Clin Invest. 1978 May;61(5):1240–1246. doi: 10.1172/JCI109040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. L., Kaltreider H. B. Cytolytic activity of pulmonary and systemic lymphoid cells from C57BL/6 mice following intrapulmonary or intraperitoneal immunization with allogeneic tumor cells. Exp Lung Res. 1980 Jun;1(2):99–110. doi: 10.3109/01902148009069640. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Beacham C. H., Gorenberg D. J. The bronchoalveolar lymphocyte. Studies on the life history and lymphocyte traffic from blood to the lung. Cell Immunol. 1977 Jun 1;31(1):48–54. doi: 10.1016/0008-8749(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Emeson E. E., Norin A. J., Veith F. J. Antigen-induced recruitment of circulating lymphocytes to the lungs and hilar lymph nodes of mice challenged intratracheally with alloantigens. Am Rev Respir Dis. 1982 Apr;125(4):453–459. doi: 10.1164/arrd.1982.125.4.453. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Martin W. J., Verbonitz M. W. Hemagglutinin-specific cytotoxic T-cell response during influenza infection. J Exp Med. 1977 Sep 1;146(3):893–898. doi: 10.1084/jem.146.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford W. L. Lymphocyte migration and immune responses. Prog Allergy. 1975;19:1–59. doi: 10.1159/000313381. [DOI] [PubMed] [Google Scholar]
- Green G. M., Jakab G. J., Low R. B., Davis G. S. Defense mechanisms of the respiratory membrane. Am Rev Respir Dis. 1977 Mar;115(3):479–514. doi: 10.1164/arrd.1977.115.3.479. [DOI] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. II. Inhibition of lymphocyte proliferation by purified macrophages from rat lung. Immunology. 1979 Jun;37(2):429–436. [PMC free article] [PubMed] [Google Scholar]
- Holt P. G., Batty J. E. Alveolar macrophages. V. Comparative studies on the antigen presentation activity of guinea-pig and rat alveolar macrophages. Immunology. 1980 Oct;41(2):361–366. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Byrd P. K., Daughety T. W., Shalaby M. R. The mechanism of appearance of specific antibody-forming cells in lungs of inbred mice after intratracheal immunization with sheep erythrocytes. Am Rev Respir Dis. 1983 Mar;127(3):316–321. doi: 10.1164/arrd.1983.127.3.316. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B., Caldwell J. L., Adam E. The fate and consequence of an organic particulate antigen instilled into bronchoalveolar spaces of normal canine lungs. Am Rev Respir Dis. 1977 Aug;116(2):267–280. doi: 10.1164/arrd.1977.116.2.267. [DOI] [PubMed] [Google Scholar]
- Kaltreider H. B. Expression of immune mechanisms in the lung. Am Rev Respir Dis. 1976 Mar;113(3):347–379. doi: 10.1164/arrd.1976.113.3.347. [DOI] [PubMed] [Google Scholar]
- Laughter A. H., Martin R. R., Twomey J. J. Lymphoproliferative responses to antigens mediated by human pulmonary alveolar macrophages. J Lab Clin Med. 1977 Jun;89(6):1326–1332. [PubMed] [Google Scholar]
- Lipscomb M. F., Toews G. B., Lyons C. R., Uhr J. W. Antigen presentation by guinea pig alveolar macrophages. J Immunol. 1981 Jan;126(1):286–291. [PubMed] [Google Scholar]
- Liu M. C., Ishizaka K., Plaut M. T lymphocyte responses of murine lung: immunization with alloantigen induces accumulation of cytotoxic and other T lymphocytes in the lung. J Immunol. 1982 Dec;129(6):2653–2661. [PubMed] [Google Scholar]
- Lyons C. R., Lipscomb M. F. Alveolar macrophages in pulmonary immune responses. I. Role in the initiation of primary immune responses and in the selective recruitment of T lymphocytes to the lung. J Immunol. 1983 Mar;130(3):1113–1119. [PubMed] [Google Scholar]
- Mayernik D. G., Ul-Haq A., Rinehart J. J. Differentiation-associated alteration in human monocyte-macrophage accessory cell function. J Immunol. 1983 May;130(5):2156–2160. [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- Morrow P. E. Lymphatic drainage of the lung in dust clearance. Ann N Y Acad Sci. 1972 Dec 29;200:46–65. doi: 10.1111/j.1749-6632.1972.tb40177.x. [DOI] [PubMed] [Google Scholar]
- Nash D. R., Holle B. Local and systemic cellular immune responses in guinea-pigs given antigen parenterally or directly into the lower respiratory tract. Clin Exp Immunol. 1973 Apr;13(4):573–583. [PMC free article] [PubMed] [Google Scholar]
- Pennline K. J., Conrad R. E., Gerber H. R., Herscowitz H. B. Suppressive effect of alveolar macrophages on the in vitro immune response of rabbit lymphocytes. J Reticuloendothel Soc. 1979 May;25(5):495–512. [PubMed] [Google Scholar]
- Shellito J., Caldwell J. L., Kaltreider H. B. Immune functions of murine alveolar macrophages: binding of lymphocytes and support of lymphocyte proliferation. Exp Lung Res. 1983 Feb;4(2):93–107. doi: 10.3109/01902148309055007. [DOI] [PubMed] [Google Scholar]
- Spencer J. C., Waldman R. H., Johnson J. E., 3rd Local and systemic cell-mediated immunity after immunization of guinea pigs with live or killed m. tuberculosis by various routes. J Immunol. 1974 Apr;112(4):1322–1328. [PubMed] [Google Scholar]
- Stanley P., Haslam E. A. The polypeptides of influenza virus. V. Localization of polypeptides in the virion by iodination techniques. Virology. 1971 Dec;46(3):764–773. doi: 10.1016/0042-6822(71)90078-x. [DOI] [PubMed] [Google Scholar]
- Thrall R. S., Peterson L. B., Braley J. F., Linehan J. H., Dawson C. A., Moore V. L. Chronic pulmonary inflammation modulates the fate of proteins administered by the respiratory tract. J Lab Clin Med. 1980 Mar;95(3):343–350. [PubMed] [Google Scholar]
- Ullrich S. E., Herscowitz H. B. Immunological function of alveolar macrophages: interaction with a soluble protein antigen and the immunogenicity of alveolar macrophage-associated antigen. J Reticuloendothel Soc. 1980 Aug;28(2):111–127. [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Weinberg D. S., Unanue E. R. Antigen-presenting function of alveolar macrophages: uptake and presentation of Listeria monocytogenes. J Immunol. 1981 Feb;126(2):794–799. [PubMed] [Google Scholar]
- Willoughby J. B., Willoughby W. F. In vivo responses to inhaled proteins. I. Quantitative analysis of antigen uptake, fate, and immunogenicity in a rabbit model system. J Immunol. 1977 Dec;119(6):2137–2146. [PubMed] [Google Scholar]
- Yap K. L., Ada G. L. Cytotoxic T cells in the lungs of mice infected with an influenza A virus. Scand J Immunol. 1978;7(1):73–80. doi: 10.1111/j.1365-3083.1978.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Yaron A., Dunham E. K., 3rd, Schlossman S. F. Synthesis and immunological properties of the oligolysyl-N-epsilon-dinitrophenyllysine and oligolysylalanylalanylalanyl-N-epsilon-dinitrophenyllysine peptide series. Biochemistry. 1974 Jan 15;13(2):347–354. doi: 10.1021/bi00699a020. [DOI] [PubMed] [Google Scholar]
- Yeager H., Jr, Sweeney J. A., Herscowitz H. B., Barsoum I. S., Kagan E. Modulation of mitogen-induced proliferation of autologous peripheral blood lymphocytes by human alveolar macrophages. Infect Immun. 1982 Oct;38(1):260–266. doi: 10.1128/iai.38.1.260-266.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


