Abstract
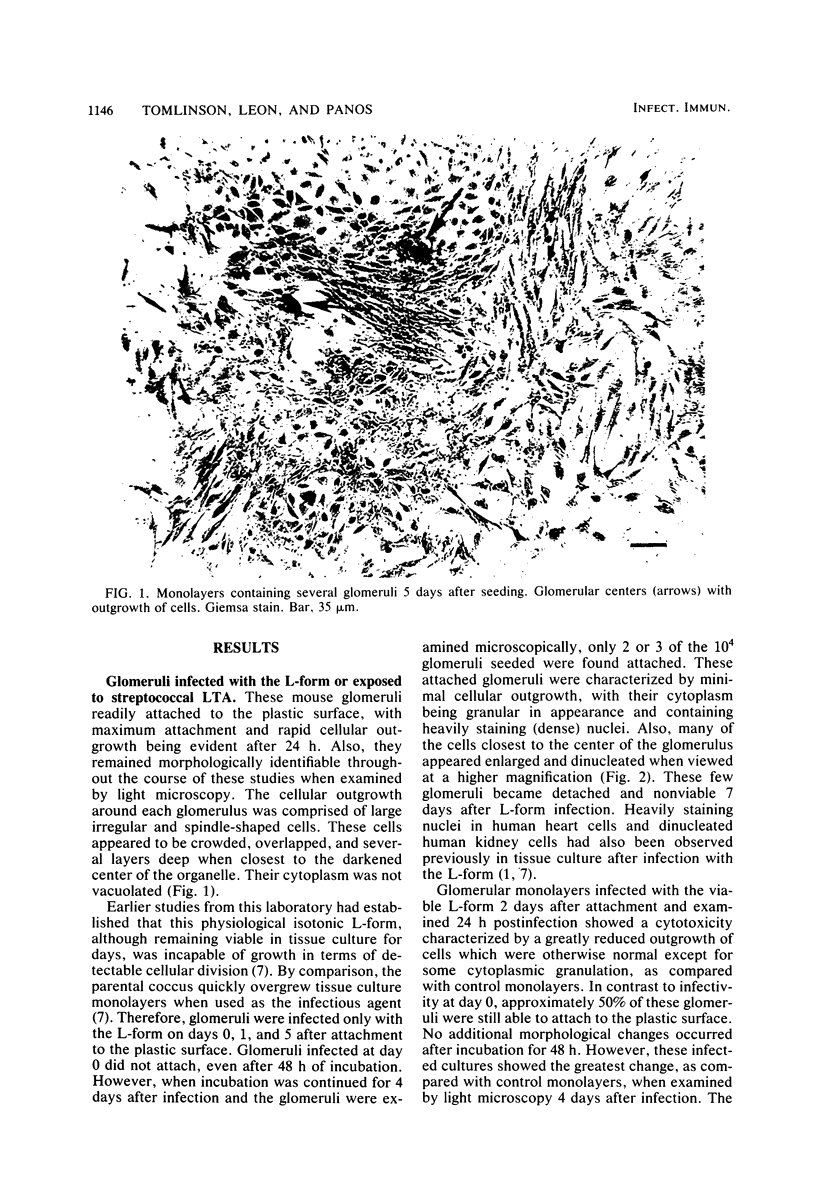
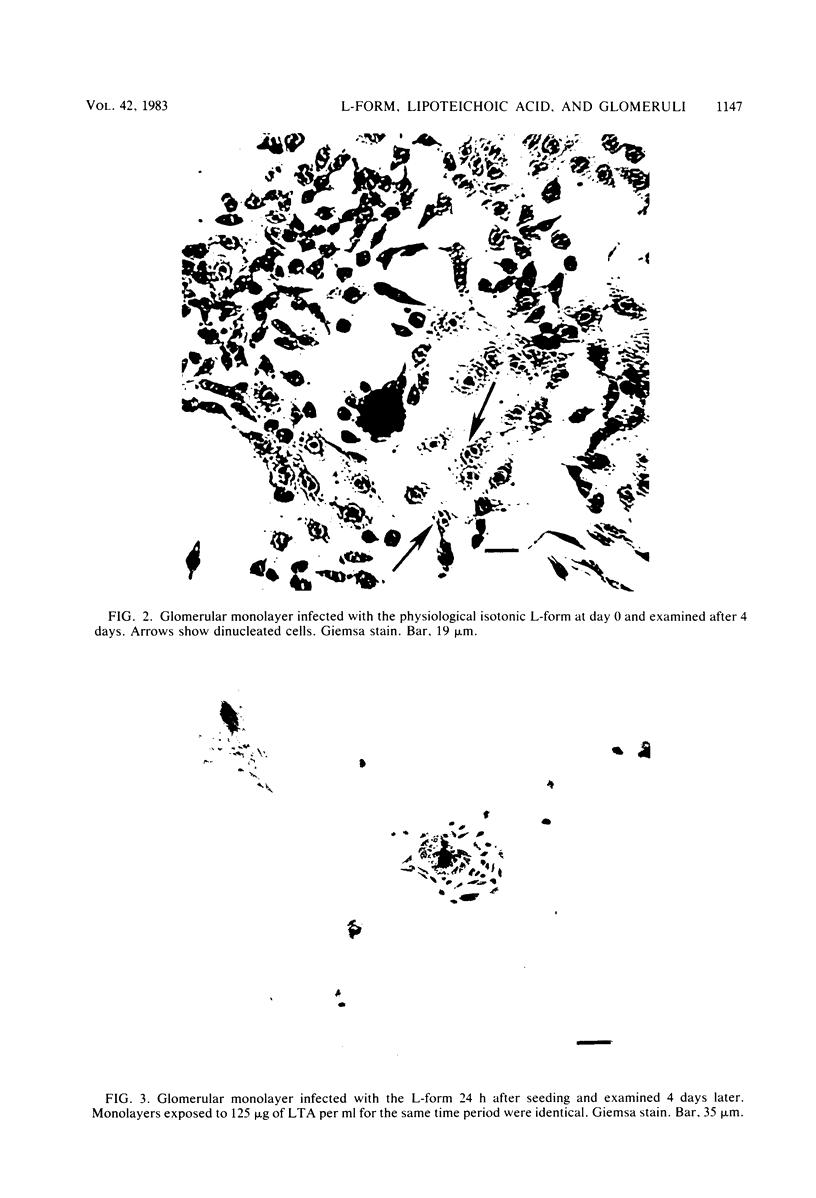
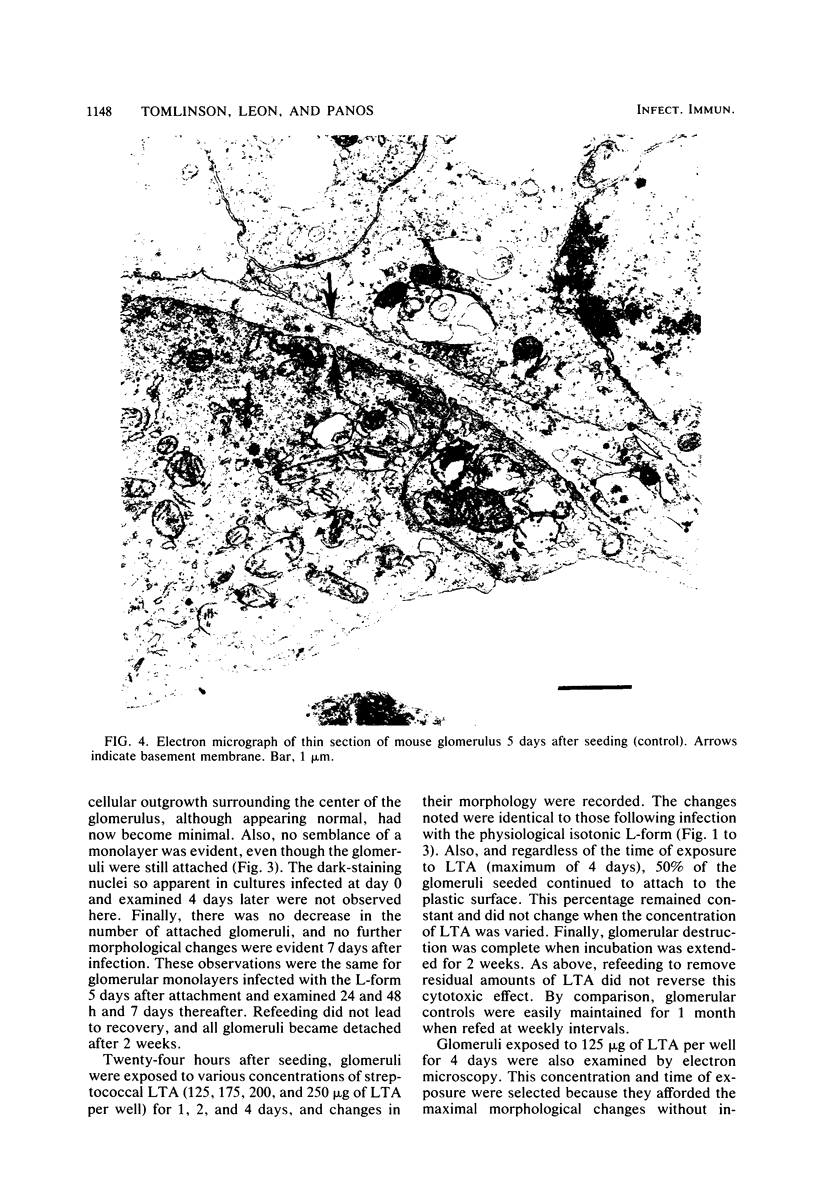
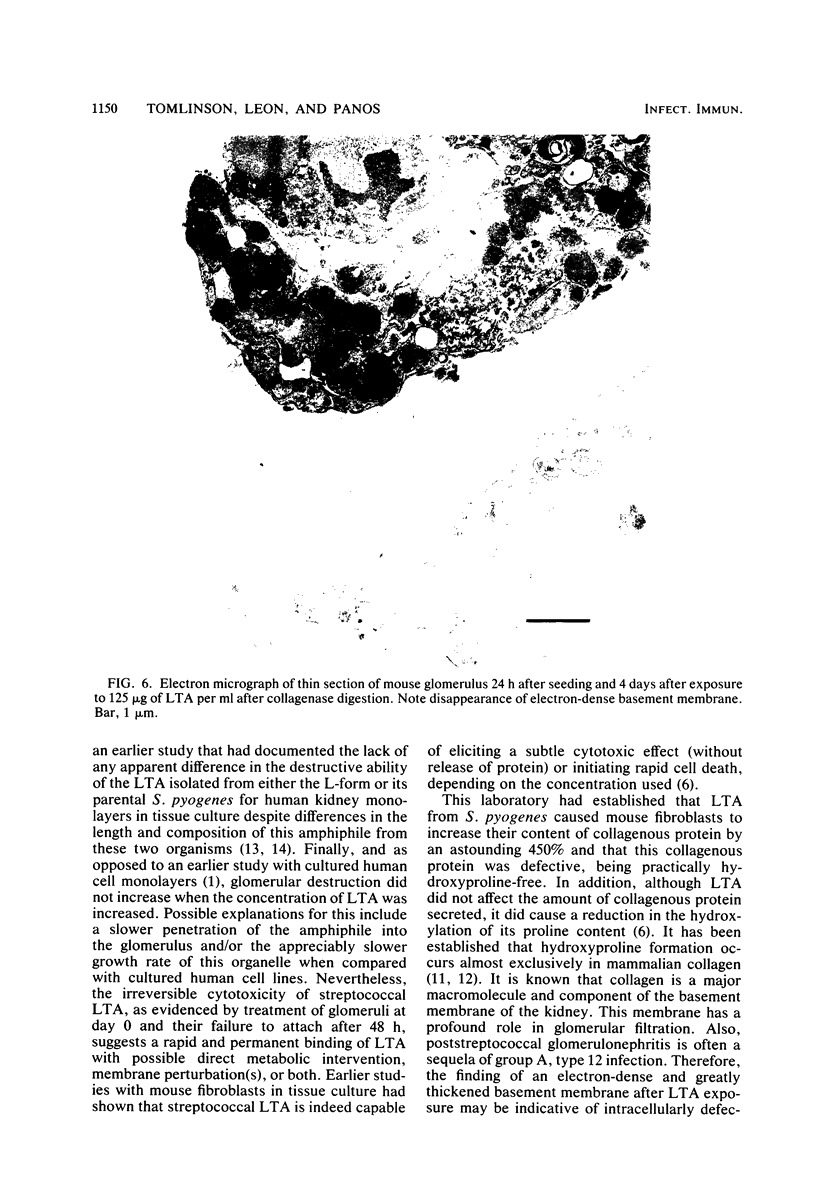
The morphology and pathology of cultured mouse glomeruli were examined at the cellular and subcellular levels after infection with a physiological isotonic L-form of Streptococcus pyogenes type 12 or exposure to streptococcal lipoteichoic acid. These changes, as viewed by light microscopy, were identical regardless of the method used to induce glomerular cytotoxicity. They were characterized by an initial reduction in the outgrowth of cells, some cellular granulation, and later, destruction of the confluent monolayer. Once initiated, cytotoxicity could not be reversed by refeedings, and complete glomerular destruction resulted after 2 weeks. Electron microscope studies revealed that the basement membrane of intact glomeruli exposed to streptococcal lipoteichoic acid had become greatly thickened (two- to fourfold) and electron dense. Our recent biochemical findings have shown that streptococcal lipoteichoic acid increases the amount of collagen formed and retained by mouse fibroblasts in tissue culture as well as causing a reduction in the hydroxylation of proline in both intracellular and secreted collagenous material (Leon and Panos, Infect. Immun. 40:785-794, 1983). These results, together with the present findings, suggest that the thickening of the glomerular basement membrane may be due to defective collagen biosynthesis as a result of streptococcal lipoteichoic acid. The use of cultured glomeruli as a model system for studying the earliest basement membrane alterations in the absence of an immune response as a result of streptococcal lipoteichoic acid is suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeVuono J., Panos C. Effect of L-form Streptococcus pyogenes and of lipoteichoic acid on human cells in tissue culture. Infect Immun. 1978 Oct;22(1):255–265. doi: 10.1128/iai.22.1.255-265.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., VERNIER R. L., GOOD R. A. An electron microscope study of the glomerulus in nephrosis, glomerulonephritis, and lupus erythematosus. J Exp Med. 1957 Nov 1;106(5):649–660. doi: 10.1084/jem.106.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J. C., 3rd, Hare T. A. Gamma-aminobutyric acid in peripheral tissue, with emphasis on the endocrine pancreas: presence in two species and reduction by streptozotocin. Diabetes. 1979 Dec;28(12):1073–1076. doi: 10.2337/diab.28.12.1073. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Harwood R., Williams I. F. The biosynthesis of basement-membrane collagen by isolated rat glomeruli. Eur J Biochem. 1975 Jun;54(2):531–540. doi: 10.1111/j.1432-1033.1975.tb04166.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leon O., Panos C. Adaptation of an osmotically fragile L-form of Streptococcus pyogenes to physiological osmotic conditions and its ability to destroy human heart cells in tissue culture. Infect Immun. 1976 Jan;13(1):252–262. doi: 10.1128/iai.13.1.252-262.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon O., Panos C. Cytotoxicity and inhibition of normal collagen synthesis in mouse fibroblasts by lipoteichoic acid from Streptococcus pyogenes type 12. Infect Immun. 1983 May;40(2):785–794. doi: 10.1128/iai.40.2.785-794.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS H. J., TERRYBERRY J. E. Counting actively metabolizing tissue cultured cells. Exp Cell Res. 1957 Oct;13(2):341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- STRUNK S. W., HAMMOND W. S., BENDITT E. P. THE RESOLUTION OF ACUTE GLOMERULONEPHRITIS. AN ELECTRON MICROSCOPIC STUDY OF FOUR SEQUENTIAL BIOPSIES. Lab Invest. 1964 May;13:401–429. [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Membrane lipoteichoic acid of Streptococcus pyogenes and its stabilized L-form and the effect of two antibiotics upon its cellular content. J Bacteriol. 1976 Aug;127(2):855–862. doi: 10.1128/jb.127.2.855-862.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabyj B. M., Panos C. Teichoic acid of a stabilized L-form of Streptococcus pyogenes. J Bacteriol. 1973 Jun;114(3):934–942. doi: 10.1128/jb.114.3.934-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]