Abstract
S-Nitrosoglutathione (GSNO) is an endogenous bronchodilator with several beneficial pulmonary effects. Levels are decreased in the asthmatic airway, and GSNO inhalation has been proposed as an asthma therapy. 5-lipoxygenase (5-LO) is the rate-limiting enzyme in the synthetic pathway for cysteinyl leukotrienes (CysLTs), bronchoconstricting agents that are overproduced in asthma. Here, we have studied the effect of GSNO on the expression of 5-LO in human airway A549 cell lines and in primary normal human tracheobronchial epithelial (NHBE) cells in vitro. GSNO at concentrations of 0.5–1 μM caused a 3- to 6-fold increase in 5-LO expression. However, GSNO at > 5 μM significantly inhibited both 5-LO expression and LT production. We also found that airway epithelial cells had γ-glutamyl transpeptidase (γ-GT) activity. The effect of 1 μM GSNO on 5-LO expression was prevented by the γ-GT inhibitor, acivicin, suggesting a convergence of GSNO and CysLT metabolic pathway that may be relevant to asthma. Our data demonstrate that GSNO levels ⩽ 1 μM, likely recapitulating those in the asthmatic airway, increase 5-LO expression, an effect that may increase inflammation and bronchoconstriction. However, GSNO at concentrations > 5 μM suppresses 5-LO expression. These data suggest that GSNO might inhibit 5-LO expression in the clinical setting.
Keywords: 5-lipoxygenase, asthma, leukotriene, S-nitrosoglutathione, S-nitrosylation
5-lipoxygenase (5-LO) is the rate-limiting enzyme in the biosynthesis of leukotrienes (LTs), molecules that cause bronchoconstriction and promote inflammation. It catalyzes the two first steps in arachidonic acid (AA) oxygenation in the pathway by which AA is converted to LTs (1, 2). This pathway involves synthesis of LTA4, which can be converted to the neutrophil chemoattractant, LTB4, or—by addition of glutathione (GSH)—the cysteinyl LT (CysLT), LTC4. LTC4 is bioactivated by cleaving the γ glutamyl bond of the added GSH to form LTD4—or LT-cysteinyl glycine. LTD4 is then cleaved by removal of the glycine, forming LTE4 (1, 3). In response to specific (inflammatory) stimuli, 5-LO undergoes a calcium-dependent translocation from the cytosol to the nuclear envelope and endoplasmic reticulum, where it associates with the 5-LO activating protein to achieve bioactivation (4). Human 5-LO has been purified as a monomeric soluble protein with an molecular weight of ∼ 78 kD (5). The 5-LO gene is not ordinarily abundantly expressed in mammals, and its expression is highly regulated. The promoter of 5-LO gene lacks the typical TATA box; however, it has highly G + C rich regions and contains several consensus-binding sites for transcription factors such as specificity protein (Sp)1 and Sp3 (6, 33).
S-nitrosothiols (SNOs) are NO-thiol adducts that serve as physiologic S-nitrosylating agents. Airway levels are difficult to establish precisely because of dilutional issues and because of ex vivo catabolism involved in studies based on bronchoalveolar lavage and tracheal aspirate samples. Concentrations of endogenous SNOs, such as S-nitrosoglutathione (GSNO), have been estimated to be in the μM range of ∼ 0.5–10 μM in the normal human airway lining fluid and rat brainstem (7, 8). Several studies have reported that airway SNO levels are decreased in patients with asthma and cystic fibrosis (CF) (9–12), and in ovalbumin-induced mouse and guinea-pig models of asthma (13, 35). Indeed, mice lacking the GSNO catabolic enzyme, GSNO reductase, are protected from ovalbumin-induced airway hyperreactivity (13). GSNO has several potential benefits for patients with asthma and CF, including bronchodilation, augmented response to β2 agonists, increased ciliary motility, inhibition of amiloride-sensitive sodium transport, increased expression, maturation and function of both wild-type and ΔF508 mutant CFTR, antimicrobial effects and stimulation of neutrophil apoptosis (8, 11, 13, 15, 17, 21, 24). Taken together, these data suggest that deficiency of GSNO and other SNOs may contribute to the pathophysiology of obstructive lung disease and that the reversal of this defect may be of therapeutic benefit (9–13, 38).
We and others have also demonstrated that the activities of certain transcription factors, such as hypoxia inducible factor 1 and nuclear factor-κB, are regulated by GSNO (19, 20). More recently, we have shown that gene regulation by Sp1 and Sp3 is differentially modified by physiologic and/or pathologic levels of GSNO (15). Because 5-LO expression is regulated—in part—by Sp1/Sp3 (6, 33), and because GSNO metabolism is abnormal in asthma, we have investigated the effect of GSNO on 5-LO expression and activity in human airway epithelial cell lines and primary normal human tracheobronchial epithelial (NHBE) cells in vitro. Here, we report that GSNO levels ⩽ 1 μM are permissive for increased 5-LO expression, whereas GSNO levels > 5 μM in the airway inhibit 5-LO expression and activity.
MATERIALS AND METHODS
Chemical Methods
GSNO was prepared by acid nitrosation followed by neutralization in 10 mM phosphate-buffered saline as previously described (8). It was stored at −80°C until use and assayed by chemiluminescence (NOA 280; Sievers Instruments, Boulder, CO) following reduction in CuCl and cysteine as previously described (9).
Cell Culture
A549 (Type II alveolar epithelial) cells were obtained from American Type Culture Collection (Manassas, VA) and grown in Ham's F12K medium with 10% (vol/vol) fetal calf serum, and 1% (vol/vol) penicillin/streptomycin (Life Technologies, Gaithersburg, MD). Cells were maintained at 37°C in a humidified atmosphere of 5% carbon dioxide and air and passaged at confluence approximately every 4 d.
Primary human tracheobronchial epithelial (NHBE) cells were obtained from Cambrex Inc. (Walkersville, MD). Lung tissue was from a disease-free, nonsmoking 51-yr-old female (Lot number: NHBE 5F1209). Cells were maintained in humidified incubator at 37°C and 5% CO2 and cultured in bronchial epithelial growth medium (BEGM; Cambrex Inc., Walkersville, MD) until cultures were 80–90% confluent.
Western Blot Analysis
Western blot analysis was performed as previously described (14, 15). In brief, whole cell extracts were prepared in 1% NP-40 (Sigma Chemical Co., St. Louis, MO) buffer containing 50 mM Tris-HCl (pH 8.0), 1% NP-40, 150 mM NaCl, 2 μM leupeptin, 1 μM aprotinin and 1 μM pepstatin (Roche Diagnostics, Mannheim, Germany), 1 μM PMSF and 2 μM Na2VO4 (Boehringer Mannheim Corp, Indianapolis, IN). Insoluble material was recovered and sheared by passage through a 25-gauge needle. Protein was quantitated by the BCA Protein Assay Kit (Sigma). Protein (25 μg) was fractioned on a 6% SDS polyacrylamide gel in 25 mM Tris, 192 mM glycine, 0.1% SDS at pH 8.3, then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) using an electrophoretic transfer cell with Tobin Transfer Buffer (25 mM Tris, 192 mM glycine, 20% methanol at pH 8.3). Blots were blocked in Tris-buffered saline–Tween 20 (TBS-T) (TBS-T = 10 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, at pH 8.0), containing 5% nonfat dried milk and probed with a 1:1,000 dilution of anti–5-LO antibodies (Research Diagnostics, Inc., Flanders, NJ) in TBS-T containing 5% nonfat milk (RT, 45 min). Blots were washed and incubated for 30 min with a 1:2,000 dilution of horseradish peroxidase–conjugated anti-mouse antibody (Pierce, Rockford, IL) in TBS-T containing 5% nonfat dried milk for 30 min. Protein was visualized by enhanced chemiluminescence (ECL; Amersham Biosciences, Piscataway, NJ) using Hyperfilm (Amersham Biosciences). Blots were stripped and probed with anti–α-tubulin antibodies (mouse monoclonal IgM, 1:5,000; Biotech, Santa Cruz, CA) as a control for protein loading.
Leukotriene Assay
Cysteinyl leukotrienes (LTC4, LTD4, and LTE4) in whole cell extract were measured using an ACE Competitive Enzyme Immunoassay (Cayman Chemical Co., Ann Arbor, MI). Reagents were prepared using ultra pure water, and the standard curve was diluted using Ham's F12K medium. Results were expressed in pg/ml of sample.
γ-GT Activity
A549 cells were assayed for γ-GT activity as previously described (16). The cells were trypsinized off the plates and assayed for γ-GT activity. One unit of enzyme was defined as the amount of enzyme that released 1 μM/min of p-nitroaniline at 25°C. The protein concentration was determined with the BCA assay (Pierce).
Statistical Analysis
Means were compared by ANOVA followed by the Student's t test. Results are expressed as means ± SEM. P < 0.05 was considered significant.
RESULTS
The Effect of GSNO on 5-LO Expression
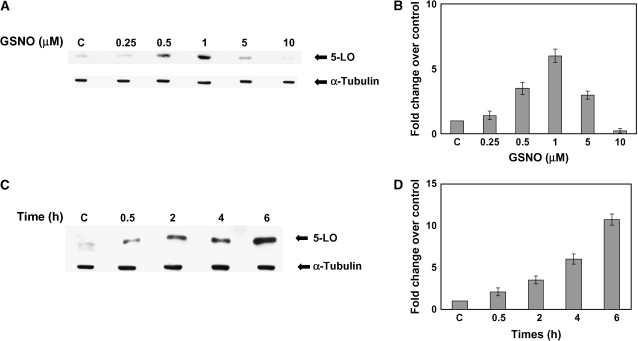
A549 cells and NHBE cells treated with 0.5 or 1 μM GSNO for 6 h had significantly elevated expression of 5-LO (P < 0.005). 5-LO expression was decreased in cells treated with 5 and 10 μM GSNO (Figures 1A and 3C). One micromolar of GSNO increased the 5-LO expression as a function of time. Induction continued for up to 6 h (Figures 1C and 1D).
Figure 1.
S-Nitrosoglutathione concentrations < 5 μM increase, while those > 5 μM decrease, 5-LO expression in A549 cells (A). Western blot analysis was performed on whole cell extracts made from A549 cells treated with various concentrations of GSNO for 6 h. Membranes were stripped and re-probed with a α-tubulin to verify that an equal amount of protein was loaded. (B) The optical densities of the bands were quantified by densitometry. (C) A time course of GSNO induction of 5-LO expression was also evaluated by Western blot analysis. Twenty-five micrograms protein was loaded in each lane for each experiment (n = 3). (D) The optical densities of the bands were quantified by densitometry.
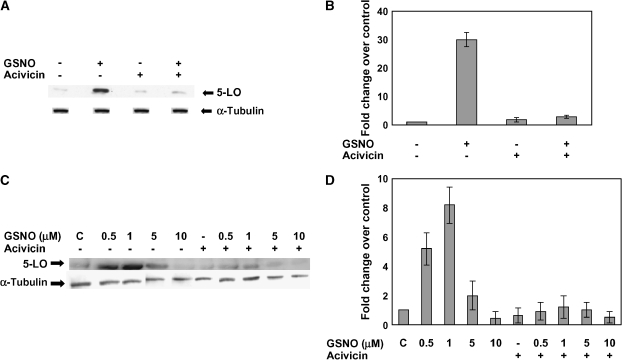
Figure 3.
S-Nitrosoglutathione induction of 5-LO expression is inhibited by acivicin and occurs in primary human tracheal epithelial (NHBE) cells. (A) Western blot analysis was performed on whole cell extracts made from A549 cells treated with 1 μM GSNO in the presence of acivicin (100 μM) for 6 h. The membrane was stripped and reprobed with α-tubulin to verify that an equal amount of protein was loaded. (B) Blots were scanned and densitometry was performed for quantification. Twenty-five micrograms protein was loaded to each lane (n = 2; P < 0.005). (C) Western blot analysis of 5-LO was also performed on whole cell extracts from NHBE cells in the absence or presence of GSNO (0.5, 1, 5, and 10 μM) and acivicin (100 μM) for 6 h. Twenty-five micrograms of protein was loaded in each lane (n = 3). (D) The membrane was stripped and reprobed with α-tubulin to verify that an equal amount of protein was loaded. Plots were scanned and densitometry was performed for quantification.
The Effect of Different S-Nitrosylating Agents on 5-LO Expression
We evaluated S-nitroso-N-acetylcysteine (SNOAC) and NO itself, as well as reduced (GSH) and oxidized (GSSG) glutathione, for their effect on 5-LO expression. Of note, SNOAC had an effect comparable to that of GSNO (Figures 2A and 2B). However, equivalent doses of free radical NO itself were inactive, and neither GSH nor GSSG had significant effects on 5-LO expression (Figures 2A and 2B), suggesting that the mechanism of GSNO action is independent of the free radical NO reactions, oxidation or glutathionylation.
Figure 2.
S-Nitrosoglutathione and S-nitroso-N-acetylcysteine are more active than NO, GSH, or GSSG in affecting 5-LO expression; the effect does not involve glutathionylation or oxidation, and it is both transcriptional and translational. (A) Western blot analysis was performed on whole cell extracts. Lane 1, control; lanes 2 and 3, GSNO at 1 and 10 μM; lanes 4 and 5, GSH at 1 and 10 μM; lanes 6 and 7, GSSG at 1 and 10 μM; lanes 8 and 9, (NO at 1 and 10 μM, prepared anaerobically from a saturated solution in deaerated water); lanes 10 and 11, S-nitroso-N-acetyl-cysteine (SNOAC) at 1 and 10 μM. The membrane was stripped and re-probed with α-tubulin to verify equal amount protein loading. Twenty-five micrograms of protein was loaded in each lane for each experiment (n = 3). (B) Blots were scanned and densitometry was performed for quantification. (C) Western blot analysis was performed on whole cell extracts made from A549 cells, treated with 15 or 25 μg/ml actinomycin D beginning 2 h before 6 h exposure to 1 μM GSNO—or in the absence of GSNO. Cells were also treated with 50 or 75 μg/ml cycloheximide for 15 min before and during a 6-h exposure to 1 μM GSNO and throughout the 6-h GSNO exposure. A, actinomycin D; C, cycloheximide. Twenty five micrograms of protein was loaded in each lane for each experiment (n = 3; P < 0.005). (D) Blots were scanned and densitometry was performed for quantification.
Transcriptional and Translational Control of 5-LO by GSNO
Both actinomycin D (15 and 25 μg/ml) and cycloheximide (50 and 75 μg/ml) inhibited the effect of 1 μM GSNO on 5-LO expression (Figures 2C and 2D). Actinomycin D and cycloheximide inhibited 5-LO expression somewhat at baseline (Figures 2C and 2D)—interestingly, cycloheximide somewhat more than actinomycin.
Effect of GSNO on CysLT Levels
CysLT production in control cells was 218 ± 10 pg/ml (mean ± SD). After a 6-h incubation with 1 μM of GSNO, CysLT levels (276 ± 21 pg/ml) were modestly higher than control values. However, higher concentrations of GSNO dose-dependently decreased CysLT levels to 181 ± 42 (5 μM) and 172 ± 13 (10 μM) pg/ml, a significant inhibition compared with the control values (P < 0.05).
The Role of γ-GT in Regulating the Effect of GSNO on 5-LO Expression
γ-GT regulates GSNO bioactivities in a variety of cell and organ systems by breaking down the cell membrane–impermeable GSNO to cell-permeable S-nitrosocysteinyl-glycine (12, 14, 20). Therefore, we evaluated the role of γ-GT in modifying the GSNO-induced effects on the 5-LO expression. We measured γ-GT activity in A549 cells. The cells had 30.9 ± 1.72 mU γ-GT activity per mg protein. Then, we treated the cells with 1 μM GSNO in the presence or absence of 100 μM acivicin, an inhibitor of γ-GT–mediated GSNO bioactivation (13, 14, 18, 20, 21). Acivicin significantly decreased GSNO induction of 5-LO expression in A549 cells (P < 0.005; Figures 3A and 3B). Of note, effect of 0.5 and 1 μM GSNO on 5-LO expression was also significantly inhibited by acivicin in NHBE cells, though the effect was incomplete (Figures 3C and 3D), suggesting that NHBE cells may have γ-GT–independent mechanisms for allowing GSNO to be active inside the cell. Consistent with an additional pathway, the effect of 10 μM GSNO to inhibit 5-LO expression was not significantly prevented by acivicin.
GSNO Regulation of 5-LO Likely Involves Sp1/Sp3 Transcription Factors
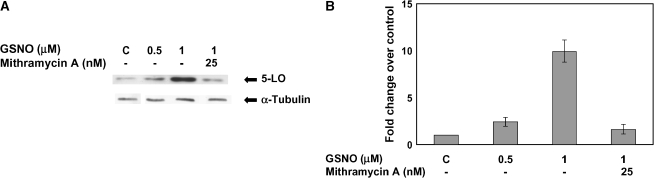
We tested the possibility that the transcriptional component of the GSNO effect on 5-LO involves the Sp binding site. We have previously shown that GSNO concentrations ⩽ 10 μM increase Sp3 DNA binding, increase Sp3-dependent gene transcription, and inhibit Sp1 DNA binding (15). Higher (10–100 μM) levels, on the other hand, decrease Sp3 binding and reverse Sp1 binding. The threshold for the inhibitory effect of GSNO on 5-LO expression is similar to the threshold for increased (inhibitory) Sp1 binding in regulation of cftr transcription (15). Other investigators have also shown that GC-rich Sp binding sites are critical to the transcriptional regulation of 5-LO (34). A549 cells were treated with Mithramycin A, a specific inhibitor of the GC-rich Sp1/Sp3 binding site (39), through the 6-h incubation period with 1 μM GSNO. Mithramycin A blocked the GSNO induction of 5-LO (P < 0.003; Figures 4A and 4B).
Figure 4.
5-LP is regulated by GSNO and mithramycin A, an Sp1/Sp3 transcription binding site inhibitor. (A) Western blot analysis was performed on whole cell extracts made from A549 cells treated with 0.5 μM and 1 μM GSNO. In addition, the cells were treated with 1 μM GSNO in the presence of 25 nM mithramycin A for a 6-h incubation period. The membrane was stripped and re-probed with α-tubulin to verify that an equal amount of protein was loaded. (B) Blots were scanned and densitometry was performed for quantification. Twenty-five micrograms protein was loaded in each lane (n = 3; P < 0.003).
Proteosome-Dependent Degradation of 5-LO
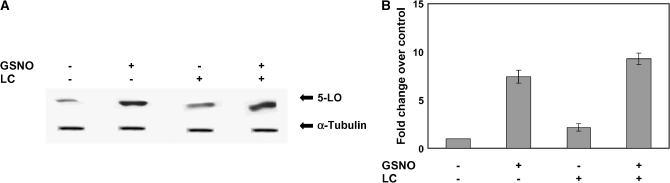
Whole cell extracts were made from A549 cells treated with GSNO in the presence or absence of the proteasome inhibitor clasto-lactacystin β-lactone (LC). The level of 5-LO protein was modestly but significantly higher in cells treated with LC. Cells treated with 1 μM GSNO and 25 μM LC had higher levels still of 5-LO protein (P < 0,003; Figures 5A and 5B); the effects were additive.
Figure 5.
Proteosomal inhibition moderately increases 5-LO expression; the effect is less than that of 1 μM GSNO, and is additive with that of 1 μM GSNO. (A) Western blot analysis was performed on whole cell extracts from A549 cells treated with the protesome inhibitor, clasto-lactacystin β-lactone (25 μM), in the presence or absence of 1 μM GSNO. The membrane was stripped and reprobed with α-tubulin to verify that an equal amount of protein was loaded. Twenty-five micrograms of protein was loaded in each lane (n = 3; P < 0.003). (B) Blots were scanned and densitometry was performed for quantification.
DISCUSSION
5-LO is the rate-limiting enzyme in the biosynthetic pathway for LTs. Cellular 5-LO expression increases in association with inflammatory responses (22). Excess production of CysLTs has been implicated in several inflammatory diseases, and there has been considerable interest in the development of 5-LO inhibitors and inhibitors of CysLT receptors for therapeutic use (23, 28, 29, 32). Suppression of LT synthesis can be achieved by inhibition of phospholipases and/or by direct inhibition of 5-LO or FLAP (29). Antagonists of cysteinyl leukotriene receptor (CysLTR) 1 are also commonly used in the management of asthma and allergic rhinitis.
Because GSNO relaxes airway smooth muscle, augments ventilation-perfusion matching, improves ciliary motility, has antimicrobial effects, and augments airway hydration, there is interest in GSNO replacement—and/or therapy with an S-nitrosylating agent resistant to catabolism—as a therapy for asthma and CF (8, 11, 13, 14, 18, 20, 38). We have recently shown that GSNO can also affect Sp1- and Sp3-regulated gene expression in the case of cftr transcription in airway epithelial cells (15). As Sp1 and Sp3 also regulate to 5-LO transcription (6, 33), and Cys LTs are important to asthma, we have studied the interaction between GSNO and 5-LO expression and activity in airway epithelial cells. We report that GSNO levels < 5 μM are associated with increased 5-LO expression, while levels > 5 μM inhibit 5-LO expression. Of note, based on airway lavage studies, airway GSNO levels are likely < 1 μM in subjects endotracheally intubated for severe asthma (10) and in subjects with both mild asthma and mild CF (9, 10, 12). In human asthma and in an ovalbumin challenge model of guinea pig asthma, GSNO catabolism is accelerated (10, 35). Further, recent evidence demonstrates that the GSNO reductase knockout mouse is resistant to experimental asthma (13). Accelerated catabolism could decrease airway GSNO levels, potentially increasing 5-LO expression. Thus, GSNO deficiency in the asthmatic airway might favor increased Cys LT production, while levels over 5 μM could inhibit Cys LT production.
Our data indicate that GSNO regulates 5-LO expression, at least in part, at the level of transcription; its expression is moderately inhibited both by actinomycin D. The human 5-LO gene promoter region lacks TATAA or CCAAT boxes, but has repeated GC-rich elements that characteristically regulate so-called “housekeeping” genes. Indeed, the transcription factors Sp1/Sp3—which bind these GC rich regions, and are important for regulation of TATA-less genes (36, 37)—are required for 5-LO transcription (33). Interestingly, naturally occurring mutations in the 5-LO promoters only slightly alter 5-LO promoter activity in reporter gene assays, but have an impact on the response of asthma to 5-LO inhibitors (27, 28, 30, 31, 34, 35). GSNO at levels < 10 μM increases Sp3 expression and binding, while higher concentrations inhibit Sp3 binding and increase Sp1 binding (15). The relevance of this regulatory Sp site to the transcriptional effects of GSNO on 5LO is suggested both by parallel effects with regard to the effects of GSNO on Sp1/Sp3 binding and 5-LO expression, and by inhibition of the GSNO effect on 5-LO expression by MMA. The biochemical mechanism by which GSNO regulates Sp activity—though proposed to involve regulation of Sp zinc-finger binding and Sp ubiquitination (15)—have not been clarified. Additional pathways may also prove relevant.
Mechanisms by which GSNO regulates protein expression are not limited to transcriptional effects (Figures 2C and 2D). We and others have shown that SNO modifications of proteins involved in ubiquitin–proteasome pathways also affect protein expression (20, 24, 25). Further, our data suggest that 5-LO expression is regulated at baseline and after exposure to 1 μM GSNO, in part, by a post-transcriptional mechanism (Figure 2). Therefore, we studied whether modification of this pathway might be involved in regulation of 5-LO expression. Our data suggest that 5-LO degradation involves, to some extent, a proteasome-dependent mechanism; however, GSNO does not substantially affect 5-LO through altering this pathway; other mechanisms are likely involved in the post-transcriptional effect of GSNO on 5-LO expression. In this regard, post-translational regulation of 5-LO function has been described (26, 28); this could be affected by GSNO, as suggested by the fact that GSNO appears to affect 5-LO activity out of proportion to its effect on 5-LO expression.
The γ-GT GSNO product, S-nitrosocysteinyl glycine, is more cell-permeable and bioactive than GSNO in many systems (12, 14, 20). Our data suggest that γ-GT function is important for the bioactivation of GSNO to improve its cell permeability and effect on 5-LO expression. Further, we have shown that there is γ-GT activity in A549 cells, and previous studies have shown that γ-GT is expressed by macrophages in human airways (16). Of note, pulmonary expression of γ glutamyl leukotrienase or γ-GT–related (γ-GT–rel) enzyme cleaves the γ-GT bond of LTC4, and our data do not exclude the possibility that γ-GT–rel—which is abundantly expressed in the airways (40)—can also cleave GSNO. The expression, activity, and interaction of these enzymes may be of substantial interest in the asthmatic airway.
In summary, levels of GSNO < 5 μM increase 5-LO expression, but do not significantly impact 5-LO activity. On the other hand, higher levels inhibit 5-LO expression and activity. The transcriptional component of this regulation likely involves, at least in part, Sp1 and Sp3, consistent with our previous report concerning the affect of GSNO on Sp1- and Sp3-dependent gene regulation. This may be of particular interest given the potential pharmacogenomic implications of mutations of the GC-rich consensus Sp1/Sp3 binding site and the 5-LO promoter (33, 34). Also, as with other studies on GSNO, γ-GT activity appears to be important for the regulation and bioactivation of GSNO. This suggests that γ glutamyl bond cleavage could be involved in the regulation both of LT activity—primarily through γ-GT–rel—and 5-LO expression through GSNO bioactivation, likely primarily through γ-GT. Taken together, these studies suggest for the first time an interaction between S-nitrosothiol metabolism and leukotriene metabolism in the asthmatic airway. It may be important to consider these interactions in designing both basic and clinical studies involving each class of compounds.
Funded by: NIH RO1 #2 RO1 HL 59337, NIH Asthma Center Grant #1U19-A134607, Cystic Fibrosis Foundation Grant #Zaman04GO, and the Ivy Foundation.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0336RC on January 13, 2006
Conflict of Interest Statement: K.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.R.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.F.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.M.G. has consulted for Nitrox, LLC., a company with an interest in developing S-nitrosoglutathione as a treatment for cystic fibrosis.
References
- 1.Drazen J, Austen KF. Leukotrienes and airway responses. Am Rev Respir Dis 1997;136:985–998. [DOI] [PubMed] [Google Scholar]
- 2.Rouzer C, Kargman S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J Biol Chem 1988;623:10980–10988. [PubMed] [Google Scholar]
- 3.Miller DK, Gillard JW, Vickers PJ, Sadowski S, Leveille C, Mancini JA, Carleson P, Dixon RA, Ford-Hutchinson AW, Fortin R. Identification and isolation of a membrane protein necessary for leukotriene production. Nature 1990;18:278–281. [DOI] [PubMed] [Google Scholar]
- 4.Rouzer C, Samuelsson B. The importance of hydroperoxide activation for the detection and assay of mammalian 5-lipoxygenase. FEBS Lett 1986;204:293–296. [DOI] [PubMed] [Google Scholar]
- 5.Radmark O, Zhang YY, Hammarberg T, Radmark O, Samuelsson B, Ng CF, Funk C, Loscalzo J. Analysis of a nucleotide-binding site of 5-lipoxygenase by affinity labeling:binding characteristic and amino acid sequences. Biochem J 2000;351:697–707. [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshiko S, Radmark O, Samuelsson B. Characterization of the human 5-lipoxygenase gene promoter. Proc Natl Acad Sci USA 2000;87:9073–9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kluge I, Gutleck-Amsler U, Zollinger M, Do KQ. S- nitrosoglutathione in rat cerebellum: identification and quantification by liquid chromatography-mass spectrometry. J Neurochem 1997;69:2599–2607. [DOI] [PubMed] [Google Scholar]
- 8.Gaston B, Reilly J, Drazen JM, Fackler J, Ramdev P, Arnelle D, Mullins ME, Sugarbaker DJ, Chee C, Singel DJ. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci USA 1993;90:10957–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dweik R, Comhair S, Gaston B, Thunnissen F, Farver C, Thomassen M, Kavuru M, Hammel J, Abu-Soud H, Erzurum S. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA 2001;98:2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaston B, Sears S, Woods J, Hunt J, Ponaman M, McMahon T, Stamler JS. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet 1998;351:1317–1319. [DOI] [PubMed] [Google Scholar]
- 11.Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med 2002;165:922–926. [DOI] [PubMed] [Google Scholar]
- 12.Grasemann H, Gaston B, Fang K, Paul K, Ratjen F. Decreased levels of nitrosothiols in the lower airways of patients with cystic fibrosis and normal pulmonary function. J Pediatr 1999;135:770–772. [DOI] [PubMed] [Google Scholar]
- 13.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science 2005;308:1618–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaman K, McPherson ME, Vaughan J, Hunt JF, Mendes F, Gaston B, Palmer L. S-nitrosoglutathione increases cystic fibrosis transmembrane regulator maturation. Biochem Biophys Res Commun 2001;284:L65–L70. [DOI] [PubMed] [Google Scholar]
- 15.Zaman K, Palmer L, Doctor A, Hunt JF, Gaston B. Concentration-dependent effects of endogenous S-nitrosoglutathione on gene regulation by specificity proteins Sp3 and Sp1. Biochem J 2004;380:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanigan MH, Frierson HF Jr. Immunohistochemical detection of γ-glutamyl transpeptidase in normal human tissue. J Histochem Cytochem 1996;44:1101–1108. [DOI] [PubMed] [Google Scholar]
- 17.Andersson C, Gaston B, Roomans GM. S-Nitrosoglutathione induces functional ΔF508-CFTR in airway epithelial cells. Biochem Biophys Res Commun 2002;297:552–557. [DOI] [PubMed] [Google Scholar]
- 18.Howard M, Fischer H, Roux J, Santos B, Gullans S, Yancey P, Welch W. Mammalian osmolytes and S-nitrosoglutathione promote ΔF508 CFTR protein maturation and function. J Biol Chem 2003;278:35159–35167. [DOI] [PubMed] [Google Scholar]
- 19.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry 2001;40:1688–1693. [DOI] [PubMed] [Google Scholar]
- 20.Palmer L, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol 2000;58:1197–1203. [DOI] [PubMed] [Google Scholar]
- 21.Lipton AJ, Johnson M, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature 2001;413:171–174. [DOI] [PubMed] [Google Scholar]
- 22.Murakami M, Austen KF, Bingham CO, Friend DS, Penkose JF, Arm DP. Interleukin-3 regulates development of the 5-lipoxygenase/leukotriene C4 synthase pathway in mouse mast cells. J Biol Chem 1995;270:22653–22656. [DOI] [PubMed] [Google Scholar]
- 23.Cashman R. Leukotriene biosynthesis inhibitors. Pharm Res 1985;5:253–261. [DOI] [PubMed] [Google Scholar]
- 24.Yao D, Gu Z, Nakamura T, Shi Z, Ma Y, Gaston B, Palmer L, Rockenstein E, Zhang Z, Masliah E, et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of Parkin regulates its E3 ligase activity. Proc Natl Acad Sci USA 2004;101:10810–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer LA, Brown-Steinke K, Gaston B. Identification of S-nitrosylated proteins in the HIF-1alpha ubiquitination pathway. Am J Respir Crit Care Med 2002;165:A627. [Google Scholar]
- 26.Pouliot M, McDonald P, Khamzina L, Borgeat P, McColl S. Granulocyte-macrophage colony-stimulating factor enhances 5-lipoxygenase levels in human polymorphonuclear leukocytes. J Immunol 1994;152:851–858. [PubMed] [Google Scholar]
- 27.Lee CW, Lewis RA, Tauber AI, Mehrotra M, Corey EJ, Austen KF. The myeloperoxidase-dependent metabolism of leukotrienes C4, D4, and E4 to 6-trans-leukotriene B4 diastereoisomers and the subclass-specific S-diastereoisomeric sulfoxides. J Biol Chem 1983;24:15004–15010. [PubMed] [Google Scholar]
- 28.Watts RA, Issac JDL. Immunotherapy of rheumatoid arthritis. Ann Rheum Dis 1992;51:577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claesson HE, Dahlen SE. Asthma and leukotrienes: antileukotrienes as novel anti-asthmatic drugs. J Intern Med 1999;245:205–227. [DOI] [PubMed] [Google Scholar]
- 30.Brach MA, de Vos S, Arnold C, Gross HJ, Mertelsmann R, Herrmann F. Leukotriene B4 transcriptionally activates interleukin-6 expression involving NF-kappa B and NF-IL6. Eur J Immunol 1992;10:2705–2711. [DOI] [PubMed] [Google Scholar]
- 31.Stankova J, Rola-Pleszczynski M. Leukotriene B4 stimulates c-fos and c-jun gene transcription and AP-1 binding activity in human monocytes. Biochem J 1992;282:625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chanarin N, Johnston SL. Leukotrienes as a target in asthma therapy. Drugs 1994;47:12–24. [DOI] [PubMed] [Google Scholar]
- 33.Silverman ES, Du J, De Sanctis GT, Radmark O, Samuelsson B, Drazen JM, Collins T. Egr-1 and Sp1 interact functionally with the 5-lipoxygenase promoter and its naturally occurring mutants. Am J Respir Cell Mol Biol 1998;19:316–323. [DOI] [PubMed] [Google Scholar]
- 34.In KH, Asano K, Beier D, Grobholz J, Finn PW, Silverman EK, Silverman ES, Collins T, Fischer AR, Keith TP, et al. Naturally occurring mutations in the human 5-lipoxygenase gene promoter that modify transcription factor binding and reporter gene transcription. J Clin Invest 1997;99:1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drazen JM, Yandava CN, Dube L, Szczerback N, Hippensteel R, Pillari A, Israel E, Schork N, Silverman ES, Katz DA, et al. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet 1999;22:168–170. [DOI] [PubMed] [Google Scholar]
- 36.Hagen G, Denning J, Preiss A, Beato M, Suske G. Functional analysis of the transcription factor Sp4 reveal properties distinct from Sp1 and Sp3. J Biol Chem 1995;271:18925–18930. [DOI] [PubMed] [Google Scholar]
- 37.Suske G. The Sp-family of transcription factors. Gene 1999;238:291–300. [DOI] [PubMed] [Google Scholar]
- 38.Fang K, Johns R, Macdonald T, Kinter M, Gaston B. S-Nitrosoglutathione breakdown prevents airway smooth muscle relaxation in the guinea-pig. Am J Physiol Lung Cell Mol Physiol 2000;279:L716–L721. [DOI] [PubMed] [Google Scholar]
- 39.Ryu H, Lee J, Zaman K, Kubilis J, Ferrante RJ, Ross BD, Neve R, Ratan RR. Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons. J Neurosci 2003;23:3597–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han B, Luo G, Shi ZZ, Barrios R, Atwood D, Liu W, Habib GM, Sifers RN, Corry DS, Lieberman M. Gamma-glutamyl leukotrienase: a novel endothelial membrane protein, is specifically responsible for leukotriene D (4) function in vivo. Am J Pathol 2002;161:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]