Abstract
Anaplastic thyroid carcinoma (ATC) is a highly aggressive form of the disease for which new therapeutic options are desperately needed. Previously, we showed that the high affinity PPARγ agonist, RS5444, inhibits cell proliferation of ATC cells via induction of the cyclin dependent kinase inhibitor p21WAF1/CIP1 (p21). We show here that upregulation of RhoB is a critical step in PPARγ-mediated activation of p21-induced cell stasis. Using multiple, independently derived ATC cell lines, we find that treatment with RS5444 leads to upregulation of RhoB and subsequent activation of p21, and that silencing of RhoB by RNAi blocks the ability of RS5444 to induce p21 and to inhibit cell proliferation. Our results show that transcriptional regulation of RhoB by the nuclear transcription factor PPARγ is responsible for induction of p21 mRNA and protein. We further implicate RhoB as a key signaling effector for growth inhibition of ATC, as treatment with a histone deacetylase (HDAC) inhibitor previously shown to increase RhoB expression in lung cancer cells caused upregulation of RhoB in ATC cells, accompanied by increased expression of p21 and inhibition of cell proliferation; this effect occurred even in ATC cells that are unresponsive to RS5444 due to lack of expression of PPARγ. Our results implicate RhoB as a novel intermediate in critical signaling pathways and as an additional target for therapeutic intervention in ATC.
Keywords: Peroxisome proliferator-activated receptor gamma, RhoB, anaplastic thyroid carcinoma, p21WAF1/CIP1, histone deacetylase inhibitor
Introduction
Thyroid cancer is the most common endocrine cancer with an approximate incidence rate of 33,550 newly diagnosed cases per year in the U.S (1). Despite a generally good prognosis, over 1,500 thyroid cancer patients die of their disease annually. Anaplastic thyroid carcinoma (ATC) is the most poorly differentiated, highly aggressive form of thyroid cancer with a median survival of about four months, a one year survival rate of less than 10%, and 99% lethality overall. If detected early, extensive surgery combined with radiotherapy offers the best chance of local disease control. To date, chemotherapy (usually doxorubicin plus cisplatin or paclitaxel) has shown only palliative benefit in advanced ATC [reviewed in (2)], but neither agent has demonstrated curative potential. More effective targeted therapies based on a better understanding of the molecular and cellular signaling pathways disrupted in ATC are therefore needed.
Peroxisome proliferator activated receptor gamma (PPARγ) agonists have chemopreventive and antitumor activity against a variety of human cancers associated with transcriptional activation of PPARγ (3-6) . These findings are in keeping with the hypothesis that PPARγ can act as a tumor suppressor in several cancers including thyroid cancer (7, 8). Mechanisms of PPARγ-mediated antitumor activity include induction of differentiation, promotion of cell cycle arrest, antiangiogenic effects and induction of apoptosis (9). In more differentiated thyroid cancers, PPARγ agonists have been shown to induce apoptosis through cytochrome C/caspase 3 and PTEN-Akt pathways (10).
We and others have recently demonstrated that the PPARγ agonist RS5444 inhibits cell proliferation and induces tumor differentiation (9, 11, 12). In our previous work, we found that RS5444 inhibited ATC tumor growth in vitro and in vivo but did not induce apoptosis as a single agent (9). We showed that RS5444 is dependent upon PPARγ for its antitumor activity since GW9662, a pharmacological antagonist of PPARγ, blocked inhibition of cell growth by RS5444 (9). We also found that the cyclin kinase inhibitor p21CIP1/WAF1 (p21) was upregulated by RS5444. To date, p21 has been implicated as a modulator of PPARγ-mediated inhibition of cell proliferation, but this evidence has been limited to correlative observations (13-16). In our recent study, we found that p21 was required for PPARγ-mediated growth inhibition by RS5444 in ATC cells, and that combinatorial treatment of ATC cells with RS5444 and paclitaxel resulted in apoptotic synergy. Silencing experiments demonstrated the requirement of p21 for this observed synergy (9), but the mechanism by which PPARγ agonists might upregulate p21 remained unknown.
RhoB is a member of the Ras superfamily of isoprenylated small GTPases, which regulate actin stress fibers and vesicle transport (17, 18). Membrane association of RhoB occurs through either geranylgeranylated (RhoB-GG) or farnesylated (RhoB-F) modifications. RhoB is required for apoptosis in transformed cells that are exposed to farnesyltransferase inhibitors, DNA-damaging agents or paclitaxel (19). In cancer cells, RhoB modulates proliferation, survival, invasion and angiogenic capacity (17). RhoB is not mutated in cancer, but its altered expression and activity appear crucial to cancer progression and therapeutic responses. Farnesyl transferase inhibitors (FTI) upregulate RhoB levels and this upregulation of RhoB can mediate phenotypic reversion, growth inhibition, cytoskeletal actin reorganization and apoptosis (20).
We now define a sequential pathway whereby the thiazolidinedione (Tzd) RS5444 acts via a PPARγ-dependent mechanism to upregulate RhoB leading to increased expression of p21 followed by attenuation of cell proliferation. The elaboration of this novel signaling pathway triggered by PPARγ agonists provides insight into how to target such agents for treatment of ATC. We now demonstrate that the high-affinity HDAC inhibitor, FK228 (a.k.a. romidepsin), previously shown to stimulate RhoB expression in lung cancer cell lines (21), also inhibits ATC cell proliferation via p21 in a RhoB-dependent fashion. These results identify RhoB upregulation as a key step for targeting ATC cell proliferation and tumor progression.
Materials and Methods
Chemicals
PPARγ agonists RS5444 and troglitazone were kindly provided by Daiichi Sankyo, Inc. GW9662 was purchased from Sigma-Aldrich (St. Louis, MO), FK228 (NSC 630176, depsipeptide or romidepsin) was a gift from Gloucester Pharmaceuticals, Inc. (Cambridge MA) and Division of Cancer Treatment and Diagnosis, National Cancer Institute. Rosiglitazone was obtained from ChemPacific (Baltimore MD).
Cell Culture
DRO90−1 (DRO) and ARO81 (ARO) anaplastic thyroid carcinoma cell lines were kindly provided by Dr. G.J. Juillard (University of California-Los Angeles) as were KTC2 and KTC3 anaplastic thyroid carcinoma cell lines by Dr. Junichi Kurebayashi of Kawasaki Medical School (22). Please note that a recent publication indicates that DRO and ARO cell lines may be of questionable thyroid origin (23). THJ-16T and THJ-11T cells were established in the Copland laboratory derived from human anaplastic thyroid carcinoma tumor tissues received from Dr. Trad Wadsworth (East Virginia Medical School) and Dr. Clive Grant (Mayo Clinic). Cells were cultured in RPMI 1640 medium (Cellgro, Herndon VA) and proliferation studies with 10 nM RS5444 and 1 ng/ml FK228 were done as previously described (9, 24). For morphology studies, cells were plated in 12-well plates at initial concentrations of 2.5 × 104 cells/well. Cells were treated with either DMSO or 10 nM RS5444 (24 hrs). After treatment, phase images were obtained on an inverted microscope (Olympus IX71, C Squared Corporation, Pittsburgh PA). For real time PCR studies, cells were plated in 60 mm plates at 50% confluence and treated with 10 nM RS5444 for indicated incubation periods. For immunoblotting analyses, cells were plated in 60 mm plates at initial concentrations of 3 × 105 cells/plate and were treated with either 10 nM RS5444, 100 nM rosiglitazone, 1 μM troglitazone, 10 μM GW9662, 1 ng/ml FK228, or indicated combinations for indicated incubation periods.
Lentiviral Infection
Lentiviral constructs for the packaging of self-inactivating lentiviruses were made in the pLKO.1 vector (SigmaAldrich, St. Louis MO). The target sequence for PPARγ was 5’– GACAACAGACAAATCACCATT –3’ and a random scrambled sequence was used for the nontarget vector (SigmaAldrich). Lentiviruses were produced by transient transfection in 293FT cells (Invitrogen) using ViraPower (Invitrogen) and Lipofectamine 2000 (Invitrogen) as per manufacturer's protocol. Target ATC cells were plated in 10 cm plates and grown to 70% confluence for infection of lentivirus along with 5 μg/ml polybrene per manufacturer's protocol. After 48 hrs, 2 μg/ml puromycin (SigmaAldrich) was added for selection of infected cells expressing shRNA.
Luciferase Reporter Gene Analysis
Specific activation of PPRE3-tk-luc (R. Evans, Salk Institute, La Jolla, CA) by RS5444 was demonstrated by dual luciferase assay. An 1876 bp RhoB promoter-luciferase (Daniel Tovar, Institut Claudius Regaud) was also examined for responsiveness to 10 nM RS5444 or 1 ng/ml FK228. Cells were transfected, treated with DMSO control (1:1000), 10 nM RS5444 or or 1 ng/ml FK228 for 24 hrs and then luciferase activity was measured as previously described (9).
siRNA Transfection
Cells were either plated in triplicate at 4.0 × 104 cells/ml in 6-well plates or 2 × 104 cells / well in 12-well plates, allowed to adhere overnight, and then transfected for 24 − 72 hrs with 2 to 5 μg of either scrambled, or RhoB (SI00058933 RhoB 1, SI00058912 RhoB 3 or SI00058926 RhoB 4) specific siRNA duplexes (Qiagen Valencia, CA) using RNAiFect reagent (Qiagen, Valencia, CA). After transfection, cells in the 6-well plates were treated with either DMSO, 10 nM RS5444 or 1 ng/ml FK228 for 24 hrs and then analyzed by either real time PCR or immunoblotting. Cells in the 12-well plates were exposed for 6 days with medium and drug changed every 48 hrs. After 6 days, cells were washed with PBS (Cellgro), trypsinized and counted by Beckman Coulter Counter.
Cell Lysis and Immunoblotting
Cellular lysates and tumor tissue extractions were collected, analyzed for protein concentration and transferred to Immobilon-P membranes as previously described (9). The membranes were hybridized with antibodies (Santa Cruz, Cell Signaling, Sigma-Aldrich) as indicated overnight at 4°C followed by treatment with species-specific horseradish peroxidase IgG (Jackson labs) for 45 mins at room temperature. Detection was performed using Supersignal West Pico chemiluminescence kit (Pierce). Protein expression from Western analysis was quantitated (Image Quant 5.0 Molecular Dynamics, GE Healthcare, Piscataway, NJ) and mean ± S.D. is reported for replicates of 3.
Plasmids
pcDNA3.1 was purchased from Invitrogen and pcDNA3.1-dominant negative RhoB was kindly provided by Séverine Steuve (Free University of Brussels). The RhoB promoter linked to a luciferase reporter gene plasmid (hRB rhoB promoter/luciferase 1876 bp) was kindly provided by Dr. Daniel Tovar (Institut Claudius Regaud, France). For transient transfection, cells were plated in 60 mm plates and transfected using Lipofectamine 2000 (Invitrogen, Carlsbad CA) for 24 hrs followed by additional 24 hrs after which cells were treated with 10 nM RS5444, 1 ng/ml FK228 or DMSO. Cells were then collected and mRNA was isolated using RNAqueous (Ambion, Austin TX).
RNA Isolation, Real Time Quantitative RT-PCR
Total mRNA was isolated from cells using RNAqueous (Ambion) per the manufacturer's protocol followed by ethanol precipitation. Two-step quantitative reverse transcriptase-mediated real-time PCR (QPCR) was used to measure changes in mRNA levels of target genes in ATC cells as previously described (9). Applied Biosystems’ assays-on-demand assay mix of primers and TaqMan® MGB probes (FAM™ dye-labeled) for RhoB (Hs00269660_s1), RhoA (Hs00357608_m1), RhoC (Hs00237129_m1), Rac1 (Hs00251654_m1), cdc42 (Hs00741586_mH), p21 (CDKN1A Hs00355782_m1) and 18S rRNA (Hs99999901_s1) were used for QPCR measurements. Data was normalized to 18s rRNA for each sample. Fold change values between treated (RS5444 or FK228) and control samples were calculated using the ΔΔCt method (25).
Xenograft Studies
Tumor implantation of DRO, ARO and THJ-11T cells for xenograft studies were performed as previously described (9).
Cell death analysis
Cells were plated and grown to 50% confluence in 100 mm plates followed by 24 hour treatment with either DMSO control, 10 nM RS5444, 1 ng/ml FK228 or 0.8% TX-100 (Sigma Aldrich). Media was collected and TX-100 samples were serially diluted as standards for cell death percentage. Media was plated and incubated with LDH (Thermoelectron) at 1:10 for 30 minutes in the dark and then the absorbance was read at 340 nm on a Spectrophotometer (Molecular Devices). For DAPI studies, cells were plated on slides at 50% confluence overnight and then incubated in DAPI (Dako) for 3 minutes followed by washing with PBS + 0.05% Tween. Apoptosis was characterized by positive DAPI staining and 3 microscopic fields in each group were used to calculate the percentage of DAPI positive cells. All slides were analyzed under fluorescence light obtained by an Olympus microscope (Olympus IX71, C Squared Corporation, Pittsburgh PA).
Statistical Analysis
Data are presented as the mean ± SD and comparisons of treatment groups were analyzed by 2 – tailed paired Student's t test. Data for comparison of multiple groups are presented as mean ± SD and were analyzed by ANOVA. p < 0.05 was considered statistically significant.
Results
Regulation of cell proliferation by RS5444 is PPARγ-dependent
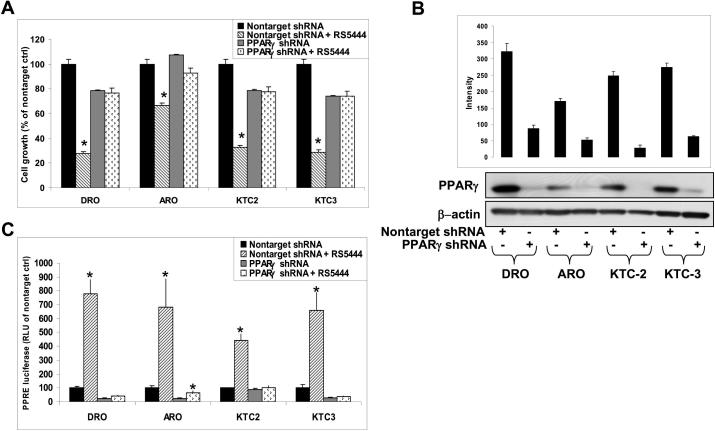
We previously demonstrated that DRO cells were growth inhibited by 10 nM RS5444 through a PPARγ-dependent mechanism (9). We now confirm and extend this finding to four human cell lines (DRO, ARO, KTC2 and KTC3) that have been engineered to stably express lentiviral shRNA against PPARγ (Figure 1A). Cells silenced for PPARγ expression (PPARγ shRNA) expressed little or no PPARγ as compared to nontarget lentiviral shRNA control cells (Figure 1B). Cells were then tested for transcriptional response to PPARγ by transient transfection of PPRE-tk-luciferase as a transcriptional reporter for PPARγ and showed loss of transcriptional response to 10 nM RS5444 (Figure 1C). These results show that PPARγ-induced growth inhibition is a general property of ATC cells.
Figure 1. RS5444 mediated growth inhibition is PPARγ-dependent.
A. Cell proliferation assay as described in Materials and Methods of PPARγ shRNA lentiviral infected cell lines were dependent upon PPARγ for RS5444-mediated growth inhibition. Cell numbers were counted on day 7 and data were plotted as percent of nontarget control ± S.D. * indicates p<0.05 when compared to untreated control. B. Western analysis confirms that these cell lines were silenced for PPARγ protein expression. Nontarget shRNA controls demonstrated endogenous PPARγ expression that is equivalent to that of noninfected cells. C. PPARγ shRNA functionally blocked PPARγ-mediated transcription as shown in these same cell lines that were transiently transfected with a PPRE3-tk-luc reporter and treated with 10 nM RS5444 for 24 hrs. Firefly luciferase activity was normalized to Renilla luciferase activity and graphed as RLU as compared to nontarget control ± S.D.
PPARγ agonists upregulate RhoB expression, which is dependent upon PPARγ
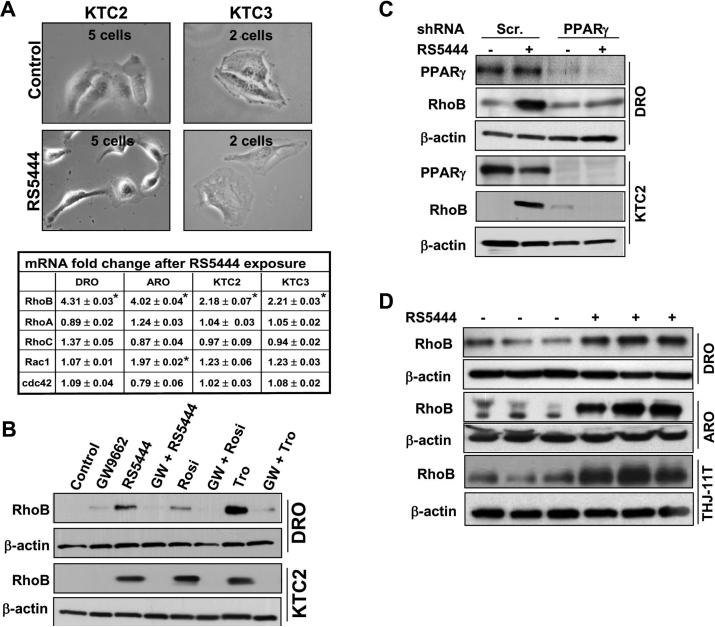
We observed altered cellular morphology following treatment with 10 nM RS5444 (Figure 2A), which led us to examine the potential involvement of members of the RhoGTPase family of small GTPases, as these are known to play roles in regulating the actin cytoskeleton (17). Since the primary effects of PPARγ are at the level of transcriptional regulation of target genes, we used Real Time PCR to rapidly screen for changes in mRNA levels of the members of the RhoGTPase family, and found that RS5444 consistently upregulated RhoB expression in all four cell lines examined (Figure 2A). Upregulation of RhoB protein was demonstrated in DRO and KTC2 cells treated for 24 hr with 10 nM RS5444 or two related Tzd PPARγ agonists, 100 nM rosiglitazone and 1 μM troglitazone (Figure 2B). These effects were confirmed to be dependent upon PPARγ, as pretreatment of cells with 10 M GW9662, an irreversible pharmacological inhibitor of PPARγ, blocked induction of RhoB (Figure 2B), as did shRNA silencing of PPARγ (Figure 2C). We also investigated these effects in mouse xenograft models where we previously identified tumor growth inhibition of established tumors and upregulation of p21 as a result of 0.025% RS5444 treatment (9), and found that RhoB expression was strongly upregulated in DRO and ARO tumors excised from mice chronically treated with 0.025% RS5444 in the diet for 4 wks (Figure 2D). We confirmed this finding in an ATC cell line (THJ-11T) created in our laboratory (Figure 2D). These results demonstrate that increased expression of RhoB is a general response to activation of PPARγ in ATC cells.
Figure 2. Changes in morphology and induction of RhoB is PPARγ-dependent.
A. Live microscopic images of KTC2 and KTC3 cell lines treated with and without 10 nM RS5444 for 24 hrs at 40X magnification revealed altered morphology. Real Time PCR for multiple RhoGTPases in cell lines treated with RS5444 for 24 hrs demonstrated an increase of RhoB mRNA with no appreciable alteration in mRNA levels of the other RhoGTPases. Experiments were performed in triplicate. * indicates p<0.05 when compared to untreated control. B. Three Tzd PPARγ agonists (10 nM RS5444, 100 nM rosiglitazone, 1 μM troglitazone) induced RhoB protein expression in DRO and KTC2 cells examined 24 hrs after treatment. When the irreversible PPARγ antagonist, GW9662, was added 5 min prior to Tzd treatment, induction of RhoB was blocked demonstrating specificity for PPARγ. C. Western analysis of lentiviral infected DRO and KTC2 cells silenced for PPARγ illustrates a lack of RhoB upregulation in the absence of PPARγ when treated with RS5444. D. Western analysis demonstrated induction of RhoB expression in tumors (DRO, ARO and THJ-11T) grown in athymic nude mice following chronic oral treatment (4 weeks) with 0.025% RS5444 versus that of vehicle control. Results from three random animals of each group are shown.
RS5444 growth arrest is mediated via RhoB
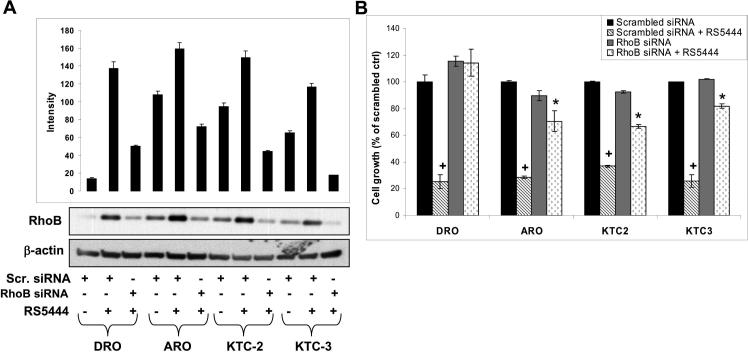
As RhoB has been shown to possess antiproliferative and proapoptotic activity in other types of cancer cells (26), we assessed the role of RhoB in the inhibition of ATC cell growth by PPARγ (Figure 3). Cells transfected with scrambled siRNA respond to 10 nM RS5444 with upregulation of RhoB protein (Figure 3A). Cells transfected with RhoB siRNA and treated with RS5444 showed RhoB levels similar to that of scrambled untreated controls (Figure 3A). Silencing RhoB expression revealed that all four lines were RhoB-dependent for RS5444 induced growth arrest (Figure 3B). Scrambled siRNA treated cells were growth inhibited by 70 − 80% after exposure to 10 nM RS5444 over 6 days with media changes and addition of 10 nM RS5444 every 48 hr (Figure 3B). Conversely, cells silenced for RhoB expression showed significantly less growth inhibition when exposed to RS5444 (Figure 3B). These results show that RhoB is an essential intermediate in PPARγ-mediated growth inhibition of ATC cells.
Figure 3. RhoB upregulation by RS5444 is necessary for inhibition of ATC proliferation.
A. Cell lines were examined by Western analysis to verify that RhoB expression remained silenced even in the presence of RS5444. Cells were transfected for 24 hrs with scrambled (scr) and RhoB siRNA and treated with 10 nM RS5444 for 24 hrs. B. Cellular proliferation of these transiently transfected cell lines demonstrated that growth inhibition is RhoB dependent. Cell numbers were counted on day 7 and data are plotted as percent of scrambled control ± S.D. + indicates p<0.01 when compared to scrambled control. * indicates p<0.05 when compared to RhoB siRNA control (gray bar) or with scrambled siRNA + RS5444.
p21 upregulation is RhoB-dependent
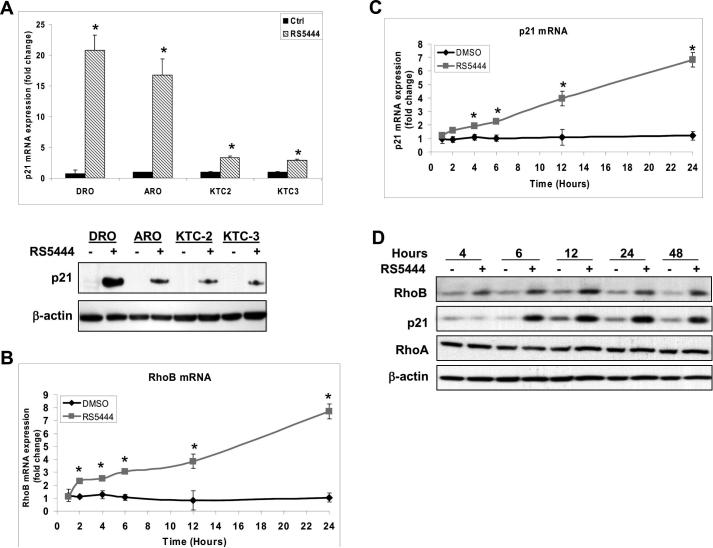
We previously discovered that RS5444 upregulated p21 protein levels in a PPARγ-dependent fashion and that growth arrest was p21-dependent (9). Here, we tested whether the induction of p21 by RS5444 was dependent upon RhoB. We confirmed in all four cell lines that a 24 hr treatment with 10 nM RS5444 upregulates p21 mRNA and protein (Figure 4A). These events were rapid since RhoB mRNA levels in DRO cells were elevated 2-fold by 2 hrs and were higher than 8-fold by 24 hrs post RS5444 treatment compared to that of time-matched vehicle controls (Figure 4B); p21 mRNA levels were elevated 2-fold at 4 hrs post RS5444 treatment and 7-fold at 24 hrs compared to that of controls (Figure 4C) indicating that RS5444 transcriptionally upregulates RhoB and p21. RhoB protein levels are elevated by 4 hrs while p21 is elevated by 6 hrs, and remain elevated through 48 hrs after a single treatment of RS5444 in DRO cells (Figure 4D). Collectively, these data demonstrate that RhoB mRNA and protein are upregulated by RS5444 before that of p21, suggesting that increased expression of RhoB is upstream of p21 upregulation. Consistent with this interpretation, we found that silencing RhoB in DRO and KTC2 cells significantly blocked upregulation of p21 mRNA by RS5444 (Figure 5A). Similarly, expression of dominant negative RhoB also blocked RS5444-induced upregulation of p21 mRNA (Figure 5B).
Figure 4. RhoB upregulation by RS5444 occurs prior to p21 upregulation.
A. Real time PCR and westerns for p21 expression in all four cell lines showed p21 induction after 10 nM RS5444 treatment for 24 hrs. Fold change values were calculated between treated and control samples and plotted as mean ± S.D; n=3. * indicates p<0.01 when compared to control. B. Real time PCR of RhoB mRNA in DRO cells upon exposure to RS5444 showed induction as early as 2 hrs. C. Real time PCR of p21 mRNA in DRO cells demonstrated induction by 4 hrs. Data was normalized to 18S and plotted as mean ± SD with n = 4. * indicates p<0.05 when compared to control. D. Immunoblotting of DRO cells treated with RS5444 demonstrated upregulation of RhoB protein by 4 hours and p21 protein by 6 hrs. No change in RhoA protein occurred between control and RS5444 treated cells.
Figure 5. RhoB upregulation by RS5444 is required to induce p21.
A. Real time PCR of p21 mRNA in DRO and KTC2 illustrates p21 mRNA's dependence upon RhoB induction when stimulated by RS5444. Cells were transiently transfected and treated as previously described. Data was plotted as fold change of p21 mRNA expression as compared to scrambled control ± S.D with n = 3. * indicates p<0.05 when compared to untreated control. + indicates p<0.05 when compared to RhoB siRNA control or to scrambled siRNA with RS5444. B. Real time PCR of p21 mRNA using dominant negative (DN) RhoB also demonstrated p21 mRNA's dependence upon RhoB induction through PPARγ. Cells were transiently transfected with either pcDNA3.1 or DN RhoB and then treated with RS5444 for 24 hrs. C. Silencing RhoB blocked RS5444 stimulated p21 protein expression in transiently transfected DRO and KTC2 cells. Protein lysates were examined for RhoB and p21 expression. RhoA expression was examined to demonstrate specificity of the RhoB siRNA.
We next evaluated the effect of RhoB silencing on protein expression levels of RhoB, p21, and RhoA (Figure 5C). We found that silencing RhoB had no effect upon protein expression for RhoA, demonstrating siRNA specificity and that stimulation with RS5444 in scrambled siRNA transfected cells demonstrated upregulation of RhoB and p21 protein (Figure 5C). Moreover, we found that RhoB siRNA inhibited the induction of p21 protein levels by RS5444. These results demonstrate that RhoB is necessary for p21 mRNA and protein upregulation by RS5444 (Figure 5C).
HDAC inhibitor FK228 also upregulates RhoB
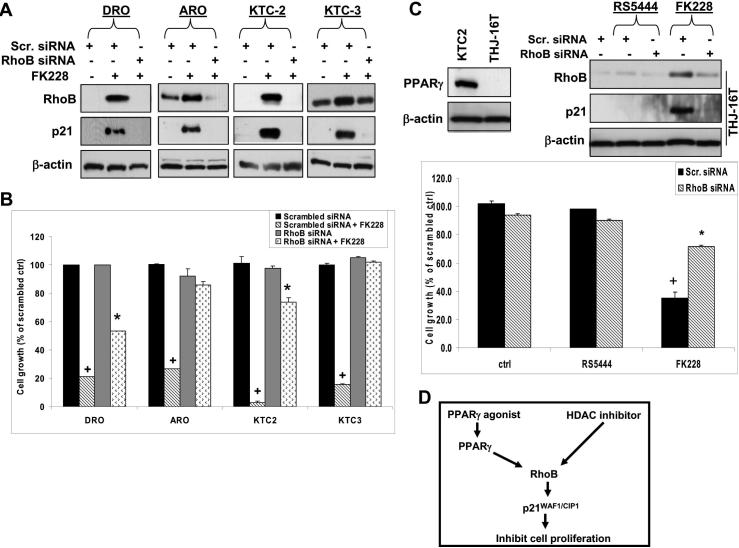
Our results showed that increased expression of RhoB is an essential upstream step for PPARγ/p21-dependent inhibition of ATC proliferation, and in doing so, we identified RhoB as a novel point of therapeutic intervention in ATC. We next wished to test whether this information could be used to identify PPARγ-independent therapeutic approaches for targeting ATC. Previous investigators found that histone deacetylases (HDACs) suppress RhoB expression in human nonsmall lung carcinoma cell lines and that HDAC inhibitors upregulate RhoB (21, 27).We found that KTC3 cells transfected with scrambled or 3 different RhoB siRNAs showed RhoB siRNA 4 almost completely inhibited the pan-HDAC inhibitor, FK228, from inducing RhoB mRNA while RhoB siRNA 3 demonstrated modest inhibition and RhoB siRNA 1 demonstrated no inhibition (Figure S1). Cell proliferation of KTC3 cells using these same siRNAs illustrated differential growth effects when treated with 1 ng/ml FK228 based upon the ability of the siRNA to silence RhoB (Figure S1). Using scrambled siRNA, cells respond to FK228 with upregulation of RhoB and p21 protein while cells transfected with RhoB siRNA 4 demonstrate silenced RhoB and lack of p21 upregulation in response to FK228 (Figure 6A), and that knockdown of RhoB significantly inhibited FK228 induced growth arrest (Figure 6B).
Figure 6. The pan-HDAC inhibitor, FK228, mediates RhoB dependent growth inhibition.
A. Western analysis of cells verified that RhoB and p21 expression remained silenced even in the presence of FK228. Cells were transiently transfected as previously described and then treated with 1 ng/ml FK228 for 24 hrs. B. Silencing RhoB blocks FK228 mediated inhibition of cell proliferation in ATC cells. Cell numbers were counted on day 7 and data were plotted as total cell number ± S.D. + indicates p<0.01 when compared to scrambled control. * indicates p<0.05 when compared to RhoB siRNA control or to scrambled siRNA with FK228. C. Western analysis verified that THJ-16T do not express PPARγ protein. THJ-16T was transiently transfected and treated as described previously. RS5444 was ineffective while FK228 upregulated RhoB and p21 protein levels. Thus, silencing RhoB in THJ-16T cells demonstrated that FK228 is RhoB dependent while RS5444 had no effect upon proliferation. Data are plotted as percent of scrambled control ± S.D. * indicates p<0.01 when compared to scrambled control. D. The signaling pathway of both PPARγ agonists and HDAC inhibitors in ATC is a linear event whereby they induce RhoB leading to p21 upregulation leading to attenuation of cell proliferation. PPARγ agonists upregulate RhoB mRNA and protein via PPARγ while an HDAC inhibitor most likely upregulates RhoB mRNA via epigenetic acetylation of the transcriptional complex regulating RhoB transcription.
These results suggest that HDAC inhibitor-based therapies could be effective against ATC that are unresponsive to PPARγ agonists. To test this possibility, we evaluated the response to FK228 in THJ-16T cells, which is a cell line derived from a primary ATC tumor in our laboratory. THJ-16T cells express no PPARγ and do not show upregulation of RhoB or inhibition of cell proliferation in response to RS5444 (Figures 6C). However, THJ-16T cells do show upregulation of RhoB and p21 in response to FK228 and silencing of RhoB expression attenuates FK228-dependent inhibition of cell proliferation (Figure 6C).
Discussion
Our novel discovery that PPARγ upregulates RhoB in ATC cells was prompted by RS5444-mediated alterations in cell morphology coupled with supporting literature demonstrating that enhanced RhoB activity was associated with growth inhibition and elevated p21 (17, 28, 29). RhoA and RhoC share ∼86% amino acid homology with RhoB and also mediate actin stress fiber formation. Other RhoGTPases, which include Rac1 and Cdc42, have been found to promote oncogenesis, invasion and metastasis (30, 31). However, RhoB is antiproliferative and proapoptotic in cancer cells (32), and overexpression of RhoB can inhibit cell migration, invasion, and metastasis (33). In head and neck, lung and brain cancers, RhoB levels are decreased with tumor progression suggesting that silencing this pathway is critical to tumor survival and progression (34-36). Our data demonstrate that RhoB mRNA and protein levels are low in ATC cell lines with upregulation upon exposure to RS5444 or FK228.
We found that RS5444 regulates RhoB at the transcriptional level with rapid upregulation of RhoB mRNA by 2 hrs of treatment and protein levels by 4 hrs. We identified two putative PPARγ response elements (−1302 to −1282; −1422 to −1402) in the RhoB promoter with 70−80% homology to a consensus PPARγ response element (PPRE). However, transient transfection of the RhoB promoter linked to a luciferase reporter (1876 bp promoter fragment) in DRO, KTC2 and KTC3 did not lead to induction of luciferase activity after 24 hr treatment with 10 nM RS5444 even though treatment with 1 ng/ml FK228 induced luciferase activity 3−9 fold (Figure S2). Thus, the transcriptional regulation of RhoB by PPARγ may be regulated by another region of the RhoB gene or by a more complicated mechanism involving structured chromatin complexes not assayable using transfected RhoB promoter linked to luciferase. RhoB transcript levels have been observed to be transcriptionally suppressed by HDAC1 and found to be positively regulated when treated with an HDAC inhibitor (trapoxin A) via an inverted CCAAT element in the RhoB promoter 451 bp upstream of the transcriptional start site (27). RhoB is an early response gene responding to environmental induction due to environment stresses, such as UV irradiation, which under this circumstance, is mediated by the RNA-binding protein HuR stabilizing RhoB mRNA (37). Oppositely, oncogenic signals mediated by the Ras/PI3K/Akt pathway suppress RhoB expression (38). Thus, while multiple mechanisms appear to regulate RhoB expression in cancer, the important consequence of our work is that RhoB can be re-expressed in ATC through the use of drugs, resulting in biologically active protein that opposes tumor growth.
We describe a novel mechanistic signaling pathway leading to tumor growth inhibition by two classes of drugs via a RhoB dependent mechanism that is upstream of p21. We show that PPARγ-mediated upregulation of p21 mRNA occurs through RhoB by using dominant negative RhoB and siRNA to silence RhoB. Two reports have identified a potential link between upregulation of p21 and RhoB (28, 29). In one report, RhoB-GG induced p21 in a p53-dependent manner, though this was dispensable because RhoB-GG still inhibited growth of p53-null cells that lacked p21 (28). The authors concluded that RhoB-GG suppressed human tumor cell proliferation by more than one mechanism and that it promoted apoptosis as well as inhibited cell cycle transit. In the other report, ectopic expression of RhoB was demonstrated to upregulate p21 (17, 28, 29). Our present study is the first to show that endogenous RhoB is directly responsible for regulation of p21 mRNA and protein levels by RS5444 and FK228 in p53-wild type (DRO and KTC2) as well as p53-mutant (ARO and KTC3) cells (39).
In a recent report, ciglitazone and rosiglitazone increased apoptosis in several ATC cell lines (40). The lack of induction of apoptosis by RS5444 (9) is striking since expression of RhoB induces apoptosis in cancer cells in cell culture [reviewed in (32)] and ectopic tumors grown in mice (41, 42). Even more surprising, DAPI staining revealed that treatment with FK228 also does not induce apoptosis in THJ-11T and THJ-16T with only slight apoptosis (∼12%) in DRO (Figure S3A). Using a LDH assay to examine overall cell death, FK228 treatment again only slightly induces cell death (∼13%) in DRO with no effect on KTC2 and KTC3 cells (Figure S3B). One study demonstrated that cyclin B1 is the target for suppression by RhoB causing apoptosis (43). It is possible that both RS5444 and FK228 could stimulate an antiapoptotic pathway antagonizing the proapoptotic properties of RhoB that is independent of the regulatory role of RhoB in cell proliferation. Alternatively, it may be that induction of apoptosis by rosiglitazone may be PPARγ/RhoB-independent. One additional consideration is that cellular localization and post-translational modification (prenylation and sumoylation) of RhoB are critical regulators of its effects (32). Site-directed mutagenesis studies demonstrated the requirement for palmitoylated cysteine 192 and prenylated cysteine 193 for the tumor suppressive and proapoptotic activities of RhoB (27, 28). Understanding these mechanisms could be important in the selection of the most potent PPARγ agonist or RhoB modulator for combinatorial therapy such as a Tzd and taxane against ATC (9). Importantly, we found that chronic daily treatment of mice harboring ATC tumors with 0.025% RS5444 in the diet retains the ability to sustain elevated RhoB over 4 weeks of treatment (Figure 2D). This also suggests that RhoB and p21 may be ideal molecular markers of response to therapy and easily measured in biopsy tissue following treatment.
In summary, we have identified a novel growth inhibitory pathway regulated by PPARγ and identified a HDAC inhibitor that upregulates RhoB mRNA and protein in human ATC cells (Figure 6D). Elevated RhoB protein is necessary for these two drugs to then upregulate p21 mRNA and protein as well as inhibit cell proliferation (Figure 6D). Thus, RhoB is implicated as a critical signaling node that could be therapeutically targeted in ATC. Importantly, it is a target that can be upregulated by multiple classes of drugs. HMG CoA reductase inhibitors (statins) (44), prenylation inhibitors (FTI, GGTI) (32), HDAC inhibitors (FK228) and now PPARγ agonists upregulate RhoB in various cancers. Although the connection to RhoB has not been made in ATC, the aforementioned classes of drugs inhibit growth in ATC cells (45-47) most likely acting in part through upregulation of functional RhoB. We are currently investigating the role of these drugs that mediate RhoB upregulation and growth inhibition in ATC. Post-translational modification and cellular localization of RhoB as a result of treatment with each of these drugs will also most likely dictate optimal antitumor activity (32) and allow for rational selection of combinatorial therapy with maximum benefit to the patient.
Supplementary Material
Acknowledgements
We thank Drs. Aubrey Thompson, Alan Fields, Norman Eberhardt, Keith Bible, Christopher Barnes and Stefan Grebe for careful reading and helpful editing of this manuscript. This work was funded in part from NIH grant P30CA15083 (Cancer Center Support Grant, RCS), Mayo Clinic Research Committee (RCS), Florida Department of Health Bankhead Coley grant (JAC, RCS) and a grant for Rare Cancers from Dr. Ellis and Dona Brunton (JAC).
Abbreviations
- PPARγ
Peroxisome proliferators-activated receptor gamma
- ATC
anaplastic thyroid carcinoma
- Tzd
thiazolidinedione
- shRNA
lentivirus shRNA
- siRNA
short interfering RNA
- DN
dominant-negative
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Smallridge RC, Marlow L, Copland JA. Anaplastic thyroid cancer: Molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2008 doi: 10.1677/ERC-08-0154. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debril M-B, Renaud J-P, Fajas L, et al. The pleiotropic functions of peroxisome proliferator-activated receptor gamma. J Mol Med. 2001;79:30–47. doi: 10.1007/s001090000145. [DOI] [PubMed] [Google Scholar]
- 4.Sertznig P, Seifert M, Tilgen W, Reichrath J. Present concepts and future outlook: Function of peroxisome proliferator-activated receptors (ppars) for pathogenesis, progression, and therapy of cancer. J of Cell Physiol. 2007;212:1–12. doi: 10.1002/jcp.20998. [DOI] [PubMed] [Google Scholar]
- 5.Tontonoz P, Spiegelman BM. Fat and beyond: The diverse biology of ppar . Annu Rev of Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 6.Koeffler HP. Peroxisome proliferator-activated receptor {gamma} and cancers. Clin Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 7.Sarraf P, Mueller E, Smith WM, et al. Loss-of-function mutations in ppar[gamma] associated with human colon cancer. Molec Cell. 1999;3:799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y, Ying H, Zhao L, et al. Ppar[gamma] insufficiency promotes follicular thyroid carcinogenesis via activation of the nuclear factor-[kappa]b signaling pathway. Oncogene. 2005;25:2736–47. doi: 10.1038/sj.onc.1209299. [DOI] [PubMed] [Google Scholar]
- 9.Copland JA, Marlow LA, Kurakata S, et al. Novel high-affinity ppar[gamma] agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21waf1//cip1. Oncogene. 2006;25:2304–17. doi: 10.1038/sj.onc.1209267. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Wang S, Wu J, Huang S. Effects of ppargamma agonists on cell survival and focal adhesions in a chinese thyroid carcinoma cell line. J Cell Biochem. 2006;98:1021–35. doi: 10.1002/jcb.20839. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Bush CR, Necela BM, et al. Rs5444, a novel ppar[gamma] agonist, regulates aspects of the differentiated phenotype in nontransformed intestinal epithelial cells. Molec and Cell Endocrinol. 2006;251:17–32. doi: 10.1016/j.mce.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Bush CR, Havens JM, Necela BM, et al. Functional genomic analysis reveals crosstalk between peroxisome proliferator-activated receptor gamma (ppargamma) and calcium signaling in human colorectal cancer cells. J. Biol. Chem. 2007;282:23387–401. doi: 10.1074/jbc.M702708200. [DOI] [PubMed] [Google Scholar]
- 13.Chang T-H, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000;60:1129–38. [PubMed] [Google Scholar]
- 14.Elnemr A, Ohta T, Iwata K, et al. Ppargamma ligand (thiazolidinedione) induces growth arrest and differentiation markers of human pancreatic cancer cells. Int J Oncol. 2000;17:1157–64. doi: 10.3892/ijo.17.6.1157. [DOI] [PubMed] [Google Scholar]
- 15.Koga H, Sakisaka S, Harada M, et al. Involvement of p21(waf1/cip1), p27(kip1), and p18(ink4c) in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology. 2001;33:1087–97. doi: 10.1053/jhep.2001.24024. [DOI] [PubMed] [Google Scholar]
- 16.Hong J, Samudio I, Liu S, Abdelrahim M, Safe S. Peroxisome proliferator-activated receptor {gamma}-dependent activation of p21 in panc-28 pancreatic cancer cells involves sp1 and sp4 proteins. Endocrinology. 2004;145:5774–85. doi: 10.1210/en.2004-0686. [DOI] [PubMed] [Google Scholar]
- 17.Prendergast GC. Actin’ up: Rhob in cancer and apoptosis. Nature Reviews Cancer. 2001;1:162–68. doi: 10.1038/35101096. [DOI] [PubMed] [Google Scholar]
- 18.Wennerberg K, Der CJ. Rho-family gtpases: It's not only rac and rho (and i like it). J Cell Sci. 2004;117:1301–12. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 19.Liu A-x, Cerniglia GJ, Bernhard EJ, Prendergast GC. Rhob is required to mediate apoptosis in neoplastically transformed cells after DNA damage. PNAS. 2001;98:6192–97. doi: 10.1073/pnas.111137198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prendergast GC, Oliff A. Farnesyltransferase inhibitors: Antineoplastic properties, mechanisms of action, and clinical prospects. Seminars in Cancer Biology. 2000;10:443–52. doi: 10.1006/scbi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 21.Mazieres J, Tovar D, He B, et al. Epigenetic regulation of rhob loss of expression in lung cancer. BMC Cancer. 2007;7:220. doi: 10.1186/1471-2407-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurebayashi J, Okubo S, Yamamoto Y, et al. Additive antitumor effects of gefitinib and imatinib on anaplastic thyroid cancer cells. Cancer Chemotherapy and Pharmacology. 2006;1:11. doi: 10.1007/s00280-006-0185-x. [DOI] [PubMed] [Google Scholar]
- 23.Schweppe RE, Klopper JP, Korch C, et al. DNA profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008:jc.2008–1102. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Copland JA, Marlow LA, Williams SF, et al. Molecular diagnosis of a braf papillary thyroid carcinoma with multiple chromosome abnormalities and rare adrenal and hypothalamic metastases. Thyroid. 2006;16:1293–302. doi: 10.1089/thy.2006.16.1293. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2-[delta][delta]ct method. Methods. 2001;25:402–08. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Wang D-A, Sebti SM. Palmitoylated cysteine 192 is required for rhob tumor-suppressive and apoptotic activities. J Biol Chem. 2005;280:19243–49. doi: 10.1074/jbc.M411472200. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Yan-Neale Y, Fischer D, et al. Histone deacetylase 1 represses the small gtpase rhob expression in human nonsmall lung carcinoma cell line. Oncogene. 2003;22:6204–13. doi: 10.1038/sj.onc.1206653. [DOI] [PubMed] [Google Scholar]
- 28.Du W, Prendergast GC. Geranylgeranylated rhob mediates suppression of human tumor cell growth by farnesyltransferase inhibitors. Cancer Res. 1999;59:5492–96. [PubMed] [Google Scholar]
- 29.Allal C, Pradines A, Hamilton A, Sebti S, Favre G. Farnesylated rhob prevents cell cycle arrest and actin cytoskeleton disruption caused by the geranylgeranyltransferase i inhibitor ggti-298. Cell Cycle. 2002;1:430–7. doi: 10.4161/cc.1.6.272. [DOI] [PubMed] [Google Scholar]
- 30.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of rac1, rhoa, and mitogen-activated protein kinases is required for ras transformation. Mol Cell Biol. 1995;15:6443–53. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitehead IP, Abe K, Gorski JL, Der CJ. Cdc42 and fgd1 cause distinct signaling and transforming activities. Mol. Cell. Biol. 1998;18:4689–97. doi: 10.1128/mcb.18.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang M, Prendergast G. Rhob in cancer suppression. Histol Histopathol. 2006;21:213–8. doi: 10.14670/HH-21.213. [DOI] [PubMed] [Google Scholar]
- 33.Jiang K, Sun J, Cheng J, et al. Akt mediates ras downregulation of rhob, a suppressor of transformation, invasion, and metastasis. Mol Cell Biol. 2004;24:5565–76. doi: 10.1128/MCB.24.12.5565-5576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazieres J, Antonia T, Daste G, et al. Loss of rhob expression in human lung cancer progression. Clin Cancer Res. 2004;10:2742–50. doi: 10.1158/1078-0432.ccr-03-0149. [DOI] [PubMed] [Google Scholar]
- 35.Forget M-A, Desrosiers RR, Del Maestro RF, et al. The expression of rho proteins decreases with human brain tumor progression: Potential tumor markers. Clin and Experimen Metast. 2002;19:9–15. doi: 10.1023/a:1013884426692. [DOI] [PubMed] [Google Scholar]
- 36.Adnane J, Muro-Cacho C, Mathews L, Sebti SM, Munoz-Antonia T. Suppression of rhob expression in invasive carcinoma from head and neck cancer patients. Clin Cancer Res. 2002;8:2225–32. [PubMed] [Google Scholar]
- 37.Westmark CJ, Bartleson VB, Malter JS. Rhob mrna is stabilized by hur after uv light. Oncogene. 2004;24:502–11. doi: 10.1038/sj.onc.1208224. [DOI] [PubMed] [Google Scholar]
- 38.Jiang K, Delarue F, Sebti S. Egfr, erbb2 and ras but not src suppress rhob expression while ectopic expression of rhob antagonizes oncogene-mediated transformation. Oncogene. 2004;23:1136–45. doi: 10.1038/sj.onc.1207236. [DOI] [PubMed] [Google Scholar]
- 39.Fagin J, Matsuo K, Karmakar A, et al. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–84. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aiello A, Pandini G, Frasca F, et al. Peroxisomal proliferator-activated receptor-{gamma} agonists induce partial reversion of epithelial-mesenchymal transition in anaplastic thyroid cancer cells. Endocrinology. 2006;147:4463–75. doi: 10.1210/en.2005-1610. [DOI] [PubMed] [Google Scholar]
- 41.Couderc B, Pradines A, Rafii A, et al. In vivo restoration of rhob expression leads to ovarian tumor regression. Cancer Gene Ther. 2008;15:456–64. doi: 10.1038/cgt.2008.12. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Sun J, Pradines A, et al. Both farnesylated and geranylgeranylated rhob inhibit malignant transformation and suppress human tumor growth in nude mice. J Biol Chem. 2000;275:17974–78. doi: 10.1074/jbc.C000145200. [DOI] [PubMed] [Google Scholar]
- 43.Kamasani U, Huang M, DuHadaway JB, et al. Cyclin b1 is a critical target of rhob in the cell suicide program triggered by farnesyl transferase inhibition. Cancer Res. 2004;64:8389–96. doi: 10.1158/0008-5472.CAN-04-2437. [DOI] [PubMed] [Google Scholar]
- 44.Holstein SA, Hohl RJ. Synergistic interaction of lovastatin and paclitaxel in human cancer cells. Mol Cancer Ther. 2001;1:141–49. [PubMed] [Google Scholar]
- 45.Furuya F, Shimura H, Suzuki H, et al. Histone deacetylase inhibitors restore radioiodide uptake and retention in poorly differentiated and anaplastic thyroid cancer cells by expression of the sodium/iodide symporter thyroperoxidase and thyroglobulin. Endocrinology. 2004;145:2865–75. doi: 10.1210/en.2003-1258. [DOI] [PubMed] [Google Scholar]
- 46.Mitsiades CS, Poulaki V, McMullan C, et al. Novel histone deacetylase inhibitors in the treatment of thyroid cancer. Clin Cancer Res. 2005;11:3958–65. doi: 10.1158/1078-0432.CCR-03-0776. [DOI] [PubMed] [Google Scholar]
- 47.Zhong W-B, Wang C-Y, Chang T-C, Lee W-S. Lovastatin induces apoptosis of anaplastic thyroid cancer cells via inhibition of protein geranylgeranylation and de novo protein synthesis. Endocrinology. 2003;144:3852–59. doi: 10.1210/en.2003-0098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.