Abstract
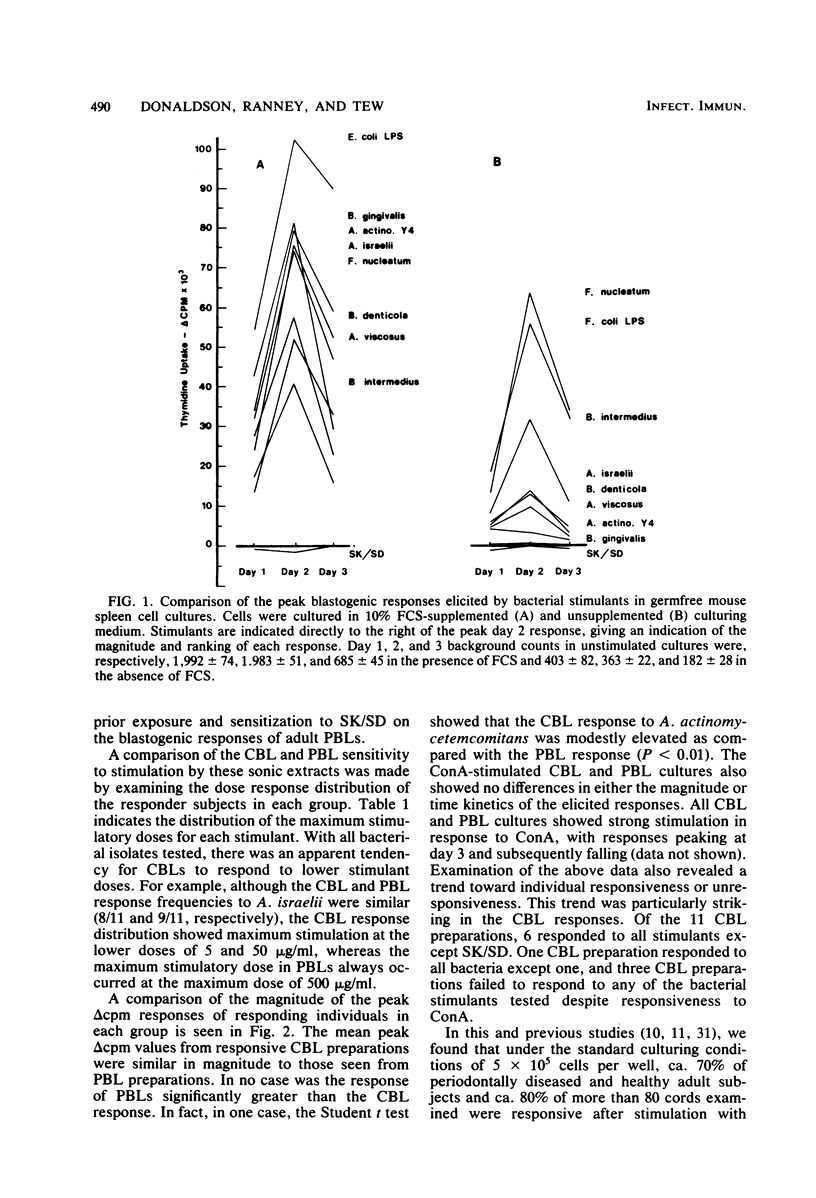
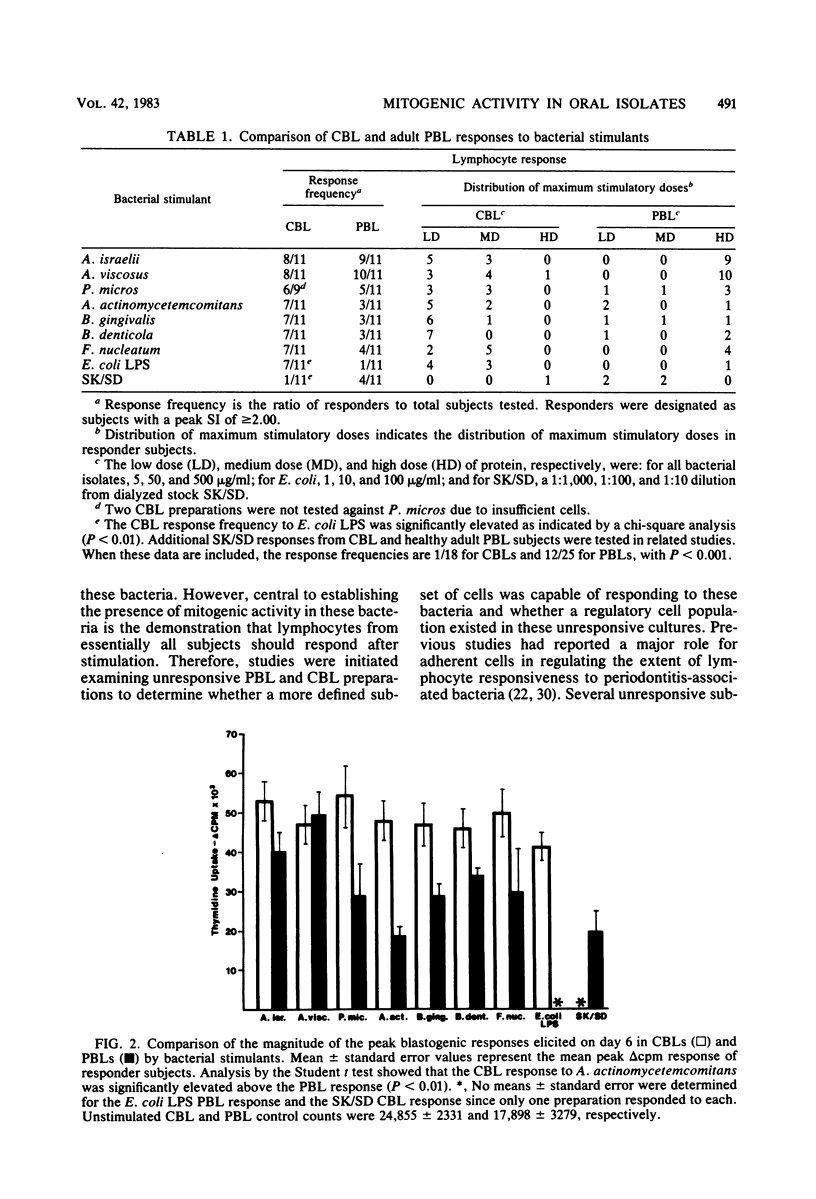
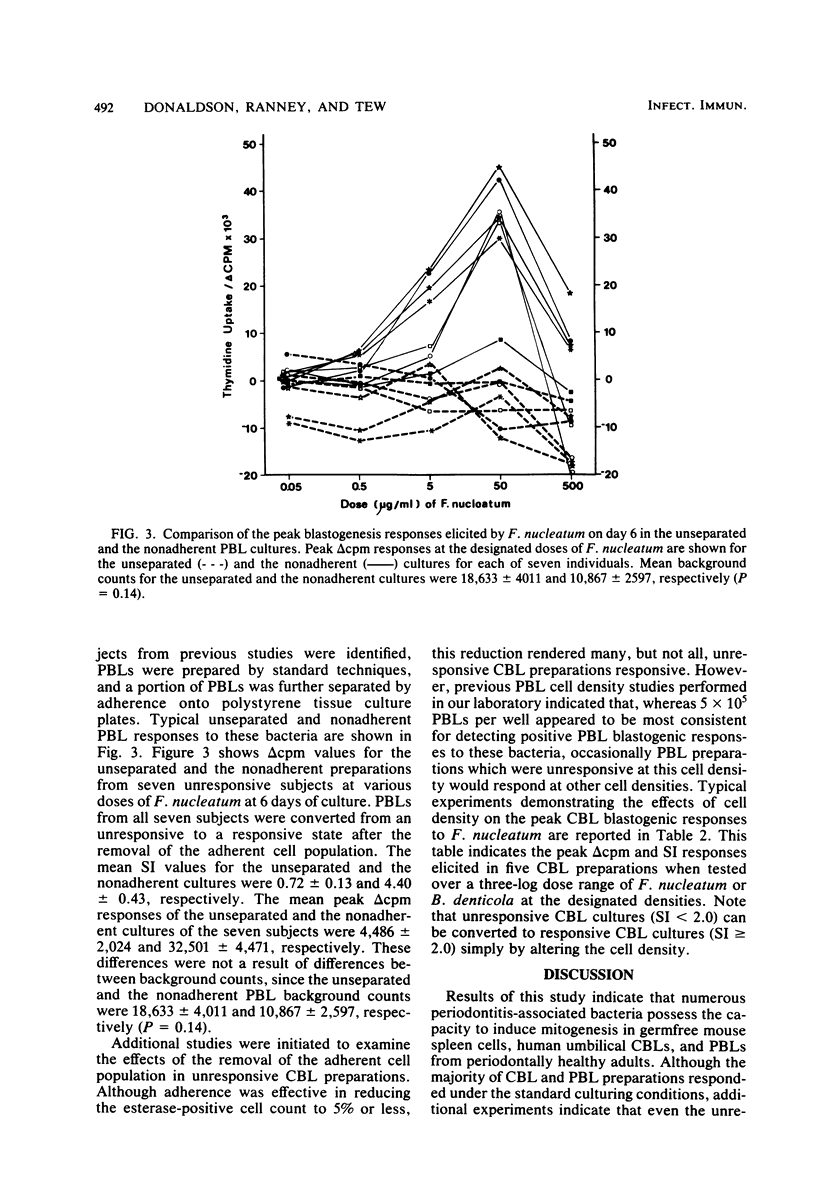
This study examines several periodontitis-associated bacterial isolates for the presence of mitogenic activity, as indicated by their capacity to stimulate unsensitized lymphocytes to undergo blastogenesis. Germfree mouse spleen cells responded vigorously to all of the bacterial sonic extracts tested. The kinetics and dose responses to these activators in germfree mouse spleen cell cultures paralleled those seen with the standard murine B-cell mitogen, Escherichia coli lipopolysaccharide. In contrast, Streptokinase-Streptodornase (Varidase; Lederle Laboratories) antigen elicited no response. Human cord blood lymphocytes also responded upon stimulation with these same bacterial isolates but failed to respond to Streptokinase-Streptodornase. The frequency, magnitude, and kinetics of these cord blood lymphocyte responses were remarkably similar to those seen with adult peripheral blood lymphocytes. However, in this and previous studies, individuals with unresponsive peripheral blood lymphocytes have been observed. Studies were initiated to determine whether these unresponsive leukocyte preparations truly lacked the capacity to respond to these bacteria or whether unresponsiveness reflected the presence of a regulatory cell population in these cultures. After the removal of the adherent cells from unresponsive peripheral blood lymphocyte cultures, the nonadherent cells were found to be responsive. Therefore, peripheral blood lymphocyte responsiveness appears to be regulated via an adherent cell population. The removal of adherent cells from unresponsive cord blood lymphocyte preparations resulted in a less consistent alteration to responsiveness. However, cord blood lymphocyte preparations unresponsive at a standard cell density were shown to be responsive at altered cell densities.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. J., Chan S. P., Socransky S. S., Oppenheim J. J., Mergenhagen S. E. Importance of Actinomyces and certain gram-negative anaerobic organisms in the transformation of lymphocytes from patients with periodontal disease. Infect Immun. 1976 May;13(5):1363–1368. doi: 10.1128/iai.13.5.1363-1368.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banck G., Forsgren A. Many bacterial species are mitogenic for human blood B lymphocytes. Scand J Immunol. 1978;8(4):347–354. doi: 10.1111/j.1365-3083.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Bick P. H., Carpenter A. B., Holdeman L. V., Miller G. A., Ranney R. R., Palcanis K. G., Tew J. G. Polyclonal B-cell activation induced by extracts of Gram-negative bacteria isolated from periodontally diseased sites. Infect Immun. 1981 Oct;34(1):43–49. doi: 10.1128/iai.34.1.43-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B., Hefti A. Are Actinomyces viscosus antigens B cell mitogens? J Immunol. 1977 Apr;118(4):1460–1465. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clagett J. A., Engel D. Polyclonal activation: a form of primitive immunity and its possible role in pathogenesis of inflammatory diseases. Dev Comp Immunol. 1978 Apr;2(2):235–241. doi: 10.1016/s0145-305x(78)80066-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Damais C., Bona C., Chedid L., Fleck J., Nauciel C., Martin J. P. Mitogenic effect of bacterial peptidoglycans possessing adjuvant activity. J Immunol. 1975 Jul;115(1):268–271. [PubMed] [Google Scholar]
- Donaldson S. L., Bick P. H., Moore W. E., Ranney R. R., Burmeister J. A., Tew J. G. Polyclonal B-cell activating capacities of gram-positive bacteria frequently isolated from periodontally diseased sites. J Periodontal Res. 1982 Nov;17(6):569–575. doi: 10.1111/j.1600-0765.1982.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Donaldson S. L., Miller G. A., Rice P. L., Ranney R. R., Tew J. G. The maintenance of B-cell and T-cell function in frozen and stored human lymphocytes. J Clin Immunol. 1981 Apr;1(2):106–112. doi: 10.1007/BF00915387. [DOI] [PubMed] [Google Scholar]
- Donaldson S. L., Ranney R. R., Burmeister J. A., Tew J. G. Blastogenic responses by lymphocytes from periodontally healthy populations induced by periodontitis-associated bacteria. J Periodontol. 1982 Dec;53(12):743–751. doi: 10.1902/jop.1982.53.12.743. [DOI] [PubMed] [Google Scholar]
- Dziarski R., Dziarski A., Levinson A. I. Mitogenic responsiveness of mouse, rat and human lymphocytes to Staphylococcus aureus cell wall, teichoic acid, and peptidoglycan. Int Arch Allergy Appl Immunol. 1980;63(4):383–395. doi: 10.1159/000232654. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Relationships between adjuvant, immunosuppressive, and mitogenic activities of staphylococcal peptidoglycan. Infect Immun. 1979 Nov;26(2):508–514. doi: 10.1128/iai.26.2.508-514.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Studies on the mechanism of peptidoglycan- and lipopolysaccharide-induced polyclonal activation. Infect Immun. 1982 Feb;35(2):507–514. doi: 10.1128/iai.35.2.507-514.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D., Clagett J., Page R., Williams B. Mitogenic activity of Actinomyces viscosus. I. Effects on murine B and T lymphocytes, and partial characterization. J Immunol. 1977 Apr;118(4):1466–1471. [PubMed] [Google Scholar]
- Horton J. E., Leikin S., Oppenheim J. J. Human lymphoproliferative reaction to saliva and dental plaque-deposits: an in vitro correlation with periodontal disease. J Periodontol. 1972 Sep;43(9):522–527. doi: 10.1902/jop.1972.43.9.522. [DOI] [PubMed] [Google Scholar]
- Horton J. E., Oppenheim J. J., Chan S. P., Baker J. J. Relationship of transformation of newborn human lymphocytes by dental plaque antigen to the degree of maternal periodontal disease. Cell Immunol. 1976 Jan;21(1):153–160. doi: 10.1016/0008-8749(76)90336-1. [DOI] [PubMed] [Google Scholar]
- Horton J. E., Oppenheim J. J., Mergenhagen S. E. A role for cell-mediated immunity in the pathogenesis of periodontal disease. J Periodontol. 1974 May;45(5):351–360. doi: 10.1902/jop.1974.45.5.351. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Kin K., Itoh Y., Kawai T., Morita M., Shioiri-Nakano K. Cellular cooperation in lymphocyte activation. IV. Requirement of cell-to-cell interaction for the activation of human T and B lymphocytes by protein A. Cell Immunol. 1980 Jan;49(1):142–153. doi: 10.1016/0008-8749(80)90064-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopatin D. E., Mangan D. F., Horner I. S. Cells involved in the mitogen-induced helper function which facilitates the blastogenic response to Actinomyces viscosus. Clin Immunol Immunopathol. 1981 Jun;19(3):394–405. doi: 10.1016/0090-1229(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Lopatin D. E. In vitro stimulation of immunoglobulin production from human peripheral blood lymphocytes by a soluble preparation of Actinomyces viscosus. Infect Immun. 1981 Jan;31(1):236–244. doi: 10.1128/iai.31.1.236-244.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patters M. R., Chen P., McKenna J., Genco R. J. Lymphoproliferative responses to oral bacteria in humans with varying severities of periodontal disease. Infect Immun. 1980 Jun;28(3):777–784. doi: 10.1128/iai.28.3.777-784.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. R., Sorensen R. U., Polmar S. H. Lymphocyte responses of human neonates to bacterial antigens. Cell Immunol. 1981 Jan 15;57(2):307–315. doi: 10.1016/0008-8749(81)90089-7. [DOI] [PubMed] [Google Scholar]
- Räsänen L., Arvilommi H. Cell walls, peptidoglycans, and teichoic acids of Gram-positive bacteria as polyclonal inducers and immunomodulators of proliferative and lymphokine responses of human B and T lymphocytes. Infect Immun. 1982 Feb;35(2):523–527. doi: 10.1128/iai.35.2.523-527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räsänen L., Karhumäki E., Majuri R., Arvilommi H. Polyclonal activation of human lymphocytes by bacteria. Infect Immun. 1980 May;28(2):368–372. doi: 10.1128/iai.28.2.368-372.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Protein A from Staphylococcus aureus-a mitogen for human T lymphocytes and B lymphocytes but not L lymphocytes. J Immunol. 1978 Jan;120(1):302–311. [PubMed] [Google Scholar]
- Smith S., Bick P. H., Miller G. A., Ranney R. R., Rice P. L., Lalor J. H., Tew J. G. Polyclonal B-cell activation: severe periodontal disease in young adults. Clin Immunol Immunopathol. 1980 Jul;16(3):354–366. doi: 10.1016/0090-1229(80)90141-5. [DOI] [PubMed] [Google Scholar]
- Stashenko P. Regulatory effect of monocytes on T cell proliferative responses to oral microbial antigens. Infect Immun. 1982 Dec;38(3):938–947. doi: 10.1128/iai.38.3.938-947.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew J. G., Miller G. A., Greene E. J., Rice P. L., Jordan W. P., Palcanis K. G., Ranney R. R. Immunological studies of young adults with severe periodontitis. II. Cellular factors. J Periodontal Res. 1981 Jul;16(4):403–416. doi: 10.1111/j.1600-0765.1981.tb00991.x. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]


