Abstract
Objective: We aimed to study the neural processing of emotion‐denoting words based on a circumplex model of affect, which posits that all emotions can be described as a linear combination of two neurophysiological dimensions, valence and arousal. Based on the circumplex model, we predicted a linear relationship between neural activity and incremental changes in these two affective dimensions. Methods: Using functional magnetic resonance imaging, we assessed in 10 subjects the correlations of BOLD (blood oxygen level dependent) signal with ratings of valence and arousal during the presentation of emotion‐denoting words. Results: Valence ratings correlated positively with neural activity in the left insular cortex and inversely with neural activity in the right dorsolateral prefrontal and precuneus cortices. The absolute value of valence ratings (reflecting the positive and negative extremes of valence) correlated positively with neural activity in the left dorsolateral and medial prefrontal cortex (PFC), dorsal anterior cingulate cortex, posterior cingulate cortex, and right dorsal PFC, and inversely with neural activity in the left medial temporal cortex and right amygdala. Arousal ratings and neural activity correlated positively in the left parahippocampus and dorsal anterior cingulate cortex, and inversely in the left dorsolateral PFC and dorsal cerebellum. Conclusion: We found evidence for two neural networks subserving the affective dimensions of valence and arousal. These findings clarify inconsistencies from prior imaging studies of affect by suggesting that two underlying neurophysiological systems, valence and arousal, may subserve the processing of affective stimuli, consistent with the circumplex model of affect. Hum Brain Mapp, 2009. © 2008 Wiley‐Liss, Inc.
Keywords: affect, valence, arousal, fMRI, circumplex
INTRODUCTION
Research in the affective neurosciences has been influenced heavily by a theoretical model of basic emotions which posits that a small set of discrete emotions accounts for all affective experiences [Ekman, 1992; Panksepp, 1992; Tomkins, 1962]. A single specific neural system is thought to subserve each of the discrete emotions, such as happiness, sadness, anger, and fear. Thus, proponents of discrete emotions have sought to identify distinct neural pathways that subserve each individual emotion. Numerous functional imaging studies have reported neural activity during the experience of specific emotions when contrasted with activity during experiences that are purportedly emotion‐neutral. Findings across these studies of individual emotions have been inconsistent, however, and they have not produced a comprehensive understanding of the neural systems that subserve emotional experience [Barrett and Wager, 2006; Berridge, 2003; Cacioppo et al., 2000; Davidson, 2003; Ortony and Turner, 1990]. Additional limitations of the theory of basic emotions include the fact that few proponents of the theory agree on which emotions are basic [Ekman, 1993]; measures of different basic emotions are highly intercorrelated [Cacioppo et al., 2000]; studies attempting to identify basic emotions have been biased by the choice of instruments used to quantify or classify emotions [Posner et al., 2005]; and compared with evidence from animal studies, direct evidence supporting the model of basic emotions in humans is limited [Berridge, 2003].
The circumplex model of affect is a competing theory of emotion [Russell, 1980]. It posits that all emotions can be described as a linear combination of two underlying, largely independent neurophysiological systems, valence and arousal. The valence system determines the degree to which an emotion is pleasant or unpleasant, and the arousal system determines the degree to which it is behaviorally activating (see Fig. 1). Upon these neurophysiological substrates, diverse cognitive and affective processes are recruited to interpret and contextualize any given emotional experience. The combination of these neurophysiological systems and cognitive interpretations determines how an individual experiences, delineates, and refines any emotional state. Emotions are therefore ambiguous, overlapping sensations that are the product of activity in neural pathways subserving valence and arousal, and they become contextualized and classified through the processing of salient situational, historical, behavioral, and physiological cues.
Figure 1.
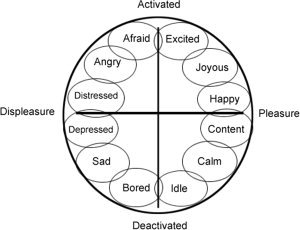
A schematic representation of the affective circumplex. The horizontal axis represents valence and the vertical axis represents arousal. Circles are drawn around each emotion to indicate that the affective states are ambiguous, overlapping categories.
By valence, we mean the positive or negative felt quality that is inherent to all emotional experiences. This quality ranges from highly pleasurable experiences, such as joy or exuberance, to highly unpleasant experiences, such as anguish or despair [Colombetti, 2005; Fridja, 1986; Russell, 2003]. We use valence and hedonic tone synonymously, but distinguish valence from the behavioral dimension of approach or withdrawal that has been described previously [Davidson et al., 1990]. Approach behaviors, such as the seeking of rewards, often suggest an underlying positive valence, and yet this is not uniformly true (consider, for example, the approach of a fearful or angry competitor). Conversely, some emotions, such as contentedness or mild serenity, are not associated clearly with either approach or withdrawal behaviors.
By “arousal,” we mean the preparedness of an organism for action [Kandel et al., 2000]. Levels of arousal can range from coma or sleep on one extreme, to intense excitement or panic on the other. This sense of “arousal” is contrasted with “attention,” which refers to the allocation of the neural resources that support the preferential orienting to, and processing of, task‐relevant stimuli [Coull, 1998; Posner, 1999]. Although attention and arousal often are intercorrelated, they are nevertheless dissociable [Sarter et al., 2001]. A delirious person, for example, may be highly aroused, and yet grossly inattentive. “Vigilance” and “salience,” on the other hand, refer to specific modes or features of attentional processing. Vigilance, for example, refers to a state of sustained attention [Sarter et al., 2001], whereas salience refers to the degree to which a stimulus attracts or biases attentional resources [Kastner and Ungerleider, 2000; Sarter et al., 2001].
A large number of neuroimaging studies have previously attempted to identify distinct and dissociable circuits that support one of several basic emotions, such as the amygdala supporting fear [Davis and Whalen, 2001; LeDoux, 2003], the subcallosal cingulate supporting sadness [Beauregard et al., 1998; Reiman et al., 1997], and the insular cortex supporting disgust [Lane et al., 1997; Sprengelmeyer et al., 1998]. These findings, however, have been highly inconsistent across studies [Barrett and Wager, 2006]. Indeed, even the most reproducible finding in imaging studies of single emotions, that of amygdala activation during the processing of fear, was reported in only 60% of studies in one meta‐analysis [Phan et al., 2002] and in less than 40% of studies in another [Murphy et al., 2003].
We suspect that these inconsistencies may be a consequence of the inherent limitations of the theory of basic emotions and of the conventional construction of functional magnetic resonance imaging (fMRI) tasks as “subtraction” paradigms. Functional imaging studies typically compare neural activity during the processing of a single emotion, such as fear, with a baseline condition that is putatively emotionally “neutral.” Whether any stimulus can be truly neutral, however, is open to debate [Damasio, 1994], and in fact, we suspect that most neutral stimuli either are emotionally ambiguous, or they induce a relative disinterest and boredom. According to the model of the affective circumplex, both of these conditions (fear and disinterest/boredom) are associated to some degree with a negative valance. Comparing brain activity during the processing of these two types of emotion would likely fail to detect differential activity related to the processing of valence, and therefore would not detect activation of the amygdala, because the valence associated with each of the conditions is relatively equal. Activation of the amygdala could be detected, however, if the valences associated with the two conditions differed substantially. Thus if the same neurophysiological systems support the processing of all emotions, as the theory of the affective circumplex suggests, the difficulties and vagaries of designing appropriate subtraction paradigms may explain the remarkably poor consistency of regional activations that have been reported during the processing of specific emotions in previous imaging studies [Murphy et al., 2003].
In contrast to the inconsistencies in findings reported from studies based on theories of basic emotions, the validity of two‐dimensional models of emotion has been supported by multiple self‐report [Feldman Barrett and Russell, 1998; Watson et al., 1988], physiological [Cacioppo et al., 2000; Lang et al., 1993], and electroencephalographic (EEG) studies of emotion [Davidson, 1984]. Their validity has also been supported by fMRI studies of emotion that have explicitly considered either valence or arousal as dimensional constructs that contribute to emotional experience [Anderson et al., 2003; Small et al., 2003]. For example, recent fMRI studies of olfaction and gustation suggest that the medial prefrontal cortex (PFC) supports the affective dimension of valence [Anderson et al., 2003; Small et al., 2003], and likewise several studies have demonstrated that activity of the dorsolateral and medial prefrontal cortices is associated with the processing of emotionally valenced words compared with neutral words [Fossati et al., 2003; Maddock et al., 2003]. Based on these and other studies [Lewis et al., 2007], we predicted that the medial PFC would subserve the affective dimension of valence. Studies have also suggested that the amygdala and anterior cingulate cortex may support valence, having demonstrated increased activity in these regions in association with positively and negatively valenced, as compared to neutral, visual stimuli [Garavan et al., 2001; Killgore and Yurgelun‐Todd, 2004]. Findings in imaging studies of arousal have been less consistent than those in imaging studies of valence. Several studies, for example, have correlated emotional arousal with activity in the primary sensory cortices, anterior cingulate cortex, and thalamus, whereas others suggest that the amygdala and parietal cortex may support the processing of arousal [Lang et al., 1998; Portas et al., 1998; Posner and Petersen, 1990; Small et al., 2003]. We suspect that one reason for these inconsistent findings is that the studies have often used ratings of affective intensity as a surrogate for arousal, while neglecting to adequately assess and control for ratings of affective valence of the stimuli, thereby confounding contributions from valence with arousal. Inconsistencies may also be the consequence of using subtraction paradigms in which the baseline stimuli are assumed to be emotionally neutral but in fact are not.
We aimed to clarify and extend findings from prior functional imaging studies that have correlated neural activity with ratings of emotional valence or arousal. Our study was not designed to prove the validity of the circumplex model or to invalidate the theory of basic emotions. Rather, we aimed to identify the neural systems that support the processing of valence and arousal across a wide range of emotional stimuli, as postulated by the circumplex model. Prior studies that have examined valence and arousal as emotional dimensions have been limited either by a reliance on off‐line ratings of emotional stimuli, or by an assessment of only one of the two affective dimensions predicated by dimensional models of emotion [Lang et al., 1998; Small et al., 2003]. To our knowledge, no studies thus far have explicitly correlated imaging‐based measures of neural activity with on‐line ratings of both valence and arousal, which the circumplex model of affect most directly predicts will reveal the underlying neurophysiological determinants of emotional experience. We sought to select and use affective stimuli that would reliably probe the full affective circumplex, and that would therefore provide the greatest ranges of valence and arousal ratings for use in parametric models. Emotion‐denoting words have been shown in several behavioral and self‐report studies to probe reliably all four quadrants (and therefore the full range of valence and arousal) of the affective circumplex [Feldman, 1995; Kring et al., 2003]. Therefore, we correlated blood‐oxygen‐level dependent (BOLD) signal during an fMRI experiment as subjects rated the valence and arousal that they attributed to written, emotion‐denoting words.
MATERIALS AND METHODS
Subjects
The procedures of this study were approved by the Institutional Review Board of the New York State Psychiatric Institute. Subjects were recruited through advertisements in the New York City community. They provided written informed consent for participation. Each subject was assessed for psychiatric disorders with the Structured Clinical Interview for DSM‐IV (SCID‐IV) [Spitzer et al., 1995]. Subjects who met DSM‐IV criteria for a current Axis I disorder or who had a lifetime history of a psychotic or substance abuse disorder, or who had a history of head trauma, seizure disorder, or other neurological disease were excluded from participating. Estimates of full scale intelligence quotient (IQ) were made using the Wechsler Abbreviated Scale of Intelligence (WASI). Handedness was assessed with the Edinburgh Handedness Inventory. Socioeconomic status (SES) was measured with the Hollingshead Index of Social Status.
Ten subjects (5 men and 5 women) were scanned, having a mean age of 25.51 ± 4.58 years (range: 19 to 34 years old). All were right‐handed, Caucasian, and native English speakers. They were of slightly above‐average intelligence (mean full scale IQ 112.4 ± 13.7) and were from households of high average SES. All were medication‐free.
Task Construction
Each trial was 38 s in duration and consisted of three distinct temporal components: (1) participants were shown a single emotion‐denoting word for 18 s, having been instructed before the experiment to reflect upon the emotion that the word described; (2) they were then shown a 9 × 9 grid displaying the dimensions of valence and arousal as visual analog scales on the x‐ and y‐axes, respectively, ranging in values from −4 to +4; (3) they then gazed at a cross‐hair at the center of an otherwise blank screen for variable durations, such that the time from offset of the prior word to presentation of the next word (i.e., the total time for rating of valence and arousal, followed by gaze fixation) equaled 20 s (see Fig. 2). The 9 × 9 grid used to collect affective ratings allowed subjects to rate simultaneously both valence and arousal with a single click of the mouse. Before the scanning session, each subject practiced a shortened version of the task using a different set of stimuli outside of the scanning environment. Subjects were instructed to try to generate the feelings described by the emotion‐denoting words and to rate the feelings using the 9 × 9 grid. Subjects were given the following written instructions: “You will be shown words that describe certain emotions. Try to think about what the emotion feels like. Some people think about situations, or draw on memories of situations, that have made them feel the emotion in the past.” Prior behavioral studies have shown that the 9 × 9 affective grid provides ratings of valence and arousal similar to those obtained when these two affective dimensions are rated separately [Russell et al., 1989].
Figure 2.
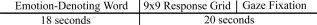
Each trial was divided into three distinct temporal components: (1) participants were shown a single emotion‐denoting word; (2) participants were shown a 9 × 9 grid displaying the dimensions of valence and arousal as visual analog scales on the x‐ and y‐axes, respectively, ranging in values from −4 to +4; (3) participants gazed at a cross‐hair at the center of an otherwise blank screen. The duration of Component 1 was 18 s. The combined duration of Components 2 and 3 was 20 s. The 9 × 9 grid was presented on screen until the subject made a selection or until a maximum time of 20 s had elapsed. Once the participant made the selection, the fixation screen was displayed for the remainder of the 20 s (i.e. the duration of Component 3 = 20 s − the duration of Component 2).
We conducted three scanning runs, each comprising 16 stimulus trials of emotion‐denoting words, for a total of 48 stimuli and associated ratings per subject. The duration of each run was 10 min 8 s. The words were selected based on behavioral studies demonstrating that they probe all four quadrants of the affective circumplex [Feldman, 1995; Feldman Barrett and Fossum, 2001]. The words were: excited, lively, cheerful, pleased, calm, relaxed, idle, still, dulled, bored, unhappy, disappointed, nervous, fearful, alert, and aroused, and each was presented three times throughout the experiment, in a pseudo‐randomized order, with the constraint that each word of the set of 16 emotion‐denoting words was presented once in each scanning run. The order of stimulus presentation was maintained across subjects.
The task was programmed in E‐Prime (v. 1.0; Psychology Software Tools, Pittsburgh, PA) running on a Dell desktop computer (Dell Computer Corp., Austin, TX). All stimuli were presented through goggles (Resonance Technology, Northridge, CA). Subjects made responses with their right hand using a computer mouse modified for use in the MRI environment.
Image Acquisition
Images were obtained on a GE Signa 3‐T whole body scanner (Milwaukee, WI) operating the E2‐M4 platform using a quadrature head coil in receive mode. T1‐weighed sagittal localizing images were used to position axial functional images parallel to the anterior–posterior commissure (AC‐PC) line. A 3D spoiled gradient recall (SPGR) image was acquired for coregistration with axial echoplanar images and a reference brain from the Montreal Neurological Institute (MNI). Axial echoplanar images (TR = 2,800 ms, TE = 25 ms, 90° flip angle, single excitation per image, slice thickness 3 mm, 0.5 mm gap, 24 cm × 24 cm field of view, 64 × 64 matrix) were obtained to provide an effective resolution of 3.75 mm × 3.75 mm × 3.5 mm and whole brain coverage, with 43 slices in each imaging volume and 218 volumes per run.
Image Preprocessing
Image processing and statistical analyses employed MATLAB 6.5 using an integrated image processing software platform that was developed in‐house, based on SPM2. All images were visually inspected and discarded if artifacts such as ghosting were found or if the subject moved more than 2.5 mm in any direction. Slice‐timing correction was applied using the middle slice of each run as the reference image. Images were motion‐corrected for three translational directions and rotations [Friston et al., 1995a]. Corrected images were reformatted to 2 mm3 voxels and then spatially normalized to the standard MNI template using a hybrid algorithm of affine transform and nonlinear warping: each subject's SPGR images were normalized to the template, and then these subject‐specific warping parameters were used to normalize the functional images to the same template. Normalized images were then spatially filtered with a Gaussian filter having a full‐width, half maximum of 8 mm. A discrete cosine transform‐based high‐pass filter with a basis function length of 128 s removed drift from the baseline image intensity and a low‐pass Butterworth filter with a cutoff frequency of 0.15 Hz removed high frequency noise. No grand mean scaling or intensity normalization was applied.
Parametric Analysis
In keeping with the general linear model [Friston et al., 1995b], we modeled the time series data for each subject with five independent functions and a constant. The variables were as follows: (1) the canonical hemodynamic response function (HRF) convolved with a box car function (BCF) of 18 s duration indexing the presentation of the affective stimulus (we will term this function A); (2) Function A weighted by the arousal rating for each stimulus; (3) Function A weighted by the valence rating for each stimulus; (4) the canonical HRF convolved with a BCF indexing the presentation of the 9 × 9 response grid (the duration of the BCF was equivalent to the time the subject required to respond, up to a maximum of 20 s), and (5) the canonical HRF convolved with a BCF indexing gaze fixation (the fixation point was displayed for a duration such that the rating period plus the gaze fixation period always equaled 20 s). Based on a priori assumptions dictated by the theoretical model of the affective circumplex that informed the design of our study, we modeled a linear relationship at each voxel between the on‐line ratings of arousal and valence with the time‐varying BOLD signal. No Euclidean normalization or parameter orthogonalization was applied. Voxel‐based correlation estimates for each subject were determined by an ordinary least squares fit.
We also assessed the correlations of BOLD signal with the extremes of emotional valence and arousal, as indexed by the absolute value of the ratings in each of these dimensions (i.e. a stimulus rated by a subject as arousal +4 and valence −3 was transformed to a +4 “extreme arousal” weighting and a +3 “extreme valence” weighting). These analyses were undertaken in light of findings from a number of animal studies of emotional processing in which subcortical brain regions contained distinct but intermingled populations of neurons, one determining positive valence and another determining negative valence [Murray, 2007]. Previous single unit recordings in the amygdala of nonhuman primates, for example, reported the existence of one population of neurons that was active during the processing of positively valenced stimuli and a second population that was active during the processing of negatively valenced stimuli during a classical conditioning experiment [Paton et al., 2006]. These two populations of neurons were interdigitated throughout the amygdala, without spatial sequestration. If these animals had been imaged using fMRI during the same experiment, the limitations in spatial resolution of fMRI would indicate the presence of progressively increasing activity in the amygdala during the presentation of progressively more positively and more negatively valenced stimuli—i.e., it would show greater amygdala activity with more extreme ratings of valence. Previous functional imaging studies, in fact, have reported increasing activation of the amygdala during both positive and negative, relative to neutral, ratings of valence [Hamann and Mao, 2002]. Therefore, we hypothesized that if individual brain regions contain these intermingled populations of neurons that are differentially sensitive to valence, then the BOLD signal in those regions should increase with ratings of valence that are progressively more positive or more negative. To model this valence‐specific effect on BOLD signal, we assessed the correlations of BOLD signal with the absolute value of valence ratings. We multiplied the absolute value of the valence rating for each stimulus with the 18‐s box‐car HRF that indexed the presentation of each stimulus. Although we did not have an a priori hypothesis regarding the correlations of BOLD signal with absolute value of arousal ratings, we did assess these correlations to maintain a parallel analytic approach across the parametric analyses for ratings of valence and arousal.
Statistical parametric maps were thresholded using the conjoint requirement of P < 0.01 and a cluster of 45 contiguous voxels. Based on Monte Carlo simulations across the entire imaging volume, this conjoint requirement yielded an effective P value < 0.05 when corrected for multiple comparisons [Forman et al., 1995; McAvoy, 2001].
Power Analysis
We conducted a comprehensive calculation of statistical power for our study. Correlations of affective ratings with BOLD signal were established within subjects in a first‐level analysis using 48 stimulus presentations per subject, with 14 imaging volumes collected per stimulus presentation. For each subject, the power to detect an effect was high (80%; two‐sided α = 0.05). Contrast images for positive and inverse correlations for each of the 10 subjects were entered into a second‐level, random effects analysis (i.e. testing whether the mean of the correlations across subjects differed significantly from zero) to assess the reproducibility and generalizability of the correlations. The power was moderate for the second level analysis (56%; two‐sided α = 0.05), thus risking the presence of false negative (type II) errors; the risk of false positives, however, was low (less than 0.05 when corrected for multiple comparisons) [Mumford and Nichols, 2008; Zarahn and Slifstein, 2001]. We can therefore be confident that the effects detected were real, although we may have missed detecting other real effects.
RESULTS
Behavioral results
On‐line ratings of valence and arousal indicated that the emotion‐denoting words probed all four quadrants of the affective circumplex (Table I). Although valence and arousal ratings correlated significantly, the magnitude of this correlation was small (correlation of valence with arousal: r = 0.27, P < 0.001; correlation of absolute valence with arousal: r = 0.10, P < 0.05; correlation of valence with absolute arousal: r = 0.04, P < 0.37). Moreover, several behavioral studies using the same affective stimuli in larger samples of subjects have demonstrated that the two dimensions are independent [Feldman, 1995; Feldman Barrett and Fossum, 2001]. We therefore treated the affective ratings as independent predictor variables in the regression model. Furthermore, inclusion of both ratings in the model isolated simultaneously the effects of each variable while covarying for the effects of the other variable.
Table I.
Mean valence and arousal rating ± SD for each emotion denoting word
| Emotion denoting word | Arousal rating | Valence rating |
|---|---|---|
| Alert | 2.80 ± 1.24 | 0.77 ± 1.43 |
| Aroused | 2.93 ± 0.69 | 2.33 ± 1.47 |
| Bored | −2.67 ± 0.66 | −2.13 ± 0.86 |
| Calm | −1.80 ± 1.24 | 2.00 ± 1.28 |
| Cheerful | 2.60 ± 0.77 | 2.93 ± 0.78 |
| Disappointed | −1.37 ± 1.47 | −2.93 ± 0.78 |
| Dulled | −2.90 ± 0.80 | −2.23 ± 0.77 |
| Excited | 3.40 ± 0.56 | 2.90 ± 0.80 |
| Fearful | 2.43 ± 0.77 | −3.37 ± 0.56 |
| Idle | −2.63 ± 1.47 | −0.33 ± 1.75 |
| Lively | 3.17 ± 0.65 | 2.00 ± 1.26 |
| Nervous | 2.69 ± 0.66 | −2.66 ± 0.67 |
| Pleased | 0.57 ± 1.36 | 2.73 ± 0.91 |
| Relaxed | −1.80 ± 1.23 | 3.03 ± 0.72 |
| Still | −2.07 ± 1.68 | 0.47 ± 1.48 |
| Unhappy | −1.37 ± 1.63 | −3.07 ± 0.64 |
Each of the 10 subjects rated the words during the scanning sessions and each word was presented three times for a total of 30 on‐line ratings per word.
Imaging results
Scatterplots depicting the mean percent change in BOLD signal for a given brain region with changes in the ratings from one affective dimension, while accounting simultaneously for the contribution from the other affective dimension, indicated that the data were well conditioned, without evidence of heteroscedasticity or undue influence from outliers [Mickey et al., 2004] (Fig. 4).
Figure 4.
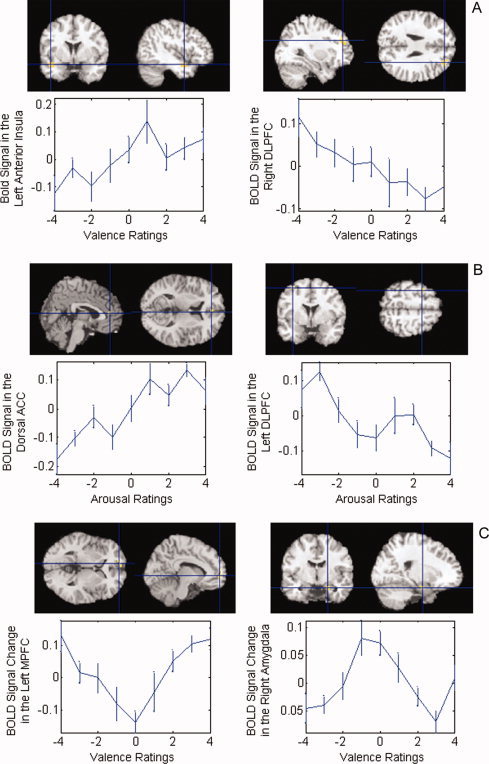
Unit change in BOLD signal per unit change in affective rating. BOLD signal intensity and affective ratings are represented on the vertical and horizontal axes, respectively. A: Valence ratings correlated positively with activity in the left anterior insula and inversely with activity in right dorsolateral prefrontal cortex (DLPFC). B: Arousal ratings correlated positively with activity in the dorsal anterior cingulate and inversely with activity in the left DLPFC. C: Valence extremely correlated positively with activity in the left medial prefrontal cortex (MPFC) and inversely with activity in the right amygdala. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Valence ratings correlated significantly with BOLD signal in the left anterior insula, and correlated inversely with BOLD signal in the right dorsolateral prefrontal cortex (DLPFC) and right precuneus gyrus (Figs. 3A and 4A, Table II). Ratings of arousal correlated positively with BOLD signal in the left parahippocampal gyrus and the dorsal anterior cingulate cortex (ACC) bilaterally. Inverse correlations of arousal with BOLD signal were detected in the left DLPFC and dorsal cerebellum (Figs. 3B and 4B).
Figure 3.
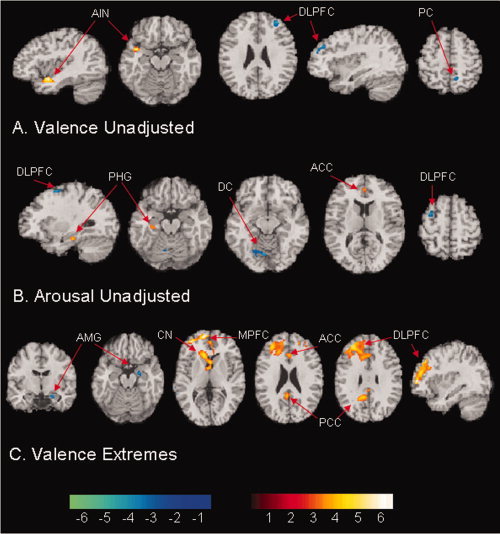
Valence unadjusted and Arousal unadjusted index voxel‐wise correlations of subjects' affective ratings and BOLD signal. Valence extremes index correlations of the absolute value of subjects' valence ratings and BOLD signal. Positive correlations are coded in red to yellow, and inverse correlations are coded in green to purple. The effective P‐value for the conjoint requirement of a statistical threshold and cluster filter across all voxels was < 0.05 [Forman et al., 1995]. A: Activity in the left anterior insula cortex (AIN) correlated with valence ratings. Inverse correlations in the right dorsolateral prefrontal cortex (DLPFC) and the right precuneus gyrus (PC) indicate that activity in these regions decreased with progressively more positive ratings of valence (or increased with more negative valence ratings) B: Activity in the left parahippocampal gyrus (PHG) and the bilateral anterior cingulate cortex (ACC) correlated with arousal ratings. The inverse correlations in left dorsolateral prefrontal cortex (DLPFC) and the dorsal cerebellum (DC) indicate that activity decreased with progressively more positive ratings of arousal (or increased with more negative ratings). C: Activity in the left dorsolateral prefrontal cortex (DLPFC), bilateral medial prefrontal cortex (MPFC), bilateral anterior cingulate cortex (ACC), and bilateral posterior cingulate cortex (PCC) correlated with extremes of valence ratings. Inverse correlation in the right amygdala (AMG) indicates that activity decreased with progressively more extreme ratings of valence.
Table II.
Only clusters greater than 45 contiguous voxels with an unadjusted P < 0.01 are reported. The coordinates and their t‐values are reported at the peak voxels in each cluster
| Bold signal correlations | Direction of correlation | Regions | Side | Brodmann's area | Cluster size (voxels) | Talairach coordinates | T‐value | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| Unadjusted valence | Positive | Anterior insula | L | 38 | 115 | −40 | 3 | −15 | 5.38 |
| Inverse | Dorsolateral prefrontal cortex | R | 10 | 173 | 32 | 50 | 25 | 4.53 | |
| Precuneus | R | 7 | 51 | 14 | −46 | 56 | 3.91 | ||
| Unadjusted arousal | Positive | Parahippocampal gyrus | L | NA | 69 | −32 | −22 | −16 | 4.00 |
| Dorsal anterior cingulate | R/L | 32 | 70 | 2 | 45 | 9 | 3.34 | ||
| Inverse | Dorsolateral prefrontal cortex | L | 6 | 46 | −32 | 5 | 57 | 4.31 | |
| Dorsal cerebellum | L | NA | 226 | −6 | −74 | −6 | 3.74 | ||
| Valence extremes | Positive | Dorsolateral prefrontal cortex | L | 10 | 4469 | −33 | 51 | 1 | 6.52 |
| Medial frontal gyrus | L | 10 | −10 | 59 | 8 | 6.23 | |||
| Dorsal anterior cingulate | R/L | 32 | 5 | 40 | 9 | 3.02 | |||
| Posterior cingulate | R/L | 7 | 358 | −2 | −49 | 39 | 4.29 | ||
| Middle frontal gyrus | R | 10 | 113 | 34 | 49 | 18 | 3.60 | ||
| Inverse | Middle temporal gyrus | L | 21 | 68 | −61 | −33 | 2 | 4.32 | |
| Amgydala | R | 34 | 84 | 35 | 1 | −20 | 4.22 | ||
L, left; R, right; R/L, Clusters detected in both hemispheres; NA, not applicable; X, Y, Z, the voxel position in standard Talairach space; One voxel = 2 mm3.
Extremes of emotional valence correlated positively with BOLD signal in the left dorsolateral and medial PFC bilaterally, in the anterior and posterior cingulate bilaterally, and in a region centered over the anterior portion of the left caudate. Inverse correlations were detected in the left medial temporal gyrus and right amygdala (Figs. 3C and 4C). Extremes of arousal did not correlate significantly with BOLD signal in any region.
DISCUSSION
Our findings suggest that distinct functional neural networks subserve the affective dimensions of valence and arousal. Ratings for valence and arousal were entered simultaneously into a single linear model, such that the neural activity correlating with the ratings in one affective dimension were dissociated from ratings in the other affective dimension.
The Valence Network
The network subserving emotional valence included the left DLPFC, bilateral medial PFC, amygdala, cingulate gyrus, insular cortex, and precuneus. Within this network, increasing activity in the left insula accompanied increasing valence (increasingly pleasant stimuli), whereas increasing activity in the right DLPFC and right precuneus accompanied decreasing valence (increasingly aversive stimuli). BOLD signal increased with progressively more extreme ratings of both positive and negative valence (i.e., with increasing absolute value of the valence ratings) in the left DLPFC and bilateral medial prefrontal cortices, left caudate nucleus, and the dorsal anterior and posterior cingulate cortices. BOLD signal declined with progressively more extreme ratings of valence in the right amygdala and left middle temporal gyrus.
The decline in amygdala activity with progressively more extreme ratings of valence may seem at odds with findings from prior studies suggesting that the amygdala activates more with more negatively valenced stimuli [Davidson, 2002; Kim et al., 2003]. We suspect that these differences can be reconciled by considering the bipolarity of the valence dimension in the affective circumplex. Our findings indicate, for example, that activity in the amygdala increases as the valence of a stimulus increases from more negative to more neutral values. This finding is consistent with previous reports that one population of neurons in the amygdala becomes increasingly more active as valence shifts in the positive (i.e. less negative) direction [Garavan et al., 2001; Murray, 2007; Paton et al., 2006]. Conversely, we also found that activity in the amygdala increases as the valence of a stimulus decreases from more positive to more neutral values, which again is consistent with previous reports that a discrete population of neurons in the amygdala, different from the first, becomes increasingly more active as valence shifts in the negative (i.e. less positive) direction [Hamann and Mao, 2002; Murray, 2007; Paton et al., 2006]. This latter set of findings is consistent with the imaging studies that report increasing activity of the amygdala during the processing of increasingly aversive stimuli [Davidson, 2002].
Consistent with the correlates of valence that we detected, multiple lesion and imaging studies have demonstrated associations of emotional valence with activation of the dorsolateral and medial prefrontal cortices and the amygdala, although the directions of these associations have varied considerably across reports [Davidson, 2002; Wager et al., 2003]. Our results may help to explain these variable findings by demonstrating that these prefrontal and amygdala regions subserve the processing of both positively and negatively valenced stimuli (i.e. regions in which BOLD signal correlated with the absolute value of valence ratings). This interpretation is supported by functional imaging studies demonstrating increased activity in the amygdala when comparing neutral stimuli with stimuli that were either positively or negatively valenced [Garavan et al., 2001; Yang et al., 2002].
Finally, the opposing directions of correlation that we found between extreme ratings of valence with prefrontal and amygdala activity (Fig. 4C) is consistent with animal models and human imaging studies that have reported a reciprocal relationship between prefrontal and amygdala activity [Davidson, 2002; Likhtik et al., 2005; Quirk et al., 2003]. Our findings suggest that as neural activity increases in prefrontal regions with increasingly positive or negative valence, activity in the amygdala concurrently declines. This is consistent with animal and human imaging studies of functional connectivity that have demonstrated inverse correlations of activity in the PFC with activity in the amygdala [Kim et al., 2003; Quirk et al., 2003; Zald et al., 1998]. Several investigators have suggested that this reciprocal relationship between activity in the PFC and amygdala may represent a regulatory, or feedback, system that serves to modulate and dampen affective responses that would otherwise be excessive [Garcia et al., 1999; Ochsner et al., 2002].
We detected positive correlations of BOLD signal with valence extremes in the dorsal anterior and posterior cingulate cortices, consistent with findings from prior imaging and electrophysiological studies of these same regions [Fossati et al., 2003; Nishijo et al., 1997]. The cingulate gyrus is an anatomically heterogeneous structure having a wide range of functions [Peterson et al., 1999]. Most investigators agree that the cingulate relays signals from the PFC to limbic structures and association cortices, such as the amygdala and precuneus, suggesting that the cingulate may transmit valence signals through the putative valence network that we have described. In opposition to our findings, however, some investigators have suggested the presence of a functional division within the ACC, in which the dorsal ACC is associated with cognitive tasks and the ventral ACC is associated with affective processing [Bush et al., 2000]. Several studies of persons with anterior cingulate lesions, however, suggest that this proposed cognitive and affective division of the ACC may be specious [Cohen et al., 1999; Fellows and Farah, 2005b]. Moreover, the control functions assigned to the dorsal ACC may extend to other domains, including the control of affect [Ochsner et al., 2002; Peterson et al., 1999]. An endogenous control system could become progressively more active with the experience of progressively greater extremes of valence in emotional stimuli.
We also found that valence ratings correlated positively with BOLD signal in the left anterior insular cortex. This finding is consistent with recent studies demonstrating involvement of the left insula in romantic love [Aron et al., 2005], addiction and reward [Naqvi et al., 2007], and positively valenced vocal prosody [Johnstone et al., 2006]. Anatomically, the insula has abundant connections with the dorsal PFC, anterior cingulate, and amygdala—other regions that we found participate in the proposed valence network [Augustine, 1985].
Activity in the right DLPFC and the right precuneus increased with the presentation of stimuli that were increasingly negatively valenced. Activation in these regions has often been detected during attentional tasks and the processing of emotional valence [Le et al., 1998; Paradiso et al., 1999; Posner and Dehaene, 1994]. We suspect that the processing of more negatively valenced stimuli recruits greater attentional resources because the affective salience of aversive stimuli tends to be greater than the salience of appetitive stimuli [Ohman et al., 2001]. Indeed, valence ratings for negatively valenced stimuli were significantly more extreme than were ratings for positively valenced stimuli (i.e., the average of the absolute values of valence ratings was greater for negatively versus positively valenced words: 2.5 ± 1.0 vs. 2.2 ± 1.3, t = 2.8; P = 0.005), suggesting that negatively valenced words may have greater emotional salience, and therefore they may recruit more attentional resources, than do positively valenced words.
Likewise, subjects with lesions to the PFC demonstrate greater apathy and attend less to novel visual stimuli than do healthy controls [Daffner et al., 2000, 2003]. This relative indifference to novel stimuli may be even more pronounced in decision‐making tasks that require subjects to assess the risks of potentially aversive or threatening stimuli [Fellows and Farah, 2005a; Manes et al., 2002]. Lastly, functional imaging studies of the right precuneus have reported activation during spatial memory tasks that require a significant reallocation of attentional resources and that are frequently associated with negatively valenced emotions, such as frustration or anger [Fletcher et al., 1995; Ghaem et al., 1997]. Anatomically, the precuneus has abundant reciprocal connections with the posterior and anterior cingulate cortices, DLPFC, and temporal lobes—regions where we found strong correlations of neural activity with ratings of valence [Cavanna and Trimble, 2006].
The Arousal Network
Significant correlations of BOLD signal with ratings of arousal suggest the existence of a unified neural network that consists of the dorsal ACC, left parahippocampus, left DLPFC, and dorsal cerebellum. These regions have been associated previously with attentional processing and error monitoring. For example, studies have associated increased ACC activation with inhibiting a prepotent, or dominant, response in favor of a subdominant one [Bush et al., 2000; Peterson et al., 1999]. Most of these studies, however, have not controlled for the arousal inherent in the emotional states that accompany attentional tasks that require resolution of cognitive and behavioral conflicts, when participants are especially prone to committing errors. These attentional states are likely highly confounded with arousal, and indeed emotion theorists have long noted that highly arousing emotions draw attention toward the arousing stimulus [James, 1890; Ohman et al., 2001]. Conversely, attention researchers note a component of arousal in certain attentional processes [Coull, 1998; Portas et al., 1998; Posner and Petersen, 1990].
The parahippocampal gyrus, an additional region where BOLD signal correlated positively with arousal ratings, is functionally closely associated with the hippocampus, which is thought to play an important role in the processing of affective experience [Richter‐Levin, 2004]. Prior studies have associated hippocampal activation with negative emotions rather than with arousal, and yet these studies have used affective probes of negative emotions that are highly arousing, such as threatening words or aversive sounds to induce fear [Buchel et al., 1999; Isenberg et al., 1999]. Consistent with our findings, electrophysiological studies using implantable electrodes have demonstrated increased hippocampal activity associated with cortical arousal [Green and Arduini, 1954], and microdialysis studies have demonstrated the increased release of acetylcholine in the hippocampus following the presentation of arousing stimuli [Inglis and Fibiger, 1995]. We also suspect that the emotion‐denoting word task used in our study recruits episodic and semantic memories, known functions of the hippocampus [Fletcher et al., 1997; Manns et al., 2003; Nadel and Moscovitch, 1997], with greater activity being associated with more arousing memories.
Activity in the left DLPFC and dorsal cerebellum correlated inversely with arousal ratings. Similar to the ACC, these regions have been implicated in attentional processing [MacDonald et al., 2000; Nigg and Casey, 2005; Posner and Petersen, 1990]. The inverse correlations indicate that activity in these regions increased as arousal levels decreased, suggesting that neural activity in these regions may have had a direct inhibitory influence on arousal. Alternatively, the findings could represent greater activity of inhibitory interneurons in these regions, which would have produced a net effect of decreasing activity in excitatory projection neurons, as arousal declined. The BOLD signal cannot distinguish whether increasing neural activity derives from excitatory or inhibitory neurons [Arthurs and Boniface, 2002].
Limitations
The design of this study was guided by the theory of the circumplex model of affect. Our findings support the theory by suggesting the existence of distinct neural pathways that subserve the affective dimensions of valence and arousal. Although the findings of this study support the postulated existence of neural pathways common to all emotions, the study was not designed to falsify basic emotion theory, and therefore it cannot gainsay the existence of discrete neural pathways that distinguish one emotion from another. In addition, the study assessed the processing of affective stimuli by presenting emotion‐denoting words to the participants and implying to them that memories might help generate the feelings described by the words. Thus the task and the stimuli used may have more readily engaged semantic and episodic memories than would have other affective probes, such as affective pictures or facial expressions, and therefore the stimuli may have engaged preferentially the neural systems that subserve the linguistic and mnemonic components of affective processing. Additional studies are needed to assess how specific the regional networks that subserve valence and arousal are to a given stimulus modality. In addition, our study design could not differentiate clearly the neural activity needed to generate valence and arousal ratings for the appraisal of emotional stimuli, from the neural activity needed actually to generate the corresponding emotional experience. This limitation, however, applies equally to a vast number of findings in affective neuroscience that require the cognitive appraisal of emotional stimuli. Lastly, given our limited sample size, we were unable to explore the effects of sex or age on our findings, which have increasingly been recognized as important influences on affective processing [Gunning‐Dixon et al., 2003; Wager et al., 2003].
CONCLUSION
Prior fMRI studies of emotion using dimensional models to explore affective experience have yielded promising results. Our findings, however, go beyond these studies in several ways. First, we demonstrate a linear relationship between neural activity and incremental changes in two affective dimensions—a “dose‐response” relationship between neural activity and affective ratings that is predicted by dimensional models of emotion. Second, functional imaging studies that have investigated dimensional models of affect have yielded inconsistent findings. Some studies, for example, have associated greater positive valence with left when compared with right prefrontal activation, whereas other studies have found the reverse [Wager et al., 2003]. We offer an explanation for these inconsistencies by indexing valence extremes and then correlating these ratings with changes in BOLD signal. Third, ours is the first study to have explicitly used a parametric design to correlate imaging‐based measures of neural activity with on‐line ratings of both valence and arousal—the approach that the circumplex model of affect most directly predicts will reveal the underlying neurophysiological determinants of emotional experience. Our findings thus support the contention that two affective dimensions, valence and arousal, can together support processing of stimuli across the range of all possible affects, consistent with the theory of the affective circumplex.
Acknowledgements
The authors thank Satie Shova for her technical assistance.
REFERENCES
- Anderson AK,Christoff K,Stappen I,Panitz D,Ghahremani DG,Glover G,Gabrieli JD,Sobel N ( 2003): Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Aron A,Fisher H,Mashek DJ,Strong G,Li H,Brown LL ( 2005): Reward, motivation, and emotion systems associated with early‐stage intense romantic love. J Neurophysiol 94: 327–337. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ,Boniface S ( 2002): How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 25: 27–31. [DOI] [PubMed] [Google Scholar]
- Augustine JR ( 1985): The insular lobe in primates including humans. Neurol Res 7: 2–10. [DOI] [PubMed] [Google Scholar]
- Barrett L,Wager T ( 2006): Structure of emotion: Evidence from neuroimaging studies. Curr Dir Psychol Sci 15: 79–83. [Google Scholar]
- Beauregard M,Leroux JM,Bergman S,Arzoumanian Y,Beaudoin G,Bourgouin P,Stip E ( 1998): The functional neuroanatomy of major depression: An fMRI study using an emotional activation paradigm. Neuroreport 9: 3253–3258. [DOI] [PubMed] [Google Scholar]
- Berridge KC ( 2003): Comparing the emotional brains of humans and other animals In: Davidson RJ,Scherer KR, Hill Goldsmith H, editors. Handbook of Affective Sciences. New York: Oxford University Press; pp 25–51. [Google Scholar]
- Buchel C,Dolan RJ,Armony JL,Friston KJ ( 1999): Amygdala‐hippocampal involvement in human aversive trace conditioning revealed through event‐related functional magnetic resonance imaging. J Neurosci 19: 10869–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G,Luu P,Posner MI ( 2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT,Berntson GG,Larsen JT,Poehlmann KM,Ito TA ( 2000): The psychophysiology of emotion In: Lewis M,Haviland‐Jones JM, editors. Handbook of Emotions, 2nd ed New York: Guilford Press; pp 173–191. [Google Scholar]
- Cavanna AE,Trimble MR ( 2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129 (Part 3): 564–583. [DOI] [PubMed] [Google Scholar]
- Cohen RA,Kaplan RF,Moser DJ,Jenkins MA,Wilkinson H ( 1999): Impairments of attention after cingulotomy. Neurology 53: 819–824. [DOI] [PubMed] [Google Scholar]
- Colombetti G ( 2005): Appraising valence. J Conscious Stud 12: 103–126. [Google Scholar]
- Coull JT ( 1998): Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 55: 343–361. [DOI] [PubMed] [Google Scholar]
- Daffner KR,Mesulam MM,Scinto LF,Acar D,Calvo V,Faust R,Chabrerie A,Kennedy B,Holcomb P ( 2000): The central role of the prefrontal cortex in directing attention to novel events. Brain 123 (Part 5): 927–939. [DOI] [PubMed] [Google Scholar]
- Daffner KR,Scinto LF,Weitzman AM,Faust R,Rentz DM,Budson AE,Holcomb PJ ( 2003): Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. J Cogn Neurosci 15: 294–313. [DOI] [PubMed] [Google Scholar]
- Damasio AR ( 1994): Descartes' Error. New York: Avon Books. [Google Scholar]
- Davidson RJ ( 1984): Affect, cognition, and hemispheric specialization In: Izard CE,Kagan J, Zajonc R, editors. Emotion, cognition, and behavior. New York: Cambridge University Press; pp 320–365. [Google Scholar]
- Davidson RJ ( 2002): Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol Psychiatry 51: 68–80. [DOI] [PubMed] [Google Scholar]
- Davidson RJ ( 2003): Seven sins in the study of emotion: Correctives from affective neuroscience. Brain Cogn 52: 129–132. [DOI] [PubMed] [Google Scholar]
- Davidson RJ,Ekman P,Saron C,Senulis J,Friesen WV ( 1990): Approach/withdrawal and cerebral asymmetry: Emotional expression and brain physiology. J Pers Soc Psychol 58: 330–341. [PubMed] [Google Scholar]
- Davis M,Whalen PJ ( 2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6: 13–34. [DOI] [PubMed] [Google Scholar]
- Ekman P ( 1992): Are there basic emotions? Psychol Rev 99: 550–553. [DOI] [PubMed] [Google Scholar]
- Ekman P ( 1993): Facial expression and emotion. Am Psychol 48: 384–392. [DOI] [PubMed] [Google Scholar]
- Feldman Barrett L,Fossum T ( 2001): Mental representations of affect knowledge. Cogn Emot 15: 333–363. [Google Scholar]
- Feldman Barrett L,Russell JA ( 1998): Independence and bipolarity in the structure of current affect. J Pers Soc Psychol 74: 976–984. [Google Scholar]
- Feldman LA ( 1995): Valence focus and arousal focus: Individual differences in the structure of affective experience. J Pers Soc Psychol 69: 153–166. [Google Scholar]
- Fellows LK,Farah MJ ( 2005a) Different underlying impairments in decision‐making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex 15: 58–63. [DOI] [PubMed] [Google Scholar]
- Fellows LK,Farah MJ ( 2005b): Is anterior cingulate cortex necessary for cognitive control? Brain 128 (Part 4): 788–796. [DOI] [PubMed] [Google Scholar]
- Fletcher PC,Frith CD,Baker SC,Shallice T,Frackowiak RS,Dolan RJ ( 1995): The mind's eye–precuneus activation in memory‐related imagery. Neuroimage 2: 195–200. [DOI] [PubMed] [Google Scholar]
- Fletcher PC,Frith CD,Rugg MD ( 1997): The functional neuroanatomy of episodic memory. Trends Neurosci 20: 213–218. [DOI] [PubMed] [Google Scholar]
- Forman SD,Cohen JD,Fitzgerald M,Eddy WF,Mintun MA,Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Fossati P,Hevenor SJ,Graham SJ,Grady C,Keightley ML,Craik F,Mayberg H ( 2003): In search of the emotional self: An FMRI study using positive and negative emotional words. Am J Psychiatry 160: 1938–1945. [DOI] [PubMed] [Google Scholar]
- Fridja NH ( 1986): The Emotions. Cambridge, England: Cambridge University Press. [Google Scholar]
- Friston KJ,Ashburner CD,Frith JB,Poline JB,Heather RS,Frackowiak RS ( 1995a) Spatial registration and normalization of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Friston KJ,Holmes AP,Poline JB,Grasby PJ,Williams SC,Frackowiak RS,Turner R ( 1995b) Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Garavan H,Pendergrass JC,Ross TJ,Stein EA,Risinger RC ( 2001): Amygdala response to both positively and negatively valenced stimuli. Neuroreport 12: 2779–2783. [DOI] [PubMed] [Google Scholar]
- Garcia R,Vouimba RM,Baudry M,Thompson RF ( 1999): The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 402: 294–296. [DOI] [PubMed] [Google Scholar]
- Ghaem O,Mellet E,Crivello F,Tzourio N,Mazoyer B,Berthoz A,Denis M ( 1997): Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport 8: 739–744. [DOI] [PubMed] [Google Scholar]
- Green JD,Arduini AA ( 1954): Hippocampal electrical activity in arousal. J Neurophysiol 17: 533–557. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM,Gur RC,Perkins AC,Schroeder L,Turner T,Turetsky BI,Chan RM,Loughead JW,Alsop DC,Maldjian J,Gur RE ( 2003): Age‐related differences in brain activation during emotional face processing. Neurobiol Aging 24: 285–295. [DOI] [PubMed] [Google Scholar]
- Hamann S,Mao H ( 2002): Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport 13: 15–19. [DOI] [PubMed] [Google Scholar]
- Inglis FM,Fibiger HC ( 1995): Increases in hippocampal and frontal cortical acetylcholine release associated with presentation of sensory stimuli. Neuroscience 66: 81–86. [DOI] [PubMed] [Google Scholar]
- Isenberg N,Silbersweig D,Engelien A,Emmerich S,Malavade K,Beattie B,Leon AC,Stern E ( 1999): Linguistic threat activates the human amygdala. Proc Natl Acad Sci USA 96: 10456–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W ( 1890): The Principles of Psychology. Vol. 1 New York: Henry Holt & Co. [Google Scholar]
- Johnstone T,van Reekum CM,Oakes TR,Davidson RJ ( 2006): The voice of emotion: An FMRI study of neural responses to angry and happy vocal expressions. Soc Cogn Affect Neurosci 1: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E,Schwartz J,Jessell T ( 2000): Principles of Neural Science. New York: McGraw Hill. [Google Scholar]
- Kastner S,Ungerleider LG ( 2000): Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23: 315–341. [DOI] [PubMed] [Google Scholar]
- Killgore WD,Yurgelun‐Todd DA ( 2004): Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage 21: 1215–1223. [DOI] [PubMed] [Google Scholar]
- Kim H,Somerville LH,Johnstone T,Alexander AL,Whalen PJ ( 2003): Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 14: 2317–2322. [DOI] [PubMed] [Google Scholar]
- Kring AM,Barrett LF,Gard DE ( 2003): On the broad applicability of the affective circumplex: Representations of affective knowledge among schizophrenia patients. Psychol Sci 14: 207–214. [DOI] [PubMed] [Google Scholar]
- Lane RD,Reiman EM,Ahern GL,Schwartz GE,Davidson RJ ( 1997): Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154: 926–933. [DOI] [PubMed] [Google Scholar]
- Lang P,Greenwald M,Bradley M,Hamm A ( 1993): Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology 30: 261–273. [DOI] [PubMed] [Google Scholar]
- Lang PJ,Bradley MM,Fitzsimmons JR,Cuthbert BN,Scott JD,Moulder B,Nangia V ( 1998): Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 35: 199–210. [PubMed] [Google Scholar]
- Le TH,Pardo JV,Hu X ( 1998): 4 T‐fMRI study of nonspatial shifting of selective attention: Cerebellar and parietal contributions. J Neurophysiol 79: 1535–1548. [DOI] [PubMed] [Google Scholar]
- LeDoux J ( 2003): The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA,Critchley HD,Rotshtein P,Dolan RJ ( 2007): Neural correlates of processing valence and arousal in affective words. Cereb Cortex 17: 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E,Pelletier JG,Paz R,Pare D ( 2005): Prefrontal control of the amygdala. J Neurosci 25: 7429–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW III,Cohen JD,Stenger VA,Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Maddock RJ,Garrett AS,Buonocore MH ( 2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F,Sahakian B,Clark L,Rogers R,Antoun N,Aitken M,Robbins T ( 2002): Decision‐making processes following damage to the prefrontal cortex. Brain 125 (Part 3): 624–639. [DOI] [PubMed] [Google Scholar]
- Manns JR,Hopkins RO,Squire LR ( 2003): Semantic memory and the human hippocampus. Neuron 38: 127–133. [DOI] [PubMed] [Google Scholar]
- McAvoy MP,Ollinger JM,Buckner RL ( 2001): Cluster size thresholds for assessment of significant activation in fMRI. Neuroimage 13: S198. [Google Scholar]
- Mickey R,Dunn O,Clark V ( 2004): Applied Statistics: Analysis of Varience and Regression. New York: Wiley‐Interscience. [Google Scholar]
- Mumford J,Nichols T ( 2008): Power calculations for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage 39: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC,Nimmo‐Smith I,Lawrence AD ( 2003): Functional neuroanatomy of emotions: A meta‐analysis. Cogn Affect Behav Neurosci 3: 207–233. [DOI] [PubMed] [Google Scholar]
- Murray EA ( 2007): The amygdala, reward and emotion. Trends Cogn Sci 11: 489–497. [DOI] [PubMed] [Google Scholar]
- Nadel L,Moscovitch M ( 1997): Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7: 217–227. [DOI] [PubMed] [Google Scholar]
- Naqvi NH,Rudrauf D,Damasio H,Bechara A ( 2007): Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT,Casey BJ ( 2005): An integrative theory of attention‐deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol 17: 785–806. [DOI] [PubMed] [Google Scholar]
- Nishijo H,Yamamoto Y,Ono T,Uwano T,Yamashita J,Yamashima T ( 1997): Single neuron responses in the monkey anterior cingulate cortex during visual discrimination. Neurosci Lett 227: 79–82. [DOI] [PubMed] [Google Scholar]
- Ochsner KN,Bunge SA,Gross JJ,Gabrieli JD ( 2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ohman A,Flykt A,Esteves F ( 2001): Emotion drives attention: detecting the snake in the grass. J Exp Psychol Gen 130: 466–478. [DOI] [PubMed] [Google Scholar]
- Ortony A,Turner TJ ( 1990): What's basic about basic emotions? Psychol Rev 97: 315–331. [DOI] [PubMed] [Google Scholar]
- Panksepp J ( 1992): A critical role for “affective neuroscience” in resolving what is basic about basic emotions. Psychol Rev 99: 554–560. [DOI] [PubMed] [Google Scholar]
- Paradiso S,Johnson DL,Andreasen NC,O'Leary DS,Watkins GL,Ponto LL,Hichwa RD ( 1999): Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry 156: 1618–1629. [DOI] [PubMed] [Google Scholar]
- Paton JJ,Belova MA,Morrison SE,Salzman CD ( 2006): The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439: 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS,Skudlarski P,Gatenby JC,Zhang H,Anderson AW,Gore JC ( 1999): An fMRI study of Stroop word‐color interference: Evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 45: 1237–1258. [DOI] [PubMed] [Google Scholar]
- Phan KL,Wager T,Taylor SF,Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Portas CM,Rees G,Howseman AM,Josephs O,Turner R,Frith CD ( 1998): A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18: 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J,Russell JA,Peterson BS ( 2005): The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev Psychopathol 17: 715–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M ( 1999): Attention In: Gazzaniga M,editor. The New Cognitive Neurosciences. Cambridge: MIT Press. [Google Scholar]
- Posner MI,Dehaene S ( 1994): Attentional networks. Trends Neurosci 17: 75–79. [DOI] [PubMed] [Google Scholar]
- Posner MI,Petersen SE ( 1990): The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Quirk GJ,Likhtik E,Pelletier JG,Pare D ( 2003): Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM,Lane RD,Ahern GL,Schwartz GE,Davidson RJ,Friston KJ,Yun LS,Chen K ( 1997): Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 154: 918–925. [DOI] [PubMed] [Google Scholar]
- Richter‐Levin G ( 2004): The amygdala, the hippocampus, and emotional modulation of memory. Neuroscientist 10: 31–39. [DOI] [PubMed] [Google Scholar]
- Russell JA ( 1980): A circumplex model of affect. J Pers Soc Psychol 39: 1161–1178. [Google Scholar]
- Russell JA ( 2003): Core affect and the psychological construction of emotion. Psychol Rev 110: 145–172. [DOI] [PubMed] [Google Scholar]
- Russell JA,Weiss A,Mendelsohn GA ( 1989): Affect grid: A single‐item scale of pleasure and arousal. J Pers Soc Psychol 57: 493–502. [Google Scholar]
- Sarter M,Givens B,Bruno JP ( 2001): The cognitive neuroscience of sustained attention: Where top‐down meets bottom‐up. Brain Res Brain Res Rev 35: 146–160. [DOI] [PubMed] [Google Scholar]
- Small DM,Gregory MD,Mak YE,Gitelman D,Mesulam MM,Parrish T ( 2003): Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39: 701–711. [DOI] [PubMed] [Google Scholar]
- Spitzer R,Williams J,Gibbon M ( 1995): Structured Clinical Interview for DSM‐IV (SCID). New York: Biometrics Research. [Google Scholar]
- Sprengelmeyer R,Rausch M,Eysel UT,Przuntek H ( 1998): Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci 265: 1927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins SS ( 1962): Affect,Imagery, Consciousness In: The Positive Affects, Vol. 1 New York: Springer. [Google Scholar]
- Wager TD,Phan KL,Liberzon I,Taylor SF ( 2003): Valence, gender, and lateralization of functional brain anatomy in emotion: A meta‐analysis of findings from neuroimaging. Neuroimage 19: 513–531. [DOI] [PubMed] [Google Scholar]
- Watson D,Clark LA,Tellegen A ( 1988): Development and validation of brief measures of positive and negative affect: The PANAS scale. J Pers Soc Psychol 54: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Yang TT,Menon V,Eliez S,Blasey C,White CD,Reid AJ,Gotlib IH,Reiss AL ( 2002): Amygdalar activation associated with positive and negative facial expressions. Neuroreport 13: 1737–1741. [DOI] [PubMed] [Google Scholar]
- Zald DH,Donndelinger MJ,Pardo JV ( 1998): Elucidating dynamic brain interactions with across‐subjects correlational analyses of positron emission tomographic data: The functional connectivity of the amygdala and orbitofrontal cortex during olfactory tasks. J Cereb Blood Flow Metab 18: 896–905. [DOI] [PubMed] [Google Scholar]
- Zarahn E,Slifstein M ( 2001): A reference effect approach for power analysis in fMRI. Neuroimage 14: 768–779. [DOI] [PubMed] [Google Scholar]


