Abstract
The interplay between pathogen effectors, their host targets, and cognate recognition proteins provides various opportunities for antagonistic cycles of selection acting on plant and pathogen to achieve or abrogate resistance, respectively. Selection has previously been shown to maintain diversity in plant proteins involved in pathogen recognition and some of their cognate pathogen effectors. We analyzed the signatures of selection on 10 Arabidopsis thaliana genes encoding defense signal transduction proteins in plants, which are potential targets of pathogen effectors. There was insufficient evidence to reject neutral evolution for 6 genes encoding signaling components consistent with these proteins not being targets of effectors and/or indicative of constraints on their ability to coevolve with pathogen effectors. Functional constraints on effector targets may have provided the driving selective force for the evolution of guard proteins. PBS1, a known target of an effector, showed little variation but is known to be monitored by a variable guard protein. Evidence of selection maintaining diversity was present at NPR1, PAD4, and EDS1. Differences in the signatures of selection observed may reflect the numbers of effectors that target a particular protein, the presence or absence of a cognate guard protein, as well as functional constraints imposed by biochemical activities or interactions with plant proteins.
NUMEROUS molecular events determine the outcome of interactions between plants and potential pathogens (Jones and Dangl 2006). On the plant side, these include proteins involved in the direct or indirect recognition of pathogens, signaling proteins that transduce recognition events, and response proteins that are induced and restrict pathogen growth as a result of their antimicrobial activity or inhibitory action on pathogenicity factors. These are matched in the pathogen by effector proteins that manipulate the host to the pathogen's advantage. Effectors may act by interfering with the resistance signaling pathway or as pathogenicity factors such as hydrolytic enzymes or inhibitors of response proteins. There are, therefore, multiple opportunities for antagonistic cycles of selection acting on plant and pathogen to affect or abrogate resistance.
The majority of host recognition genes cloned to date encode nucleotide binding site–leucine rich repeat (NBS–LRR) proteins. Analyses of NBS–LRR paralogs have revealed hypervariability in the predicted solvent exposed residues in the LRR region (e.g., Jones and Jones 1997; Kuang et al. 2004). This is consistent with diversifying selection indicative of simultaneous selection acting on both pathogen effectors and host recognition proteins in their respective attempts to avoid and maintain detection. Evolutionary analyses of NBS–LRR genes, however, are complicated by extensive gene duplication, variable rates of sequence exchange between paralogs, and indistinguishable orthologous relationships. Therefore, evolutionary analyses of individual genes in clusters are problematic. Strong diversifying selection has, however, been detected for at least two low-copy genes, RPP13 in Arabidopsis thaliana and L in flax, in which sequence exchange between paralogs has been rare (Ellis et al. 1999; Rose et al. 2004; Dodds et al. 2006). Furthermore, several single-copy NBS–LRR-encoding loci have presence–absence polymorphisms that correlate with resistance/susceptibility phenotypes, limited diversity within each allelic class, and ancient polymorphisms (Stahl et al. 1999; Tian et al. 2002; Shen et al. 2006). Frequency-dependent selection is thought to generate and maintain the diversity at R-gene loci exhibiting both patterns of variation (Michelmore and Meyers 1998; Stahl et al. 1999).
Only a few response genes have demonstrated strong evidence of selection (Tiffin and Moeller 2006). Positive selection has been detected at specific amino acid sites within cell wall degrading enzymes and inhibitors such as class I chitinases, endo-β-1,3-glucanases, and polygalacturonase inhibitor proteins (PGIPs) (Stotz et al. 2000; Bishop et al. 2000, 2005; Bishop 2005). However, both β-1,3-glucanases and PGIPs may also have roles in pathogen detection, which could influence the signatures of selection at these loci (Cote et al. 1998; Rose et al. 2002).
Until recently, there had been no previous reports on the evolution of genes encoding signal transduction proteins in plants. These proteins play a central role in both basal and gene-for-gene resistance [pathogen-associated molecular pattern (PAMP) and effector-triggered immunity] as they are responsible for relaying signals of pathogen recognition to initiate the defense response that includes release of reactive oxygen species, production of pathogenicity-related proteins, activation of systemic acquired resistance, cell wall modification, and programmed cell death (Jones and Dangl 2006). There are at least three main defense signaling pathways that involve salicylic acid, jasmonic acid, and ethylene as signaling molecules. Although several key components have been identified and ordered within a given pathway, complete characterization of all proteins involved, dissection of the intricacies of each pathway, and understanding the cross talk between them is still in progress.
The majority of host proteins known to be targeted by virulence effectors have a role in plant defense responses (Speth et al. 2007; Zhang et al. 2007). Furthermore, several of the genes encoding defense signaling proteins were identified from mutant screens in which resistant plants became susceptible (Glazebrook 1999, 2001). Therefore, such genes represent points of vulnerability that could be targeted by pathogen effectors to suppress resistance. Consistent with this idea, several effectors are known to interfere with programmed cell death associated with both host and nonhost resistance (Alfano and Collmer 2004; Mudgett 2005; Grant et al. 2006). Therefore, selection pressures would be expected to act on the plant to negate the action of effectors resulting in changes in signaling proteins or other defense components. The possibilities for modifications of signaling proteins may be constrained due to functional restrictions imposed by the need to interact with multiple upstream and downstream signaling proteins. This may have been a selective force for the evolution of recognition (guard) proteins that monitor the status of conserved signaling proteins and trigger a resistance response upon detection of effector action (Van Der Hoorn et al. 2002). This results in the potential coevolution of at least three components: the signal transduction protein, the pathogen effector that targets the signaling protein, and the recognition protein that guards the signaling protein. Analyses of sequence diversity of genes encoding defense signaling components were needed to complement the studies of recognition and response proteins. Such studies provide insights into whether defense signaling proteins are being targeted by effectors and whether there is evidence for functional constraints on their evolution.
Recently, Bakker et al. (2008) reported the analysis of ∼600-bp fragments of 27 defense genes in A. thaliana and concluded that the majority appeared to be experiencing purifying selection with only weak evidence of positive selection acting on a few genes. However, only short fragments representing approximately one-third of the coding region of each gene were analyzed. Therefore, if only specific regions of these proteins coevolve with pathogens or interacting partners, signatures of selection would have been missed.
We analyzed the entire genic region of genes encoding 10 proteins involved in defense signaling, 2 of which are known targets of effectors, to determine if the evolutionary patterns of signaling genes deviated from genomewide patterns and expectations based on neutral theory. We exploited data from a recent survey of genomewide polymorphism in A. thaliana (Nordborg et al. 2005) to test empirically for departures from expectations derived from data sampled across the genomes of the genotypes studied. This provided the opportunity to distinguish the effects of natural selection from those of demographic history. We also employed several standard tests of neutrality. The majority of the 10 genes encoding signal transduction proteins did not have evolutionary histories sufficiently distinct from random reference loci to reject neutrality; however, at least three members of the salicylic acid signaling pathway exhibited significant departures from neutral expectations.
MATERIALS AND METHODS
Plant material:
A. thaliana accessions (supplemental Table S3) were selected to represent the species geographic range. This included six accessions previously determined to have a high-average genetic distance to other accessions (Ei-2, N13, Gu-0, Lz-0, Wei-0, and Yo-0; Sharbel et al. 2000) and several parental accessions for genetic mapping populations (Col-0, Cvi-0, Ler, Nd-1, C24, Ws-0, Shahdara, and Bay-0). All accessions were obtained from the Arabidopsis Biological Research Center and grown in Promix (Premier Horticulture, Canada) with vermiculite and slow-release fertilizer (Osmocote) in growth chambers (constant 22°, 50 to 60% relative humidity, fluorescent lighting, 120 to 150 μmol m−2 s−1, 16-hr day length). Five individuals of A. lyrata from Spitersulen, Norway, were kindly provided by O. Savolainen for an outgroup. DNA was isolated using a modified CTAB protocol.
SNP discovery and genotyping:
Primer combinations (supplemental Table S4) were designed to the fully sequenced A. thaliana Col-0 genome for amplification of overlapping products spanning 10 defense signaling genes: COI1, ENHANCED DISEASE RESISTANCE 1 (EDR1), EDS1, MITOGEN-ACTIVATED PROTEIN KINASE 4 (MPK4), NONRACE SPECIFIC DISEASE RESISTANCE 1 (NDR1), NPR1, PAD4, PBS1, RIN4, and TGA2. Amplicons were purified using ExoI and SAP (USB, Cleveland) and sequenced using ABI BigDye Terminators, version 3.1. Sequence fidelity was confirmed by sequencing independent amplicons. To determine haplotype phase of heterozygous material, full-length genes were amplified using either Platinum or AccuPrime High-Fidelity polymerases (Invitrogen, Carlsbad, CA) with minimal (25) amplification cycles and cloned into either pGEM-T (Promega, Madison, WI) or pCR2.1-TOPO vectors (Invitrogen). A minimum of eight clones for each gene were sequenced in both directions. Sequences were submitted to GenBank under accession numbers EF470606–EF470864. Evidence of heterozygosity was only evident in two A. thaliana genes: EDS1 amplified from Wei-0 and RIN4 amplified from C24.
Sequence alignment and statistical analysis:
Fourteen of the 20 accessions used in this study overlapped with those included in a genomewide survey of A. thaliana polymorphism composed of 876 coding and noncoding sequences of ∼500 bp in length (Nordborg et al. 2005). To avoid sampling bias, we analyzed identical sample sets for the reference loci as were used for the defense genes. Furthermore, because the level of polymorphism in A. thaliana is strongly negatively correlated with gene density (Nordborg et al. 2005), we decided to compare genic regions directly to other genic regions. Therefore, from the 876 loci, all loci that did not contain sequence information for all 14 accessions and did not have >300 bp of consensus coding sequence were excluded. This resulted in a final reference set containing 355 loci (supplemental Table S1). Multiple sequence alignment and contigs editing were performed using Sequencher version 4.6 (Gene Codes v4.6) software. Mulitple alignment using fast Fourier transform (MAFFT; http://www.ebi.ac.uk/Tools/mafft/index.html) produced identical alignments after manual adjustment on the basis of visual inspection confirming the robustness of these alignments. Both data sets were analyzed for the following statistics using the compute and polydNdS programs in the analysis package of Libosequence (Thornton 2003) or DNAsp version 4.10 (Rozas et al. 2003) excluding consensus positions containing ambiguities: nucleotide diversity (π) (Nei 1987), Tajima's D (Tajima 1989), and πA:πS. Analyses performed including consensus positions containing ambiguities and/or using 400 bp of consensus as a stringency cutoff showed similar distributions. Furthermore, comparison of statistics for defense genes to genomewide distributions generated using all the available reference loci did not alter the results obtained with the smaller set of 355 loci.
The multilocus HKA test was performed on silent sites using a program available from J. Hey (Rutgers University, Piscataway, NJ). Pairwise intergenic HKA tests were performed on silent sites using DNAsp version 4.50.1 (Rozas et al. 2003); significance values were calculated assuming the β-distribution (Tajima 1989).
The McDonald–Kreitman test was performed using DnaSP version 4.10 (McDonald and Kreitman 1991; McDonald 1998); significance values were calculated using Fisher's exact test and the neutrality index was calculated according to Rand and Kann (1996). To obtain outgroup sequences of the 355 reference loci, these loci were blasted against the trace files for the whole genome sequencing project of A. lyrata at NCBI. Traces yielding significant hits (<1e-20) were assembled into contigs using CAP3 (100-bp minimal overlap; 95% identity; Huang and Madan 1999). Loci for which a definitive ortholog could not be established or the ortholog contained a frameshift or premature stop codon were excluded from further analysis. Of the 298 remaining loci, 44 loci invalidated the contingency table because they were either monomorphic within the coding sequence of A. thaliana or void of both nonsynonymous polymorphism and divergence.
Tests for heterogeneity in the polymorphism-to-divergence ratio were performed using DNASlider (McDonald 1998). Because recombination rate has an effect on the sliding G tests, simulations were run under six different recombination parameters (1, 2, 4, 8, 16, and 32) and the recombination parameter yielding the greatest P was reported.
All tests were performed on the entire set of 20 accessions as well as the reduced set of 14 used for comparison to the reference set. All results reported in direct comparison to the reference set were calculated with the reduced set of 14; all results reported using an outgroup i.e., MK and sliding G tests were performed on the full set of 20 individuals. Both sets of analyses gave similar results and are reported in Tables 1 and 2.
TABLE 1.
Summary statistics across the putative target genes (n = 20)
| θ/bp based on
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Gene | Length (bp) | S | Sn | πall | πAa | πSa | πA:πSa | D |
| COI1 | 3146 | 22 (22) | 0.0020 | 0.0016 | 0.0001 | 0.0072 | 0.0197 | −0.6641 |
| 1779a | 12a (12a) | (0.0022) | (0.0019) | (0.0002) | (0.0080) | (0.0255) | (−0.6655) | |
| EDR1 | 5180 | 73 (72) | 0.0042 | 0.0036 | 0.0017 | 0.0065 | 0.2105 | −0.6022 |
| 2814a | 29a (28a) | (0.0046) | (0.0035) | (0.0013) | (0.0060) | (0.2121) | (−1.0782) | |
| EDS1 | 2213 | 68 (66) | 0.0087 | 0.0083 | 0.0075 | 0.0111 | 0.6690 | −0.1838 |
| 1869a | 54a (52a) | (0.0094) | (0.0090) | (0.0079) | (0.0119) | (0.6738) | (−0.1901) | |
| MPK4 | 1812 | 41 (40) | 0.0065 | 0.0079 | 0.0009 | 0.0153 | 0.0568 | 0.8542 |
| 1134a | 12a (12a) | (0.0071) | (0.0060) | (0.0008) | (0.0119) | (0.0661) | (−0.6825) | |
| NDR1 | 722 | 6 (6) | 0.0023 | 0.0027 | 0.0021 | 0.0057 | 0.3587 | 0. 4827 |
| 657a | 5a (5a) | (0.0026) | (0.0029) | (0.0021) | (0.0063) | (0.3320) | (0.3828) | |
| NPR1 | 2624 | 87 (84) | 0.0097 | 0.0120 | 0.0036 | 0.0273 | 0.1318 | 0. 9643 |
| 1797a | 42a (40a) | (0.0105) | (0.0122) | (0.0038) | (0.0281) | (0.1340) | (0.7444) | |
| PAD4 | 2861 | 36 (35) | 0.0036 | 0.0027 | 0.0029 | 0.0032 | 0.9101 | −1.0378 |
| 1620a | 20a (20a) | (0.0039) | (0.0034) | (0.0038) | (0.0038) | (0.9960) | (−0.6413) | |
| PBS1 | 3115 | 8 (8) | 0.0007 | 0.0008 | 0.0002 | 0.0000 | NA | 0.1303 |
| 1374a | 1a (1a) | (0.0008) | (0.0007) | (0.0001) | (0.0000) | NA | (−0.5093) | |
| RIN4 | 1610 | 70 (70) | 0.0130 | 0.0097 | 0.0039 | 0.0211 | 0.1828 | −1.0439 |
| 633a | 21a (21a) | (0.0145) | (0.0116) | (0.0048) | (0.0243) | (0.1957) | (−0.8737) | |
| TGA2 | 2058 | 13 (13) | 0.0018 | 0.0011 | 0.0004 | 0.0022 | 0.1604 | −1. 3714 |
| 993a | 4a (4a) | (0.0020) | (0.0013) | (0.0003) | (0.0025) | (0.1374) | (−1.5015) | |
Statistics in parentheses are for comparison with the reference loci (n = 14). S, number of segregating sites; Sn, nucleotide diversity calculated by S; πall, πA, and πS, nucleotide diversity calculated by average pairwise comparison on the basis of all nonsynonymous sites and synonymous sites, respectively; D, Tajima's D.
Statistics applied to coding sequence only.
TABLE 2.
Values used for McDonald–Kreitman test and its significance for the full data set n = 20 and comparison with reference loci (n = 14)
| Fixed
|
Polymorphic
|
Significance: | |||
|---|---|---|---|---|---|
| Gene | N | S | N | S | Fisher's exact |
| EDS1 | 72 (72) | 48 (48) | 45 (43) | 12 (12) | P < 0.05 |
| 40 (38) | 11 (11) | ||||
| EDS1 exon 2 | 15 (15) | 23 (23) | 14 (13) | 1 (1) | P < 0.001 |
| 14 (13) | 1 (1) | ||||
| NPR1 | 64 (64) | 57 (57) | 15 (14) | 29 (28) | P < 0.01 |
| 15 (14) | 29 (28) | ||||
| NPR1 exon 2 | 34 (34) | 20 (20) | 4 (4) | 22 (22) | P < 0.001 |
| 4 (4) | 22 (22) | ||||
| PAD4 | 61 (61) | 54 (54) | 16 (16) | 4 (4) | P < 0.05 |
| 16 (16) | 4 (4) | ||||
| PAD4 exon 1 | 10 (10) | 17 (17) | 8 (8) | 2 (2) | P < 0.05 |
| 8 (8) | 2 (2) | ||||
| PBS1 | 4 (4) | 36 (36) | 1 (1) | 0 (0) | NA |
| 1 (1) | 0 (0) | ||||
| RIN4 | 11 (11) | 9 (9) | 13 (13) | 12 (12) | NS |
| 11 (11) | 10 (10) | ||||
Data on top line includes polymorphic count from both species; data on bottom line include polymorphic count from A. thaliana only. Degree of significance remained the same regardless of sample set and/or inclusion of polymorphic data from one or both species. N, nonsynonymous; S, synonymous; NA, not applicable; NS, not significant.
Linkage disequilibrium (squared allele frequency correlation, r2) between pairs of polymorphic sites within genes was analyzed and plotted using TASSEL 1.9.6. Polymorphic sites with a rare allele frequency <0.10 were excluded from analysis. Tassel calculates P-values using Fisher's exact test for biallelic sites. For multiallelic sites, repeated permutations of the alleles are used to calculate empirical P-values. The genomic sequence of EDS1 from A. lyrata revealed the absence of intron 3 relative to the A. thaliana gene. This intron was also absent from the EDS1-like paralogs in both species indicating that the ancestral gene structure probably consisted of only 3 exons. This insertion does not disrupt the reading frame; however, the sequence for 10 codons directly flanking the intron:exon borders is substantially altered resulting in numerous replacement changes. Because these substitutions may have resulted from the insertion event, these sites were excluded from the tests for selection.
Gene conversion was analyzed using Genconv (Sawyer 1989), a program that identifies stretches of identical sequence that are statistically longer than expected for that pair of sequences.
RESULTS
Most genes encoding signal transduction proteins did not deviate significantly from genomewide patterns of polymorphism:
To determine if host proteins targeted by pathogen effectors are subjected to greater selective pressures than those acting upon the genome as a whole, genes encoding two known host targets of virulence effectors of Pseudomonas syringae [RPM1-INTERACTING PROTEIN 4 (RIN4) and AvrPphB SUSCEPTIBLE 1 (PBS1)] and eight defense proteins from multiple signal transduction defense pathways, were selected for analysis with an emphasis of the salicylic acid pathway (Figure 1); these eight genes have major phenotypic consequences when mutated and are, therefore, potential effector targets. These 10 genes were completely sequenced from a panel of 20 diverse accessions of A. thaliana and analyzed for several summary statistics diagnostic of nonneutral evolution: estimates of nucleotide diversity (π), Tajima's D test for neutrality, and πA:πS. These statistics were compared to the same analyses of 355 randomly distributed coding loci (Nordborg et al. 2005) to assay for deviations from average genomewide patterns of polymorphism (Table 1, supplemental Table S1, supplemental Figure S1). For the nucleotide diversity and Tajima's D statistics, none of the genes analyzed departed significantly from genomewide patterns of polymorphism; all 10 were distributed within the middle 90% of the genomewide distribution (Table 1, supplemental Figure S1). Only CORONITINE INSENSITIVE 1 (COI1) fell within the 5% tails of the genomewide distribution for πa:πs; this is suggestive of strong purifying selection acting on this gene. Therefore, these test statistics indicate that 9 of the 10 genes analyzed encoding defense signal transduction proteins are evolving consistent with expectations based on genomewide observations.
Figure 1.—
Position of proteins involved in the defense signal transduction pathways. Proteins encoded by the genes analyzed in this study are circled. The signaling molecules, salicylic acid (SA), jasmonic acid (JA), and ethylene (C2H4), are boxed. The positions of PBS1 and RIN4 in these pathways are not currently known.
Most genes encoding signal transduction proteins did not deviate from neutral expectations:
To test if the patterns of polymorphism within genes encoding signal transduction proteins deviated from neutral expectations, we employed four tests for neutrality, Tajima's D (Tajima 1989), the MK test (McDonald and Kreitman 1991), the HKA test (Hudson et al. 1987), and the maximum sliding G test (McDonald 1998). Tajima's D tests for a disparity between two estimators of nucleotide polymorphism: nucleotide diversity (π) and Watterson's estimator (θ). Because θ does not take allele frequency into account, these estimates will differ significantly from one another in the presence of demographic and evolutionary forces that affect allele frequency in a population. A negative Tajima's D value indicates an excess of rare frequency alleles while a positive value suggests an excess of intermediate frequency alleles. The MK test analyzes deviations from neutral expectations that ratios of nonsynonymous-to-synonymous polymorphism within species and divergence between species for a given DNA sequence are predicted to be similar in closely related species. This is because synonymous and nonsynoymous sites within a given gene are expected to have a shared evolutionary history due to tight linkage. An advantage to the MK test is that it is not sensitive to demography making it useful for detecting selection. Likewise, the maximum sliding G and HKA tests investigate heterogeneity in polymorphism to divergence within and among loci, respectively. The expected number of polymorphisms and fixations are both proportional to the mutation rate; therefore, in the absence of selection and after taking recombination and sampling into account, a uniform ratio of polymorphism to divergence is expected to persist within and among different gene loci. The maximum sliding G test was proposed as an alternative to the intragenic HKA test to avoid arbitrary partitioning of the gene (McDonald 1998). A disadvantage of the HKA test is that demographic factors such as migration can give significant results leading to a false indication of selection. Because tests like HKA and Tajima's D can be affected by factors other than selection, we utilized multiple test statistics to develop the most parsimonious explanations for our data.
With the exception of Tajima's D, all of these statistical tests required an outgroup. Homologs from nine genes were sequenced from three to five individuals of A. lyrata for use as outgroups. NDR1 could not be amplified from A. lyrata despite attempts with multiple primer combinations; therefore, the homolog was obtained from genomic trace files. Outgroups for 298 of the 355 reference loci were also obtained from genomic trace files (see materials and methods). Phylogenetic analyses (neighbor joining; MEGA4) confirmed that A. lyrata was a suitable outgroup for the defense genes in our study; for all defense genes analyzed, clear bifurcating trees supported by 100% bootstrapping indicated that all alleles within species were more similar than between species (data not shown). Furthermore, when data was available within A. lyrata, it confirmed that a negligible amount of polymorphism was shared between these species.
None of the loci had significant Tajima's D (Table 1). Five loci, COI1, EDR1, MPK4, NDR1, and TGA2, did not show any evidence of nonneutral evolution in any of the tests. The MK test produced significant results for three loci, NPR1, EDS1, and PAD4 (Table 2); the maximum sliding G test was also significant for these 3 genes indicating intragenic heterogeneity in polymorphism to divergence. A multilocus HKA test performed on all 10 genes was highly significant (P = 0.00004) indicating heterogeneity in the ratio of polymorphism-to-divergence ratios among genes. Individual intergenic HKA tests based on silent sites were applied to all pairwise comparisons among these loci to determine which genes were responsible for this deviation from neutrality. Fourteen of the 45 total comparisons were significant at the 5% level supporting the multilocus analysis; however, only four comparisons remained significant after Bonferroni's correction for multiple tests. All four involved either PBS1 or RIN4 indicating that PBS1 and RIN4 are the major contributors to the significance of multilocus test with NPR1 having minor contributions. This is confirmed by a nonsignificant (P = 0.1515) multilocus HKA test on the remaining 7 genes.
COI1 is required for jasmonate signaling in response to necrotrophic pathogens and herbivores as well as fertility (Xie et al. 1998). Given its involvement in two critical aspects of species survival, defense, and reproduction, a low πA:πS value, suggestive of strong purifying selection, is not surprising. However, without any additional support for a deviation from nonneutral evolution, the data as a whole is insufficient to reject neutrality.
PBS1 encodes a protein kinase that is a known target of P. syringae effector, AvrPphB. Highly significant (P < 0.001) intergenic HKA results for three pairwise comparisons involving PBS1 (Table 3) indicate that this locus has significantly lower polymorphism relative to divergence and, therefore, is evolving differently than the other genes studied. All results from the additional neutrality tests on this locus were not significant; however, this is not unexpected. PBS1 was the least diverse of all the signaling genes analyzed (Table 1). Only eight sites segregated among the >3000 bp sampled at PBS1, compromising the power of the test for neutrality using Tajima's D. Similarly, only one polymorphism was located in the coding region providing insufficient power for the MK test. Furthermore, a homogenous intragenic polymorphism-to-divergence ratio (max sliding G test) would not be unexpected for a locus that has recently undergone a selective sweep. Therefore, PBS1 deviated from neutral expectations in the one test capable of detecting a significant departure with this data set. Either positive selection or purifying selection can account for low levels of nucleotide diversity. Since purifying selection is presumed to act predominantly on nonsynonymous sites, the absence of synonymous polymorphism is more consistent with a selective sweep acting on PBS1 or a linked gene. However, the level of nucleotide diversity among introns (π = 0.00121) at PBS1 is an order of magnitude higher than that among coding sites (π = 0.00014); this is suggestive of strong purifying selection rather than a selective sweep, which would cause a reduction in diversity at all nucleotide sites linked to the advantageous allele. Furthermore, regions affected by a selective sweep would be characterized by an excess of rare alleles; however, Tajima's D for PBS1 is positive for n = 20 (0.1303) and similar to the genome average of −0.5846 for n = 14 (−0.5093). Bakker et al. (2008) also concluded that PBS1 was under purifying selection. Studies of nucleotide diversity in regions flanking PBS1 are needed to distinguish between the possibilities of strong purifying selection or a selective sweep at this locus.
TABLE 3.
Probability values for pairwise HKA tests for full-length loci
| COI1 | EDS1 | EDR1 | MPK4 | NDR1 | NPR1 | PAD4 | PBS1 | RIN4 | TGA2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| COI1 | NA | |||||||||
| EDS1 | 0.0278 | NA | ||||||||
| EDR1 | 0.1330 | 0.4624 | NA | |||||||
| MPK4 | 0.0303 | 0.9929 | 0.5175 | NA | ||||||
| NDR1 | 0.4154 | 0.4745 | 0.7610 | 0.5101 | NA | |||||
| NPR1 | 0.0045 | 0.4158 | 0.0965 | 0.4569 | 0.1990 | NA | ||||
| PAD4 | 0.7757 | 0.0608 | 0.2197 | 0.0688 | 0.5611 | 0.0105 | NA | |||
| PBS1 | 0.2606 | 0.0017 | 0.0195 | 0.0007a | 0.0633 | 0.0005a | 0.1668 | NA | ||
| RIN4 | 0.0004a | 0.2105 | 0.0194 | 0.2648 | 0.1162 | 0.6041 | 0.0016 | 0.0000a | NA | |
| TGA2 | 0.9252 | 0.0541 | 0.1543 | 0.0526 | 0.4053 | 0.0116 | 0.7259 | 0.3097 | 0.0032 | NA |
NA, not applicable.
Significant with Bonferroni correction.
In contrast to PBS1, the HKA test with RIN4 indicated significantly higher polymorphism relative to divergence. RIN4 was more diverse than 93.5% of the reference loci (Table 1, supplemental Figure S1) and, therefore, it is unlikely that a lack of power could explain the failure to reject neutrality in these tests. Consequently, although the HKA test detected deviation from neutrality at this locus, the data from the multiple tests are insufficient to draw conclusions about nonneutral evolution. Because of the potential nonneutral evolution of PBS1 and RIN4, they were excluded as the second locus in subsequent HKA analyses of other genes.
Evidence of balancing selection acting on NPR1:
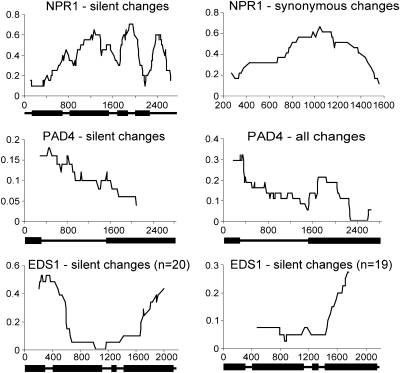
NONEXPRESSOR of PR GENES (NPR1) is a critical component of plant defense required for salicylic acid-mediated systemic acquired resistance, the elicitation of induced systemic resistance by Rhizobacterium spp., and the interaction between defense signaling pathways (Dong 2004). Pairwise comparisons with the full-length NPR1 locus and the seven other signaling genes in our study did not give significant HKA results (Table 3). However, significant intragenic heterogeneity in the polymorphism-to-divergence ratio among both silent and synonymous sites (Gmax, P = 0.0394 and P = 0.0163, respectively; Figure 2) indicated that different regions of NPR1 may have distinct evolutionary histories; therefore, the combined data across the locus could have masked deviations from neutrality in individual regions within the locus. The NPR1 locus was partitioned into three regions on the basis of intron position as well as concordance between increased levels of polymorphism relative to divergence and linkage disequilibrium (LD) decay (Figures 2 and 3). No significant HKA results were detected in comparisons using the 5′ flanking region through intron 1 of the NPR1 locus; however, three significant results (P < 0.005) were obtained after Bonferroni's correction when the same seven loci were compared against both exon 2–exon 4 and the 3′ flanking region (Table 4). Therefore, both intra- and intergenic heterogeneity tests indicate that the central portion of the NPR1 locus exhibited evolutionary patterns distinct from other loci. A peak of elevated polymorphism relative to divergence is consistent with the accumulation of silent site mutations as a result of linkage to an ancient polymorphism or haplotype under balancing selection.
Figure 2.—
Sliding-window plots of the polymorphism-to-divergence ratio of NPR1, PAD4, and EDS1. The proportion of polymorphic changes within A. thaliana relative to fixed differences between A. thaliana and A. lyrata were plotted for each window of 20 variable sites (40 for PAD4). Note that the plots in the second column are not the same for each gene. Corresponding gene structure is shown at the bottom of the plots: exons are indicated by boxes and noncoding regions by lines.
Figure 3.—
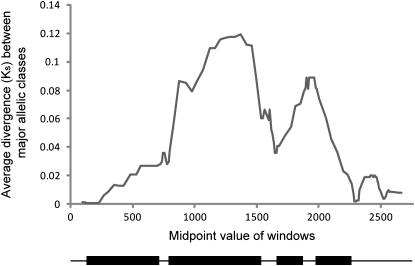
Sliding-window analysis of silent site divergence between the two major allelic classes at NPR1. Values plotted are the midpoints of windows 150 silent-sites wide at 10-bp increments. Corresponding gene structure is shown at the bottom of the plots: exons are indicated by boxes and noncoding regions by lines.
TABLE 4.
Probability values for intergenic HKA tests for partitioned loci
| COI1 | EDS1 | EDR1 | MPK4 | NDR1 | NPR1 | PAD4 | TGA2 | |
|---|---|---|---|---|---|---|---|---|
| 5′ EDS1 | 0.0691 | NA | 0.7626 | 0.7647 | 0.6441 | 0.2620 | 0.1366 | 0.1051 |
| 3′ EDS1 | 0.0020a | NA | 0.1327 | 0.5377 | 0.2542 | 0.9952 | 0.0070a | 0.0066a |
| 5′ NPR1 | 0.1236 | 0.6104 | 0.9515 | 0.6493 | 0.7584 | NA | 0.2219 | 0.1574 |
| NPR1 E2–E4 | 0.0003a | 0.1916 | 0.0149 | 0.2510 | 0.1108 | NA | 0.0010a | 0.0025a |
| 3′ NPR1 | 0.0012a | 0.4351 | 0.09 | 0.5006 | 0.2299 | NA | 0.0046a | 0.0064a |
NA, not applicable.
Significant with Bonferroni correction.
Comparison of the ratios of nonsynonymous-to-synonymous polymorphism and divergence also implicated exon 2 as a site of selection within NPR1. Application of the MK test to the entire gene indicated a significantly (P = 0.0325; Table 2) higher ratio of nonsynonymous-to-synonymous fixed substitutions vs. polymorphic differences [neutrality index (N.I.) = 0.4]. The significance level was substantially increased (P = 0.00009; N.I. = 0.1; Table 2) when the MK test was applied solely to exon 2. In comparison, MK analysis of only 11 (4.3%) of the reference loci were significant at a P-value < 0.05 and even the reference loci with the greatest significance (P = 0.0028) was less significant than exon 2 of NPR1 by more than an order of magnitude (supplemental Table S2). Therefore, this evolutionary signature is unlikely to have occurred by chance. A higher ratio of nonsynonymous-to-synonymous fixed substitutions can result from either adaptive fixation of advantageous mutations between species or selection against replacement polymorphisms within species. Adaptive fixation would be consistent with an “arms race” model of evolution in which strong positive selection in response to pathogen pressure drove the divergence between species. However, under positive selection the age of alleles observed at a locus is expected to be reduced. Sliding window analysis of nucleotide differences between the two major allelic classes observed at NPR1 revealed a dramatic increase in silent site polymorphism extending across the region from exon 2 through exon 4 (Figure 3). The level of divergence between the two allelic classes reaches a maximum (Ks = 0.1194) centered in exon 2, which is similar to the level of divergence between A. thaliana and A. lyrata (Ks = 0.1220) for NPR1. Both allelic classes are similarly divergent from A. lyrata (Ks = 0.1273 and 0.1299) suggesting that introgression of one allelic class from A. lyrata is unlikely. This is more consistent with balancing selection acting to maintain two allelic classes within A. thaliana. However, adaptive evolution between species and balancing selection within species are not mutually exclusive. It is plausible that ancient adaptive evolution occurred after speciation followed by balancing selection of the two major haplotypes in A. thaliana. The data for balancing selection within A. thaliana suggests that the adaptive evolution likely occurred in the A. lyrata lineage. Further comparisons including other related species are needed to distinguish between the various possibilities.
Balancing selection is further supported by significantly high levels (P < 10e-4) of LD extending across the majority of the coding region and the presence of an intermediate frequency, biallelic haplotype structure within exon 2 (Figure 3 and supplemental Figure S2). This haplotype structure extends across the region encoding the ankyrin repeats (Cao et al. 1997; Ryals et al. 1997), which is necessary for interaction of NPR1 with TGA transcription factors (Zhang et al. 1999; Zhou et al. 2000). When two alleles are maintained in a population through balancing selection, the regions flanking the selected site are expected to be defined by the accumulation of linked neutral mutations resulting in a biallelic haplotype structure (Charlesworth 2006). However, biallelic haplotype structures can also be explained by neutral processes in highly selfing species such as population substructure or introgression (Purugganan and Suddith 1999; Aguade 2001). Demographic processes are expected to affect a genome, and consequently a locus, as a whole and, therefore, are insufficient to explain the strong selection patterns specific to exon 2 of the NPR1 locus. Given that the MK, HKA, and max sliding G tests all rejected the null hypothesis of neutrality at the NPR1 locus, the most parsimonious conclusion is a deviation from neutrality. Furthermore, Tajima's D (1.5507) for the region spanning exon 2–exon 4, although not significant, falls within the 5% tail of the genomewide distribution for this statistic, indicating an excess of intermediate frequency alleles characteristic of balancing selection.
Balancing selection can result from several different evolutionary scenarios that act to maintain diversity at a locus. Transient balancing selection, such as diversifying selection, can result in πA:πS values >1; however, long-term balancing selection, such as frequency-dependent selection, could result in the accumulation of linked silent sites to the selected substitution while also allowing deleterious nonsynonymous changes to be removed by purifying selection, resulting in πA:πS values <1. The low πA:πS value observed for NPR1 (πA:πS = 0.128) is consistent with long-term maintenance of nonsynonymous variation at a limited number of sites. The polymorphic residues of the NPR1 protein were examined to investigate which might be subject to selection. Eight replacement substitutions were present at intermediate frequencies; five showed significant (P < 0.01) association to the observed haplotype structure (K178R, I183V, S268W, M367L, and F507H; supplemental Figure S2). Three of these are conservative changes (K178R, I183V, and M367L) and, therefore, less likely to be under selection; furthermore, two are located in exon 1 (K178R and I183V) where LD showed signs of decay. A fourth, F507H, is a slightly nonconservative substitution located in exon 4, which is of unknown function. The remaining replacement substitution, S268W, is located within the ankyrin repeat in exon 2 and is highly correlated (r2 = 0.90; P < 0.001) with the two major allelic classes observed at NPR1. Serine is a small polar residue that is often located at the beginning of α-helices because of its ability to form a hydrogen bond with the protein backbone. S268 is part of a tetrapeptide motif that is believed to form a tight turn that initiates the first α-helix of the ankyrin repeat (Sedgwick and Smerdon 1999). A substitution to tryptophan, a nonpolar residue with a large bulky side chain, would likely disrupt the functional or structural integrity of this domain. The interaction of the ankyrin repeat region of NPR1 with the transcription factor, TGA2, enhances binding to the promoter elements of pathogenesis-related (PR) genes and is, therefore, thought to be critical for defense gene activation (Després et al. 2000). Several single point mutations within the ankyrin repeats of NPR1 have been previously demonstrated to abolish interaction with TGA factors (Zhang et al. 1999; Zhou et al. 2000). Therefore, natural variation within this region is expected to affect the expression profile in response to pathogens by altering the affinity of NPR1 for a subset of transcription factors. It will be interesting to study whether effector molecules target NPR1 and if natural variation in the ankyrin repeat region influences the plant–pathogen interaction phenotype.
Evidence of nonneutral evolution at PAD4 and EDS1:
Several analyses are consistent with nonneutral evolution at ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1). Individual pairwise comparisons using full-length EDS1 against the other seven genes did not yield significant HKA results (Table 3). However, intragenic sliding Gmax analysis indicated a significant (P = 0.0004) decrease in silent site diversity relative to divergence within the central portion of the gene (Figure 2). One accession, Ws-0, contributed the majority of within-species diversity in the 5′ region of the gene (Hap 11, supplemental Figure S3). Plots of the polymorphism-to-divergence ratio with and without Ws-0 in the analysis indicated that while Ws-0 alone was responsible for the increased polymorphism detected in the 5′ region of the gene, the heterogeneity in polymorphism to divergence across the gene was still significant without Ws-0 in the data set (P < 0.044; Figure 2). The EDS1 locus was, therefore, partitioned into two regions demarcated by the sharp rise in polymorphism starting at the junction between intron 3 and exon 4. HKA tests were performed to analyze each region separately with the other seven signaling genes. None of the pairwise comparisons with exon 1–intron 3 were significant; however, three comparisons using exon 4 and the 3′ flanking region rejected neutrality (P < 0.007; Table 4). At3g48080, a paralog demonstrating 76% nucleotide identity to EDS1, is located adjacent to EDS1 on chromosome 3 in Col-0. Analysis using the coding sequence of At3g48080 predicted a single putative gene conversion event (P = 0.0038) between At3g48080 and the Nd-1 allele involving 42 bp from the 3′ region of exon 4. When Nd-1 was removed from the sample, HKA tests using exon 4 through the 3′ flanking region of EDS1 in comparison with PAD4 and TGA2 still produced significant results (P = 0.0224 and P = 0.0296, respectively); however, neither result remained significant after Bonferroni correction. Within gene heterogeneity for silent sites also ceased to be significant when Nd-1 is removed from the sample (Gmax, P = 0.0583). Therefore, gene conversion can at least partially explain both inter- and intragenic heterogeneity in polymorphism to divergence.
The MK test of EDS1 indicated a significantly higher nonsynonymous-to-synonymous ratio of polymorphism than divergence (P = 0.0169; N.I. = 2.6; Table 2) for the entire region; however, the deviation was particularly evident within exon 2 (P = 0.0005; N.I. = 18.7; Table 2). These results remain highly significant (entire gene, P = 0.0187; exon 2, P = 0.0012) even with the removal of Ws-0, which contributed 23 of the 32 (72%) singleton polymorphisms detected in the sample. The MK test applied to exon 2 of EDS1 is more significant than that for any of the 254 reference loci (supplemental Table S2), although less significant than that seen for exon 2 of NPR1. A significant ratio of nonsynonymous-to-synonymous polymorphic differences vs. fixed substitutions can result from either diversifying selection within A. thaliana or a relaxation of constraints on nonsynonymous changes during divergence that render replacement polymorphisms nearly neutral within A. thaliana. Selection efficiency is largely dependent on the product of the effective population size and the degree of selection (Glemin 2007). Therefore, the reduced effective population size in A. thaliana, a self-fertilizing species, could at least partially account for reduced selection efficiency relative to A. lyrata, a self-incompatible species (Glemin 2007). However, differences in mating system should affect all genes under selection; therefore, the presence of EDS1 exon 2 at the extreme tail of the distribution for the MK test and the πA:πS statistic (see below) suggests that mating system alone is unlikely to be sufficient to account for the observed deviations from neutrality.
Gene duplication can also result in relaxed selective constraints in duplicated genes. Relaxed constraints would ultimately result in either the acquisition of deleterious mutations that abrogate function (nonfunctionalization) or advantageous mutations that generate novel function (neofunctionalization). The location of EDS1 adjacent to At3g48080 on chromosome 3 and the high identity between the two at both the nucleotide (76%) and amino acid (70%) levels indicate these copies are indeed likely to have arisen from gene duplication. However, it is unlikely that gene duplication of the progenitor EDS1 was a recent event. First, orthologs of both EDS1 and At3g48080 are present in A. lyrata indicating that gene duplication occurred before speciation, ∼5 million years ago (Koch et al. 2000). Second, recently fixed duplicate genes are expected to have reduced diversity and an excess of rare alleles (Thornton 2007). Neither of these hold true for EDS1, which had a higher nucleotide diversity level than 86% of the reference loci and a positive Tajima's D, after the removal of Ws-0 (entire locus, D = 0.8851; exon 2, D = 1.3340). Furthermore, the A. thaliana paralogs appear to be evolving independently. Little LD is observed beyond 1 kb at EDS1, and LD is almost completely decayed between the 3′ region of EDS1, which is proximal to At3g48080, and the remainder of the gene (Figure 4). Therefore, although LD between the paralogs was not directly tested, LD within EDS1 indicates that recombination between the paralogs is likely sufficient to reduce the effects of hitchhiking and background selection. In addition, despite support for one putative gene conversion event in exon 4 (see above), evidence suggests that gene conversion events between the paralogs have been rare. The insertion event generating intron 3 in EDS1 would be predicted to compromise gene conversion (Teshima and Innan 2004). Also, divergence of the coding regions between paralogs (K = 0.16810) is greater than divergence between orthologs (K = 0.07894). Therefore, it is unlikely that nonindependent evolution of the paralogs could have generated the strong evolutionary signature observed in exon 2.
Figure 4.—
TASSEL plots of pairwise association. r2 values are above the diagonal. P-values are below the diagonal. Sites located in exons are indicated by black bars above and to the right of the matrix. Exon 3 of EDS1 was monomorphic and, therefore, is not represented. Arrows indicate consensus positions of nonsynonymous substitutions: NPR1 398, 406, 653, 667, 1002, 1298, 1416, 1939, and 1940; PAD4 208, 210, 248, 266, 299, 1512, and 1713; and EDS1 119, 639, 667, 734, 780, 781, 811, 849, 982, 1075, 1119, 1443, 1482, 1620, 1673, 1815, 2019, 2028, 2044, 2058, 2059, 2060, 2119, and 2147.
Several additional observations are inconsistent with the relaxation of selective constraints being the dominant evolutionary force shaping the pattern of polymorphism observed at EDS1. If nonsynonymous polymorphisms were nearly neutral, the expected ratio of nonsynonymous-to-synonymous polymorphism would be ∼2:1 and not 4:1 as observed for the entire EDS1 gene. A πA:πS of 1.085 across the entire gene indicates similar rates of change among nonsynonymous and synonymous sites and is consistent with the relaxation of selective constraints at EDS1; however, the ratio of nonsynonymous-to-synonymous pairwise diversity would be expected to be uniform across the gene. Contrary to this expectation, the πA:πS value for exon 2 (3.56) was not only substantially higher than that for the other regions of EDS1 (exon 1–intron 3 πA:πS = 1.24; 3′ flanking πA:πS = 0.60) but was also greater than the πA:πS values observed for all of the reference loci.
Selection acting to maintain diversity (balancing selection) within exon 2 of EDS1, however, could account for its evolutionary signature. One scenario is that the frequency of different EDS1 alleles fluctuates between or within populations in response to variation in effector alleles or oscillation in pathogen load (Holub 2001). Several observations are consistent with diversifying selection, a form of balancing selection. These include, a high number of protein variants (14 total; 11 when singleton replacements are not considered), a πA:πS value (3.56) substantially >1 and also greater than those observed for all of the reference loci, and a skew toward intermediate frequency alleles (Tajima's D after removal of Ws-0: entire locus, D = 0.8851; exon 2, D = 1.3340). Furthermore, in the absence of relaxed selective constraints, a significant ratio of nonsynonymous-to-synonymous polymorphic differences relative to fixed substitutions (entire gene, 4:1; exon 2, 14:1) is suggestive of selection acting to maintain nonsynonymous diversity in A. thaliana. However, if a selective advantage for novel alleles is present at this locus, the hitchhiking effect, which is expected to be observed among neutrally linked sites surrounding the selected region (Nordborg et al. 1996), has been diminished by recombination because little LD is observed beyond 1 kb (Figure 4).
PHYTOALEXIN DEFICIENT 4 (PAD4) is an ancient paralog of EDS1. EDS1 and PAD4 participate at similar positions in the salicylic acid signaling pathway and have been shown to interact in vivo (Wiermer et al. 2005). Both are required for similar aspects in basal and TIR-NBS–LRR mediated defense responses including salicylic acid accumulation and intensification of processing reactive oxygen intermediates (Wiermer et al. 2005). Therefore, similar patterns of evolution might be expected for these two proteins. A significant ratio of nonsynonymous-to-synonymous polymorphic differences vs. fixed substitutions was observed for PAD4 (MK test, P = 0.0284; N.I. = 3.5; Table 2) indicating different selective pressures on replacement changes between and within species. Similar to EDS1, the MK test revealed nonneutral evolution specific to the 5′ region (exon 1, P = 0.0293; N.I. = 6.8) of the coding sequence. The majority of mutations causing replacement substitutions (69%), including all seven present at intermediate frequencies (f ≥ 0.25), are located within the first third of the coding region (exon 1 plus the first 241 bps of exon 2). In contrast, the C-terminal encoding two-thirds of PAD4 are relatively conserved with all polymorphisms occurring as singletons except two as doublets. Consequently, Tajima's D is positive for the 5′ region proposed to be under selection (D = 0.2646) despite being negative for the entire gene (D = −1.0430) and highly negative in the 3′ portion of the gene (intron 1 onwards, D = −1.6370). This is suggestive of different constraints acting upon the N- and C-terminal encoding regions of PAD4.
Although a relaxation of selective constraints during divergence could account for πA:πS ratio close to 1 for the entire gene (πA:πS = 1.084), the πA:πS ratio for the first third of the gene (πA:πS = 1.309) falls within the 5% tail of the genomewide distribution for this statistic. Furthermore, the observed ratio of nonsynonymous-to-synonymous polymorphism (4:1) is higher than one would expect under complete relaxation of selective constraints. Therefore, the greater rate of nonsynonymous relative to synonymous polymorphism in the N-terminal encoding portion of the gene is more suggestive of diversifying selection. Diversifying selection is further supported by the high degree of LD stretching across exon 1 and including the first part of exon 2 (Figure 4). Furthermore, the observed heterogeneous polymorphism-to-divergence ratio among all sites (Gmax, P = 0.0135; Figure 2) indicates an increased level of polymorphism in the 5′ region of the gene which is consistent with an accumulation of polymorphism among sites linked to selected alleles as would be expected under diversifying selection. However, this test was no longer significant when applied to silent sites only and no significant results were obtained from intergenic HKA tests applied to either the full-length or partitioned PAD4 locus against the seven other signaling genes (Tables 3 and 4). PAD4 shows nucleotide sequence similarity to only one other gene in Col-0 (At2G43470); however, this similarity (59%) is limited to 300 bp of the intron. The limited level of homology and the location on different chromosomes indicate that gene conversion is an unlikely source of the heterogeneity observed in PAD4. Therefore, the collective analyses indicate that, similar to EDS1, diversifying selection may be acting to maintain diversity in the 5′ region of PAD4.
The regions possibly under selection in EDS1 and PAD4 include predicted acyl hydrolase domains (Falk et al. 1999; Jirage et al. 1999). However, all but one of the intermediate frequency, nonsynonymous changes in PAD4 are located outside the borders of this domain and, therefore, selection may be acting on the flanking N-terminal region. Furthermore, acyl hydrolase activity has not been detected for either protein (Wiermer et al. 2005). Therefore, the function of these regions and the significance of their signatures of selection are unclear. No linkage disequilibrium (r2 = 0; P > 0.1) was detected between polymorphisms within EDS1 and PAD4 providing no evidence of these two genes coevolving within A. thaliana. Evidence of selection on PAD4 was concentrated at the 5′ region of the gene; consistent with this, deletion of the first 15 amino acids of this region abrogates PAD4 interaction with EDS1 (Feys et al. 2001). Recently, a third protein, SENESCENCE ASSOCIATED GENE 101 (SAG101), was identified as forming heterodimers with EDS1 in vivo (Feys et al. 2005; Xing and Chen 2006). SAG101 is partially redundant with PAD4 in both basal and TIR–NBS–LRR mediated defense (Feys et al. 2005). It will be interesting to determine whether there is a similar pattern of diversity in SAG101 and whether there is any evidence for coevolution of these three proteins.
DISCUSSION
Plant genes involved in pathogen recognition and signal transduction are markedly different in their evolutionary dynamics. Recognition genes often exhibit frequent gene and intragenic duplications and recurrent sequence exchange among paralogs (Kuang et al. 2004; Mondragón-Palomino and Gaut 2005). Less than one-third of the 149 NBS–LRR encoding genes in the A. thaliana accession Col-0 are single-copy and more than half of these are predicted to be either duplicated in or absent from the genomes of other accessions of A. thaliana (Bakker et al. 2006). Consequently, only a small, biased subset of 18 NBS–LRR encoding genes is amenable to standard tests of nonneutral evolution, which assume identity by decent. Therefore, direct comparison between recognition and signaling gene evolution is not possible because it is not appropriate to analyze the evolution of NBS–LRR encoding genes using the same analyses employed in this study. In contrast to NBS–LRR encoding genes, genes encoding signal transduction proteins predominantly exist as identifiable orthologs that persist throughout distantly related plant species. Our results demonstrate that within A. thaliana, the majority of these genes appear to be evolving in a manner consistent with genomewide patterns of polymorphism. Similar evolutionary dynamics have also been observed among recognition and signaling genes from insects. Their recognition genes were characterized by species and lineage-specific duplications and obvious orthology was rare among Drosophila melanogaster and two species of disease carrying mosquitoes; conversely, there were clear orthologies among all 36 immunity signaling genes in the three species (Waterhouse et al. 2007).
The evolutionary dynamics distinguishing recognition and signaling genes may be a consequence of their different roles in the defense pathway. The defense system as a whole requires flexibility to evolve in response to changes in the pathogen. The predominant selective force acting on genes that encode NBS–LRR proteins may be a consequence of their primary function in the direct or indirect detection of pathogen effectors. High copy number of recognition genes and elevated sequence variability among paralogs could provide plants with the flexibility required to adapt to the evolution of virulence factors from diverse microbial pathogens. In contrast, selective forces acting on the downstream signaling genes may be more a consequence of their primary role as signal transducers. The obvious orthology of signaling genes is consistent with indispensible roles in plant defense against multiple pathogens. Such components would be predicted to have less flexibility to adapt to changes in individual pathogens because modifications could potentially compromise the effectiveness of the defense pathway against other pathogens. If effector target sites are located within a region necessary for proper protein function, the ability of a protein to evolve in response to the pathogen would be limited. No polymorphisms were observed in this study within the nine C-terminal amino acids required for RIN4 membrane localization and association with RPS2; the target sites in RIN4 for at least two effectors, avrRpt2 and AvrB, are also highly conserved (Chisholm et al. 2005; Day et al. 2005; Kim et al. 2005). The existence of the RPS2 and RPM1 guard proteins that monitor for cleavage or phosphorylation of RIN4 may alleviate selective pressures that would otherwise be imposed on these areas. Alternatively, RIN4 has recently been suggested to act as a decoy protein; conservation of the effector target sites in RIN4 would alleviate selection pressures on the primary effector targets (Van der Hoorn and Kamoun 2008).
Four of the 10 signaling genes in this study showed no evidence of nonneutral evolution and evidence for 2 others was minimal and not confirmed by additional tests. These results are consistent with the recent study of Bakker et al. (2008). The general conservation of signal transduction proteins may indicate that these proteins are not targeted by pathogens, that the evolution of such proteins is functionally constrained, or that the selection history of these genes cannot be detected through our analyses. All of the genes encoding signal transduction proteins analyzed here, with the exception of RIN4 and a member of the TGA family of bZIP transcription factors (TGA2), were originally identified as mutations in the defense pathway (Century et al. 1995; Parker et al. 1996; Glazebrook 1999, 2001). They are, therefore, potential points of vulnerability within the plant defense system that may be targeted by pathogen effectors to suppress resistance. Evolutionary constraints may result from the need to maintain specific molecular activities as well as multiple interactions with other signaling components.
Two proteins in our study, PBS1 and RIN4, are known targets of effector molecules of P. syringae. Our results indicate that PBS1 is subjected to selective forces acting to decrease rather than increase the level of diversity at this locus. The dearth of variation at PBS1 is in contrast to RPS5 that encodes the cognate guard protein that detects cleavage of PBS1 by AvrPphB (Shao et al. 2003). RPS5 is characterized by increased diversity around the deletion junction of a presence/absence polymorphism with nearly all of the variation occurring between allelic classes. This pattern of polymorphism is consistent with negative frequency-dependent selection acting to maintain diversity in populations as a presence/absence polymorphism of the RPS5 protein (Tian et al. 2002).
The other known target protein in our study, RIN4, is a negative regulator of innate immunity that is targeted by multiple effectors and guarded by at least two NBS–LRR proteins (Belkhadir et al. 2004). RIN4 was at the opposite end of the diversity spectrum from PBS1 with a higher level of nucleotide diversity than 93.5% of the reference loci (Table 1 and supplemental Figure S1). Although intergenic HKA tests indicated that RIN4 could be evolving differently than other nuclear genes, this observation was not supported by any other tests for nonneutral evolution. Evidence of selection has been reported, however, for two NBS–LRR proteins that monitor RIN4. RPM1 monitors phosphorylation of RIN4 by AvrB or AvrRpm1; RPM1 is maintained as a presence/absence polymorphism consistent with negative frequency-dependent selection (Stahl et al. 1999; Mackey et al. 2002). RPS2 monitors cleavage of RIN4 by AvrRpt2; RPS2 is also believed to be maintained through balancing selection of resistance and susceptibility alleles (Axtell and Staskawicz 2003; Mackey et al. 2003; Mauricio et al. 2003). Therefore, neither of the known targets of P. syringae effectors in this study, RIN4 or PBS1, showed evidence of evolution acting to maintain diversity at these loci, whereas evidence of balancing selection has been reported for all three genes encoding NBS–LRR proteins that monitor these targets.
Our data emphasize the need to analyze variation within the entire gene to assess evolutionary histories. We detected patterns of evolution specific to regions in NPR1, PAD4, and EDS1. Heterogeneous patterns of evolution within these genes suggest that only certain regions have coevolved with pathogens or interacting partners. NPR1, PAD4, and EDS1 showed significant and strong evidence of selection. Only weak evidence of selection was detected at NPR1 and PAD4 by Bakker et al. (2008); however, only small fragments of the coding sequence of each gene were analyzed in their study. We also detected significant deviation from neutral expectations and extreme values for several summary statistics (Tajima's D, MK, and πA:πS) for exon 2 of EDS1; this was missed by Bakker et al. (2008) who only analyzed a fragment specific to exon 4.
The strong evolutionary signatures observed at these loci are consistent with their evolution being influenced by pathogen effectors and indicates that a primary role in signal transduction does not preclude response to selection. Similarly, at least three genes encoding proteins in the Toll and IMD immunity-response signaling pathways in Drosophila spp. show evidence of positive selection (Jiggins and Kim 2007). Mutations in NPR1, PAD4, and EDS1 compromise plant defense indicating that they could be vulnerable to pathogen effectors (Cao et al. 1994; Glazebrook et al. 1996). Proteins not monitored by guard proteins might be expected to show signatures of selection. The significant evolutionary signals observed for NPR1, PAD4, and EDS1 are consistent with the current lack of known guard proteins for these potential targets. Protein–protein interaction studies in yeast and in planta are now necessary to determine which signaling proteins are effector targets and which have cognate R genes.
The evolution of signal transduction proteins is the result of the complex interplay of multiple factors not limited to functional constraints and evolutionary pressures imposed by changes in pathogen effector proteins. Other factors such as the level of gene flow in both host and pathogen populations, habitat and population size, availability of alternative hosts, etc. could also influence the signatures of selection. It will be informative to determine whether similar patterns of selection as seen in A. thaliana are recapitulated in other species that have different pathogens, demographics, ecologies, and mating systems.
Acknowledgments
We thank B. Gaut (University of California, Irvine) for insightful discussions, A. Kozik and H. Xu for bioinformatics support, and A. Chen for technical assistance. This research was supported by award DBI0211923 from the National Science Foundation Plant Genome Program.
References
- Aguade, M., 2001. Nucleotide sequence variation at two genes of the phenylpropanoid pathway, the FAH1 and F3H genes, in Arabidopsis thaliana. Mol. Biol. Evol. 12 1–9. [DOI] [PubMed] [Google Scholar]
- Alfano, J. R., and A. Collmer, 2004. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 42 385–414. [DOI] [PubMed] [Google Scholar]
- Axtell, M. J., and B. J. Staskawicz, 2003. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112 369–377. [DOI] [PubMed] [Google Scholar]
- Bakker, E. G., C. Toomajian, M. Kreitman and J. Bergelson, 2006. A genome-wide survey of R gene polymorphism in Arabidopsis. Plant Cell 18 1803–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker, E. G., M. B. Traw, C. Toomajiam, M. Kreitman and J. Bergelson, 2008. Low levels of polymorphism in genes that control the activation of defense response in Arabidopsis thaliana. Genetics 178 2032–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir, Y., Z. Nimchuk, D. A. Hubert, D. Mackey and J. L. Dangl, 2004. Arabidopsis RIN4 negatively regulates disease resistance mediated by RPS2 and RPM1 downstream or independent of the NDR1 signal modulator and is not required for the virulence functions of bacterial type III effectors AvrRpt2 or AvrRpm1. Plant Cell 16 2822–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, J. G., 2005. Directed mutagenesis confirms the functional importance of positively selected sites in polygalacturonase inhibitor protein. Mol. Biol. Evol. 22 1531–1534. [DOI] [PubMed] [Google Scholar]
- Bishop, J. G., A. M. Dean and T. Mitchell-Olds, 2000. Rapid evolution in plant chitinases: Molecular targets of selection in plant-pathogen coevolution. Proc. Natl. Acad. Sci. USA 97 5322–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, J. G., D. R. Ropoll, S. Bashir, C. M. B. Damasceno, J. D. Seeds et al., 2005. Selection of glycine β-1,3-endoglucanase genes differentially inhibited by a Phytopthora glucanase inhibitor protein. Genetics 169 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, H., S. A. Bowling, A. S. Gordon and X. Dong, 1994. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6 1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, H., J. Glazebrook, J. D. Clarke, S. Volko and X. Dong, 1997. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88 57–63. [DOI] [PubMed] [Google Scholar]
- Century, K. S., E. B. Holub and B. J. Staskawicz, 1995. NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a functional pathogen. Proc. Natl. Acad. Sci. USA 92 6597–6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, D., 2006. Balancing selection and its effects on sequences in nearby genome regions. PLoS Genet. 64 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm, S. T., D. Dahlbeck, N. Krishnamurthy, B. Day, K. Sjolander et al., 2005. Molecular characterization of proteolytic cleavage sites of the Pseudomonas syringae effector AvrRpt2. Proc. Natl. Acad. Sci. USA 102 2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote, F., K. S. Ham, M. S. Hahn and C. W. Bergmann, 1998. Oligosaccharide elicitors in host-pathogen interactions: generation, perception, and signal transduction. Subcell. Biochem. 29 385–432. [DOI] [PubMed] [Google Scholar]
- Day, B., D. Dahlbeck, J. Huang, S. T. Chisholm, D. Li et al., 2005. Molecular basis for the RIN4 negative regulation of RPS2 disease resistance. Plant Cell 17 1292–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després, C., C. DeLong, S. Glaze, E. Liu and P. R. Fobert, 2000. The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a Subgroup of the TGA family of bZIP transcription factors. Plant Cell 12 279–290. [PMC free article] [PubMed] [Google Scholar]
- Dodds, P. N., G. L. Lawrence, A.-M. Cantanzariti, T. Teh, C.-I. A. Wang et al., 2006. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. USA 103 8888–8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, X., 2004. NPR1, all things considered. Curr. Opin. Plant Biol. 7 547–552. [DOI] [PubMed] [Google Scholar]
- Ellis, J. G., G. J. Lawrence, J. E. Luck and P.N. Dodds, 1999. Identification of regions in alleles of the flax rest resistance gene L that determine differences in gene-for gene specificity. Plant Cell 11 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk, A., B. J. Feys, L. N. Frost, J. D. G. Jones, M. J. Daniel et al., 1999. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA 96 3292–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, B. J., L. J. Moisan, M.-A. Newman and J. E. Parker, 2001. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J. 20 5400–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, B. J., M. Wiermer, R. A. Bhat, L. J. Moisan, N. Medina-Escobar et al., 2005. Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17 2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook, J., 1999. Genes controlling expression of defense responses in Arabidopsis. Curr. Opin. Plant Biol. 2 280–286. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J., 2001. Genes controlling expression of defense responses in Arabidopsis – 2001 status. Curr. Opin. Plant Biol. 4 301–307. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J., E. E. Rogers and F. M. Ausubel, 1996. Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glemin, S., 2007. Mating systems and the efficacy of selection at the molecular level. Genetics 177 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, S. R., E. J. Fisher, J. H. Change, B. M. Mole and J. L. Dangl, 2006. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 60 425–449. [DOI] [PubMed] [Google Scholar]
- Holub, E.B., 2001. The arms race is ancient history in Arabidopsis, the wildflower. Nat. Rev. Genet. 2 516–527. [DOI] [PubMed] [Google Scholar]
- Huang, X., and A. Madan, 1999. CAP3: A DNA sequence assembly program. Genome Res. 9 868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. R., M. Kreitman and M. Aguade, 1987. A test of neutral molecular evolution based on nucleotide date. Genetics 116 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiggins, F. M., and K. W. Kim, 2007. A screen for immunity genes evolving under positive selection in Drosophila. J. Evol. Biol. 20 965–970. [DOI] [PubMed] [Google Scholar]
- Jirage, D., T. L. Tootle, T. L. Reuber, L. N. Frost, B. J. Feys et al., 1999. Proc. Natl. Acad. Sci. USA 96 13583–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. A., and J. D. G. Jones, 1997. The role of leucine-rich repeat proteins in plant defenses. Adv. Bot. Res. 24 89–167. [Google Scholar]
- Jones, J. D.G., and J. L. Dangl, 2006. The plant immune system. Nature 44 323–329. [DOI] [PubMed] [Google Scholar]
- Kim, H.-S., D. Desveaux, A. U. Singer, P. Patel, J. Sondek et al., 2005. Te Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc. Natl. Acad. Sci. USA 102 6496–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, M. A., B. Haubold and T. Mitchell-Olds, 2000. Mol. Biol. Evol. 17 1483–1498. [DOI] [PubMed] [Google Scholar]
- Kuang, H., S. S. Woo, B. C. Meyers, E. Nevo and R. W. Michelmore, 2004. Multiple genetic processes result in heterogeneous rates of evolution within the major cluster disease resistance genes in lettuce. Plant Cell 16 2870–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey, D., Y. Belkhadir, J. M. Alonso, J. R. Ecker and J. L. Dangl, 2003. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112 379–389. [DOI] [PubMed] [Google Scholar]
- Mackey, D., B. F. Holt, III, A. Wiig and J. L. Dangl, 2002. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108 743–754. [DOI] [PubMed] [Google Scholar]
- Mauricio, R., E. A. Stahl, T. Korves, D. Tian, M. Kreitman et al., 2003. Natural selection for polymorphism in the disease resistance gene Rps2 of Arabidopsis thaliana. Genetics 163 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. H., 1998. Improved tests for heterogeneity across a region of DNA sequence in the ratio of polymorphism to divergence. Mol. Biol. Evol. 15 377–384. [DOI] [PubMed] [Google Scholar]
- McDonald, J. H., and M. Kreitman, 1991. Adaptive protein evolution at the Adh locus in Drosophila. Nature 351 652–654. [DOI] [PubMed] [Google Scholar]
- Michelmore, R.W., and B. C. Meyers, 1998. Clusters of resistance genes in plants evolve by divergent selection and a bit-and-death process. Genome Res. 8 1113–1129. [DOI] [PubMed] [Google Scholar]
- Mondragón-Palomino, M., and B. S. Gaut, 2005. Gene conversion and the evolution of three leucine-rich repeat gene families in Arabidopsis thaliana. Mol. Biol. Evol. 22 2444–2456. [DOI] [PubMed] [Google Scholar]
- Mudgett, M. B., 2005. New insights to the function of phytopathogenic bacterial type III effectors in plants. Annu. Rev. Plant Biol. 56 509–531. [DOI] [PubMed] [Google Scholar]
- Nei, M., 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
- Nordborg, M., B. Charlesworth and D. Charlesworth, 1996. Increased levels of polymorphism surrounding selectively maintained sites in highly selfing species. Proc. R. Soc. Lond. B 263 1033–1039. [Google Scholar]
- Nordborg, M., T. T. Hu, Y. Ishino, J. Jhaveri, C. Toomajian et al., 2005. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 3 e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J. E., E. B. Houlb, L. N. Frost, A. Falk, N. D. Gunn et al., 1996. Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purugganan, M. D., and J. I. Suddith, 1999. Molecular population genetics of floral homeotic loci: departures from the equilibrium-neutral model at the APETALA3 and PISTELLATA genes of Arabidopsis thaliana. Genetics 151 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand, D. M., and L. M. Kann, 1996. Excess amino acid polymorphism in mitochondrial DNA: contrasts among genes from Drosophila, mice, and humans. Mol. Biol. Evol. 13 735–748. [DOI] [PubMed] [Google Scholar]
- Rose, J. K. C., K. S. Ham, A. G. Darvill and P. Albersheim, 2002. Molecular cloning and characterization of glucanase inhibitor proteins: coevolution of a counter-defense mechanism in plant pathogens. Plant Cell 14 1329–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, L. E., P. D. Bittner-Eddy, C. H. Langley, E. B. Holub, R.W. Michelmore et al., 2004. The maintenance of extreme amino acids diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics 166 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas, J., J. C. Sanchez-DelBarrio, X. Messeguer and R. Rozas, 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19 2496–2497. [DOI] [PubMed] [Google Scholar]
- Ryals, J., K. Weymann, K. Lawton, L. Friedrich, D. Ellis et al., 1997. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor IκB. Plant Cell 9 425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer, S, 1989. Statistical tests for detecting gene conversion. Mol. Biol. Evol. 6 526–538. [DOI] [PubMed] [Google Scholar]
- Sedgwick, G., and S. J. Smerdon, 1999. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biol. Sci. 24 311–316. [DOI] [PubMed] [Google Scholar]
- Shao, F., C. Golstein, J. Ade, M. Stoutemyer, J. E. Dixon et al., 2003. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301 1230–1233. [DOI] [PubMed] [Google Scholar]
- Sharbel, T. F., B. Haubold and T. Mitchell-Olds, 2000. Genetic isolation by distance in Arabidopsis thaliana: biogeography and postglacial colonization of Europe. Mol. Ecol. 9 2109–2118. [DOI] [PubMed] [Google Scholar]
- Shen, J., H. Araki, L. Chen, J.-Q. Chen and D. Tian, 2006. Unique evolutionary mechanism in R-genes under the presence/absence polymorphism in Arabidopsis thaliana. Genetics 172 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth, E. B., N. L. Lee and S. Y. He, 2007. Pathogen virulence factors as molecular probes of basic plant cellular functions. Curr. Opin. Plant Biol. 10 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, E. A., G. Dwyer, R. Mauricio, M. Kreitman and J. Bergelson, 1999. Dynamics of disease resistance polymorphisms at the Rpm1 locus of Arabidopsis. Nature 400 667–671. [DOI] [PubMed] [Google Scholar]
- Stotz, H. U., J. G. Bishop, C. W. Bergmann, M. Koch, P. Albersheim et al., 2000. Identification of target amino acids that affect interactions of fungal polygalacturonases and their plant inhibitors. Phys. Mol. Plant Pathol. 56 117–130. [Google Scholar]
- Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima, K. M., and H. Innan, 2004. The effect of gene conversion on the divergence between duplicated genes. Genetics 166 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton, K., 2003. Libsequence: a C++ class library for evolutionary genetic analysis. Bioinformatics 19 2325–2327. [DOI] [PubMed] [Google Scholar]
- Thornton, K., 2007. The neutral coalescent process for recent gen duplications and copy-number variants. Genetics 177 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, D., H. Araki, E. Stahl, J. Bergelson and M. Kreitman, 2002. Signature of balancing selection in Arabidopsis. Proc. Natl. Acad. Sci. USA 99 11525–11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin, P., and D. A. Moeller, 2006. Molecular evolution of plant immune system genes. Trends Genet. 12 662–670. [DOI] [PubMed] [Google Scholar]
- Van der Hoorn, R. A. L., P. J. G. M. De Wit and M. H. A. Joosten, 2002. Balancing selection favors guarding resistance proteins. Trends Plant Sci. 7 67–71. [DOI] [PubMed] [Google Scholar]
- Van der Hoorn, R. A. L., and S. Kamoun, 2008. From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell 20 2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, R. M., E. V. Kriventseva, S. Meister, Z. Xi, K. S. Alvarez et al., 2007. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316 1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiermer, M., B. J. Feys and J. E. Parker, 2005. Plant immunity: the EDS1 regulatory node. Curr. Opin. Plant Biol. 8 383–389. [DOI] [PubMed] [Google Scholar]
- Xie, D. X., B. F. Feys, S. James, M. Nieto-Rostro and J. G Turner, 1998. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280 1091–1094. [DOI] [PubMed] [Google Scholar]
- Xing, D. H., and Z. X. Chen, 2006. Effects of mutations and constitutive overexpression of EDS1 and PAD4 on plant resistance to different types of microbial pathogens. Plant Sci. 171 251–262. [Google Scholar]
- Zhang, J., F. Shao, Y. Li, H. T. Cui, L. Chen et al., 2007. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1 175–185. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., W. Fan, M. Kinkema, X. Li and X. Dong, 1999. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the Pr-1 gene. Proc. Natl. Acad. Sci. USA 96 6523–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J.-M., Y. Trifa, H. Silva, D. Pontier, E. Lam et al., 2000. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 gene required for induction by salicylic acid. Mol. Plant-Microbe Interact. 13 191–202. [DOI] [PubMed] [Google Scholar]