Abstract
We cloned a new inhibitor of apoptosis protein (IAP) homolog, SfIAP, from Spodoptera frugiperda Sf-21 cells, a host of insect baculoviruses. SfIAP contains two baculovirus IAP repeat domains followed by a RING domain. SfIAP has striking amino acid sequence similarity with baculoviral IAPs, CpIAP and OpIAP, suggesting that baculoviral IAPs may be host-derived genes. SfIAP and baculoviral CpIAP inhibit Bax but not Fas-induced apoptosis in human cells. Their apoptosis-suppressing activity in mammalian cells requires both baculovirus IAP repeat and RING domains. Further biochemical data suggest that SfIAP and CpIAP are specific inhibitors of mammalian caspase-9, the pinnacle caspase in the mitochondria/cytochrome c pathway for apoptosis, but are not inhibitors of downstream caspase-3 and caspase-7. Thus the mechanisms by which insect and baculoviral IAPs suppress apoptosis may involve inhibition of an insect caspase-9 homologue. Peptides representing the IAP-binding domain of the Drosophila cell death protein Grim abrogated human caspase suppression by SfIAP and CpIAP, implying evolutionary conservation of the functions of IAPs and their inhibitors.
Caspases are a family of intracellular proteases responsible for execution of the apoptotic program (1, 2). Some viruses harbor genes that encode caspase inhibitory proteins, thereby suppressing host defense mechanisms that otherwise would eliminate virus-infected cells by apoptosis. Examples of viral caspase inhibitors include the baculoviral p35 protein (3) and the crmA protein of the Orthopoxvirus family cowpox virus (4). Inhibitor of apoptosis protein (IAP) family proteins were first discovered in baculoviruses (5, 6). Genetic complementation analysis revealed that the IAP genes of the CpGV and OpMNPV baculoviruses can rescue p35-deficient viruses, maintaining host cell survival so that viral replication occurs successfully (5, 6). However, IAPs are structurally distinct from p35 and CrmA. Baculoviral IAPs contain two tandem copies of a baculovirus IAP repeat (BIR) domain followed by a C-terminal RING domain. Mutagenesis studies suggest a requirement for both the BIR and RING domains for their antiapoptotic function in insect cells.
Cellular IAP homologs are found in many animal species, including Drosophila, mammals, and humans (reviewed in refs. 7 and 8). All cellular IAPs contain one to three copies of a BIR domain, and most also contain a RING domain located near their C termini. Several human IAPs, including XIAP, cIAP1, and cIAP2, can directly bind and inhibit certain caspases, including caspase-3, caspase-7, and caspase-9 (9–12). Deletional analysis indicates that the second BIR domain (BIR2) of XIAP is sufficient for inhibiting mammalian caspase-3 and caspase-7 (13), whereas a fragment of XIAP encompassing the third BIR domain (BIR3) and RING domain specifically inhibits mammalian caspase-9 (9). The IAPs of Drosophila (DIAP1) and baculovirus (OpIAP; CpIAP) may also inhibit some caspases (14–16).
Apoptosis-inducing genes that encode IAP-binding proteins have been identified in Drosophila, including Reaper, Hid, and Grim (17). The Reaper, Hid, and Grim proteins contain homologous ≈14-aa N-terminal domains, which are both necessary and sufficient for binding DIAP1 and for inducing apoptosis (18, 19). Recent data suggest that Reaper, Hid, and Grim induce apoptosis by inhibiting IAPs, thus interfering with IAP-mediated suppression of caspases (20).
Spodoptera frugiperda (fall armyworm) is a Lepidopteran host of the Autographa californica nuclearpolyhedrovirus (AcMNPV), a member of the baculovirus family. Despite extensive use of S. frugiperda-derived cells for studies of apoptosis regulation by baculoviruses (reviewed in ref. 7), no endogenous apoptosis-regulating genes have been identified in these insect cells, with the exception of Sf-caspase-1 (21). Here we report the cDNA cloning and characterization of a cellular IAP from S. frugiperda (SfIAP), demonstrating that SfIAP and its baculovirus counterpart CpIAP are direct inhibitors of mammalian caspase-9 and that these IAPs are suppressible by Grim peptides.
Materials and Methods
Cloning of SfIAP.
mRNA was isolated from Sf-21 cells by using a kit from Qiagen. Degenerate primers were designed according to the consensus amino acid sequences between baculoviral IAPs and Drosophila IAPs, 5′-GC(A/C/G/T)GA(A/C/G/T)GC(A/C/G/T)GG(A/C/G/T)TT(T/C)TT(T/C)TA-3′ and 5′-AC(A/C/G/T)AC(A/G)TG(A/C/G/T)CC(A/G)CA(A/C/G/T)GG-3′. Reverse transcription–PCR was performed for 35 cycles by using 94°C for 45 sec, 46°C for 1 min, and 72°C for 1 min. Amplified fragments were blunt-end-cloned into HincII site of pTZ19 and then sequenced. To obtain full-length SfIAP cDNAs, 5′ RACE and 3′ RACE were performed from Sf-21 mRNA by using commercial kits (GIBCO/BRL; Takara) and 5′-TACGTCATGGTTCTCCCAA-3′ and 5′-TCGATGACTCAAAGTTGTG-3′ as internal PCR primers.
Plasmid Constructions.
A cDNA fragment encompassing the complete ORF of SfIAP was PCR-amplified and subcloned into the EcoRI-XhoI sites in pcDNA3-myc and pGEX4T-1. The complete ORF of CpIAP was subcloned into the EcoRI-EcoRV sites of pcDNA3-myc or the EcoRI-SalI sites in pGEX4T-1. Plasmids encoding fragments of the SfIAP and CpIAP, including BIR1+2 (amino acid residues 1–323 for SfIAP; residues 1–220 for CpIAP) and RING (residues 324–377 for SfIAP and residues 221–275 for CpIAP), were amplified by PCR by using primers containing either start or stop codons as appropriate and subcloned into pcDNA3-myc or pGEX4T-1 plasmids.
Protein Expression and Purification.
pGEX4T-1-SfIAP and pGEX4T-1-CpIAP plasmids were introduced into E. coli strain BL21 (DE3) containing the plasmid pT-Trx. Glutathione S-transferase (GST) fusion proteins were obtained by induction with 0.05 mM isopropyl β–thiogalactoside at 25°C for 8 hr and then purified by using glutathione-Sepharose (22). The catalytic domains of caspase-3, caspase-7, caspase-8, and caspase-9 were expressed, purified by Ni-chelation affinity-chromatography, and quantified as described (23–25).
Protein-Binding Assay.
pcDNA3-caspase-9-flag was in vitro-transcribed and in vitro-translated in the presence of [35S]l-methionine by using the TNT kit from Promega. Protein was exchanged into buffer (20 mM Hepes, pH 7.5/10 mM KCl/1.5 mM MgCl2/1 mM EDTA/1 mM DTT) with Bio-spin P-6 columns (Bio-Rad). GST-SfIAP, GST-CpIAP, GST-XIAP, or GST-XIAPBIR1 (10 μg) immobilized on glutathione-Sepharose (5 μl) was incubated with in vitro-translated caspase-9 (4 μl) in 100 μl of caspase assay buffer (20 mM Hepes, pH 7.4/10% sucrose/0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/1 mM EDTA/100 mM NaCl) for 1 hr at 4°C. Beads were washed two times with 0.5 ml of 50 mM Tris, pH 7.5/150 mM NaCl/2 mM DTT and analyzed by SDS/PAGE–autoradiography.
Cell Extracts and Caspase Assays.
Cytosolic extracts were prepared by using human embryonic kidney (HEK) 293 cells (11). For initiating caspase activation, either 100 nM purified recombinant caspase-8 or 1 μM horse heart cytochrome c (Sigma) plus 1 mM dATP was added to extracts (10). Caspase activity was assayed by release of 7-amino-4-trifluoromethyl-coumarin (AFC) from Ac-DEVD-AFC or Ac-LEHD-AFC (Calbiochem), using a spectrofluorimeter (26).
Cell Culture, Transfections, and Apoptosis Assays.
Insect Sf-21 cells were maintained at 27°C in Excell 401 medium (JRH Biosciences, Lenexa, KS) supplemented with 2.5% FBS. vP35del, containing a deletion in the p35 gene, was propagated in TN-368 cells (3). Plasmids encoding full-length or deletion mutants of SfIAP (1 μg) were cotransfected with 1 μg vP35del viral DNA into Sf-21 cells by using Lipofectin from GIBCO. Occlusion body formation was observed under light microscopy 3 days posttransfection.
293 and 293T cells were maintained in DMEM (Irvine Scientific) supplemented with 10% FBS, 1 mM l-glutamine, and antibiotics. 293 cells (106) were cotransfected by using Superfect (Qiagen) with 0.1 μg of green fluorescence protein (GFP) marker plasmid pEGFP (CLONTECH), 0.25 μg of either pcDNA3-Bax or pcDNA3-Fas, and 1.5 μg of either pcDNA3 myc-SfIAP or pcDNA3 myc-CpIAP. Alternatively, cells were cotransfected with 0.35 μg of pcDNA3-caspase-9-Flag and 2.1 μg of either SfIAP or CpIAP. Both floating and adherent cells were recovered 24–36 hr posttransfection and pooled, and the percentage of GFP-positive cells with nuclear apoptotic morphology was determined by staining with 0.1 μg/ml 4′-6-diamidino-2-phenylindole (mean ± SD; n = 3) (10–13). In some cases, lysates were prepared from transfected cells, normalized for total protein content, and analyzed by SDS/PAGE–immunoblotting (10–13).
Results
Cloning of SfIAP.
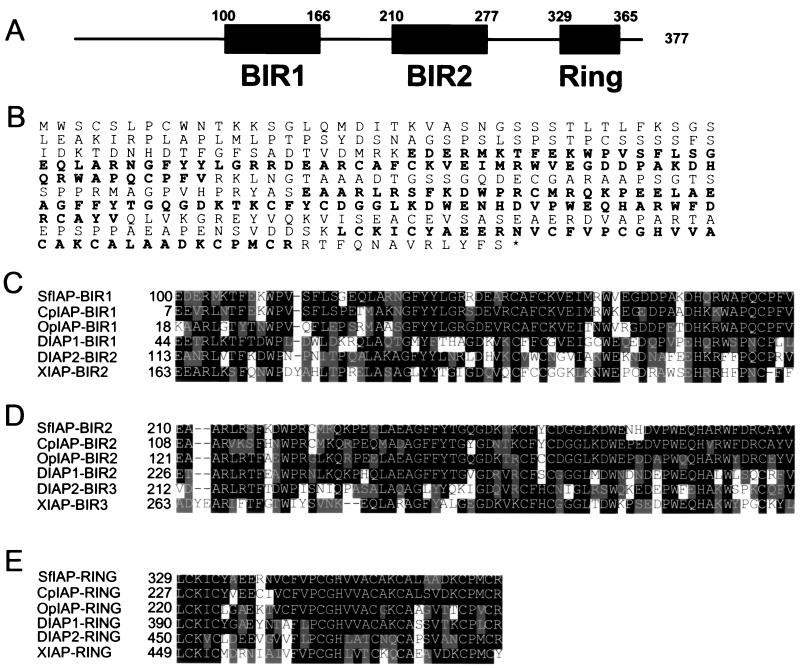
The full-length SfIAP cDNA (GenBank accession no. AF186378) contains a continuous ORF encoding a protein of 377 aa (Fig. 1). This ORF is initiated by an AUG within a favorable context for translation (27) and is preceded by upstream stop codons in all three reading frames. Similar to baculoviral IAPs, the predicted SfIAP protein contains two BIR domains, followed by a RING domain near its C terminus (Fig. 1 A and B). Within the BIR and RING regions, SfIAP shares 85% amino acid identity (90% similarity) with baculoviral CpIAP and 70% identity (80% similarity) with OpIAP (Fig. 1 C– E). Similar to CpIAP and OpIAP, the full-length SfIAP protein, but not fragments comprising either the two BIRs or the RING domain, successfully complemented p35 deficiency of mutant AcMNPV baculovirus (28), restoring replication of this mutant virus, as evidenced by occlusion body formation in Sf-21 cells (not shown). Thus, SfIAP shares sequence and functional similarity with its baculovirus counterparts, suggesting that baculoviral IAPs arose from host IAP genes such as SfIAP.
Figure 1.
Predicted domain topology and amino acid sequence of SfIAP. (A) The BIR and RING domains of SfIAP are depicted. (B) The predicted amino acid sequence of SfIAP is presented. Sequence alignments of the BIR1 (C), BIR2 (D), and RING (E) domains of SfIAP with the corresponding domains of other IAP-family members are shown. Black indicates identity and gray indicates homology.
SfIAP and CpIAP Inhibit Bax-Induced but Not Fas-Induced Apoptosis in Mammalian Cells.
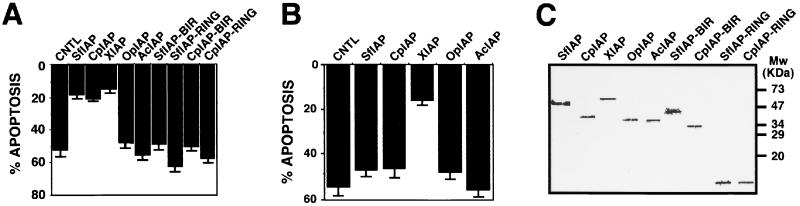
Baculoviral IAPs can protect mammalian cells from some apoptotic stimuli (29–31). To explore whether SfIAP has similar properties, SfIAP was coexpressed in HEK293 cells with either Fas or Bax, representing prototypical apoptotic stimuli that trigger alternative apoptosis pathways that utilize caspase-8 and caspase-9, respectively, as their apical proteases (reviewed in ref. 2). SfIAP, CpIAP, and human XIAP inhibited Bax-induced apoptosis (Fig. 2A). In contrast, the BIR and RING truncation mutants of SfIAP and CpIAP failed to suppress apoptosis induced by Bax, as did the full-length IAP proteins (OpIAP; AcIAP) from other baculovirus strains. Unlike Bax, SfIAP and CpIAP were ineffective at suppressing Fas-induced apoptosis in HEK293 cells (Fig. 2B), whereas XIAP did protect against Fas. Immunoblot analysis confirmed production of these various IAP proteins, excluding differences in protein levels as an explanation (Fig. 2C). Thus, SfIAP and CpIAP can block mammalian apoptosis but inhibit only a subset of the cell-death pathways suppressed by their human counterpart, XIAP.
Figure 2.
SfIAP and CpIAP protect cells against Bax- but not Fas-induced apoptosis. Expression plasmids encoding Bax (A) or Fas (B) were cotransfected into 293 cells with the indicated myc-tagged IAP expression plasmids. Percentage apoptosis was measured 24–36 hr later by 4′-6-diamidino-2-phenylindole staining (mean ± SD; n = 3). (C) Lysates were prepared from 293 cells 1 day posttransfection, normalized for total protein content (50 μg), and analyzed by SDS/PAGE–immunoblotting by using anti-myc antibody with enhanced chemiluminescence-based detection to demonstrate that the desired protein was expressed in each case.
Recombinant SfIAP and CpIAP Suppress Caspase Activation Induced by Cytochrome c (Cyt c) in Vitro.
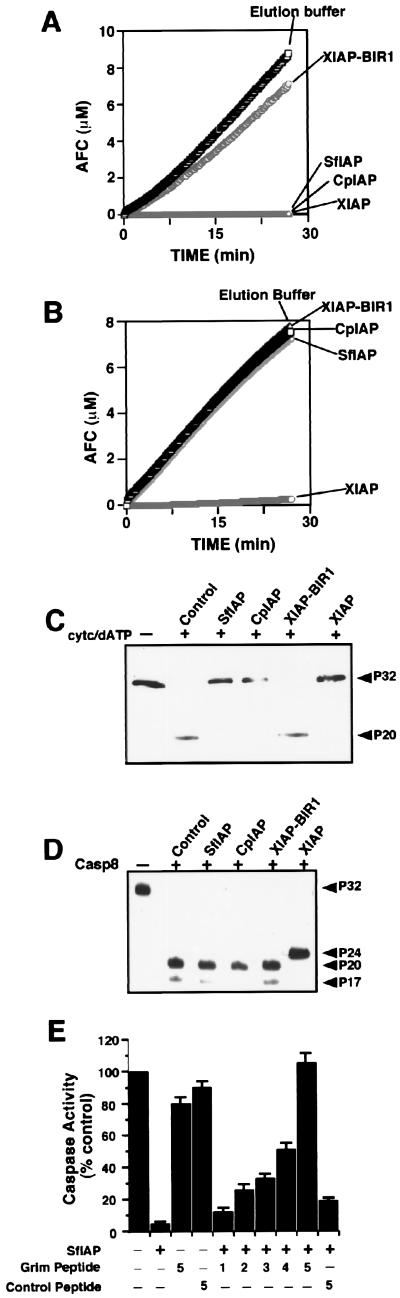
To further explore the mechanisms by which SfIAP and CpIAP inhibit mammalian apoptosis, we used a cell-free system in which exogenously added active caspase-8 or Cyt c, an agonist of the caspase-9-activating protein Apaf-1 (32), induces activation of caspase-3 and similar effector proteases, as measured by hydrolysis of Ac-DEVD-AFC (26). In cell extracts treated with Cyt c, recombinant SfIAP, CpIAP, and XIAP completely blocked the hydrolysis of Ac-DEVD-AFC (Fig. 3A). A control protein consisting of the BIR1 domain of XIAP did not interfere with caspase activity, demonstrating the specificity of these results. In contrast, recombinant SfIAP and CpIAP had no effect on DEVD-cleaving proteases in cell extracts activated by caspase-8 (Fig. 3B). Immunoblot analysis of caspase-3 processing confirmed the findings of the protease assays (Fig. 3 C and D). Taken together, these data suggest that SfIAP and CpIAP inhibit the caspase cascade activated by Cyt c pathway at a step upstream of caspase-3 but do not suppress the cascade initiated by caspase-8.
Figure 3.
SfIAP and CpIAP suppress Cyt c- but not caspase-8-induced activation of effector caspases. Recombinant SfIAP or CpIAP (2 μM) was added to cytosolic extracts (10 mg/ml) from HEK293 cells concurrently with the addition of 1 μM Cyt c/10 mM dATP (A) or 100 ng/ml active caspase-8 (B). After incubation at 30°C for 10 min, aliquots were withdrawn and assayed for caspase activity, continuously monitoring release of AFC from Ac-DEVD-AFC substrate (100 μM) beginning from the time of substrate addition. SD from 10 replicates was smaller than the points on the graphs. (C and D) Aliquots of cell extracts from the experiments performed above were analyzed by SDS/PAGE–immunoblotting by using anticaspase-3 antiserum. Arrowheads indicate the pro- and processed forms of caspase-3. In cell extracts activated by Cyt c or active caspase-8, ≈32-kDa procaspase-3 was processed to yield ≈17- to 20-kDa forms of the large subunit, indicative of active caspase-3 (the ≈12-kDa subunit of caspase-3 is undetectable with this anticaspase-3 antibody). Recombinant SfIAP and CpIAP suppressed the processing of procaspase-3 in Cyt c-treated (C) but not in caspase-8-treated (D) extracts, whereas XIAP interrupted processing at an intermediate step (≈24-kDa band), as demonstrated previously (10, 11). (E) Grim peptide or control peptide (2–10 μM) was added with or without recombinant SfIAP (2 μM) protein to HEK293 cell lysates concurrently with the addition of Cyt c/dATP. Lysates were incubated at 30°C for 10 min, and aliquots then were withdrawn and assayed for DEVD-cleaving caspase activity as above, measuring rates from the linear portion of enzyme progress curves.Data are presented as a percentage relative to control reactions in which Cyt c/dATP were alone. Numbers below diagram indicate molar ratio of peptides relative to SfIAP protein.
Grim Peptide Negatively Regulates SfIAP.
We tested the effects of a peptide corresponding to the IAP-binding domain of Grim (18 N-terminal aa of Grim) on SfIAP-mediated suppression of caspases in Cyt c-stimulated cell extracts. Although recombinant SfIAP inhibited Cyt c-induced activation of DEVD-cleaving caspases, addition of Grim peptide abrogated this inhibitory effect of SfIAP in a concentration-dependent manner, with a 5-fold molar excess of Grim peptide relative to SfIAP resulting in complete restoration of caspase activity (Fig. 3E). In contrast, several control peptides of similar length had no effect (Fig. 3E and data not shown). These results support the hypothesis that the fly apoptosis inducer Grim is an inhibitor of S. frugiperda and baculoviral IAPs.
SfIAP and CpIAP Directly Bind and Inhibit Mammalian Caspase-9.
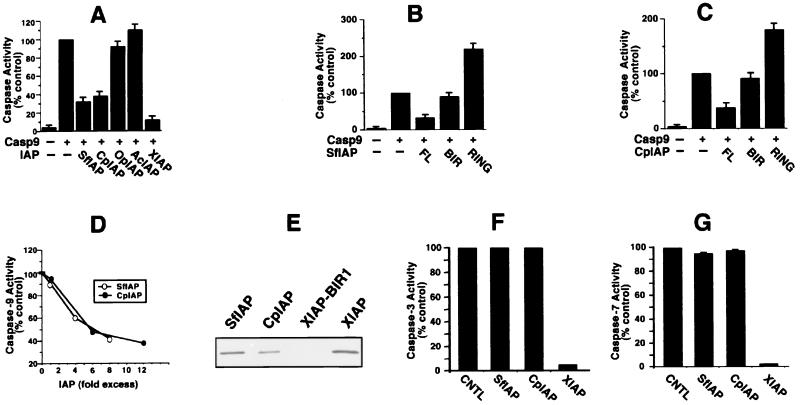
The observation that SfIAP and CpIAP suppress apoptosis induced by Bax and block caspase activation induced by Cyt c suggested that these IAPs might inhibit caspase-9, the pinnacle caspase in the mitochondrial/Cyt c pathway. To test this hypothesis, 293T cells were transfected with plasmids encoding procaspase-9 alone or in combination with SfIAP and CpIAP. Caspase activity (Fig. 4) and apoptosis (not shown) then were assayed 24–36 hr later. Both SfIAP and CpIAP, as well as human XIAP (included as a positive control), markedly suppressed activation of downstream DEVD-cleaving caspases induced by overexpression of caspase-9 (Fig. 4A) and also inhibited caspase-9-induced apoptosis (not shown). In contrast, OpIAP and AcIAP failed to suppress caspase activation or apoptosis, demonstrating the specificity of these results. Immunoblotting confirmed expression of all these IAP-family proteins at comparable levels (Fig. 2 and not shown). Unlike full-length SfIAP and CpIAP, fragments containing only the BIR domains were unable to suppress activation of DEVD-cleaving caspases induced by overexpression of procaspase-9 (Fig. 4 B and C). Moreover, fragments of SfIAP and CpIAP containing only the RING domain enhanced rather than suppressed caspase activation (Fig. 4 B and C) and apoptosis (not shown).
Figure 4.
SfIAP and CpIAP directly suppress caspase-9 but not caspase-3 or caspase-7. Caspase-9 expression plasmids were cotransfected into 293 cells with plasmids encoding various full-length IAPs (A) or fragments of SfIAP (B) comprising only the BIR domains (residues 1–323) or RING domain (residues 324–377) or fragments of CpIAP containing only BIR (residues 1–220) or RING (residues 221–275) domains (C). After 24–36 hr, lysates were prepared and assayed for DEVD-cleaving caspase activity, as above. Data are presented as a percentage relative to caspase activity of cells transfected with caspase-9 alone (mean ± SD; n = 3). (D) Recombinant active caspase-9 was added at 0.2 μM because of its lower specific activity and incubated at 37°C with Ac-LEHD-AFC substrate (100 μM) in the presence or absence of various concentrations (0.2–1.6 μM) of SfIAP (○) or (0.2–2.4 μM) of CpIAP (●). AFC release was measured continuously, and data are expressed as a percentage relative to control reactions lacking IAPs, using rates determined from the linear portion of enzyme progress curves. Various control GST-fusion proteins had no inhibiting effect (not shown). (E) [35S]Caspase-9 in reticulocyte lysate was incubated with GST-fusion proteins immobilized on glutathione-Sepharose: GST-SfIAP, GST-CpIAP, GST-XIAP-BIR1, and GST-XIAP. (F) Recombinant active caspase-3 (2.6 nM) was incubated at 37°C with Ac-DEVD-AFC substrate (100 μM) in the presence or absence of 0.05 μM GST-XIAP, 0.5 μM GST-SfIAP (200-fold molar excess relative to caspases), or 0.5 μM GST-CpIAP (≈200-fold molar excess). AFC release was measured as above. (G) Caspase-7 (7 nM) was incubated at 37°C with Ac-DEVD-AFC substrate (100 μM) in the presence or absence of 0.14 μM GST-XIAP, 0.7 μM GST-SfIAP (100-fold molar excess relative to caspases), or 0.6 μM GST-CpIAP (≈100-fold molar excess). AFC release was measured as above.
To directly examine the possibility that SfIAP and CpIAP inhibit caspase-9, enzyme inhibition assays were performed by using bacteria-produced active recombinant caspase-9, purified recombinant SfIAP and CpIAP, and Ac-LEHD-AFC as a caspase-9 substrate. SfIAP and CpIAP inhibited active recombinant caspase-9 in a concentration-dependent manner (Fig. 4D). The molar excess of SfIAP and CpIAP relative to caspase-9 producing ≈50% inhibition was 8- and 12-fold, respectively, similar to the results reported previously for XIAP (10).
SfIAP and CpIAP also specifically bound caspase-9, as determined by experiments in which [35S]caspase-9 was incubated with GST-SfIAP and GST-CpIAP, as well as positive (GST-XIAP) and negative (GST-XIAP-BIR1) control proteins (Fig. 4E). Unlike XIAP, SfIAP and CpIAP neither bound (not shown) nor inhibited recombinant-purified caspase-3 and caspase-7 (Fig. 4 F and G), whereas human XIAP did. Thus, XIAP inhibits a broader range of mammalian caspases than SfIAP and CpIAP.
Discussion
Studies of baculoviruses were among the first to demonstrate that suppression of host cell apoptosis is often a critical aspect of the virus life cycle (3). The genomes of numerous baculoviruses contain at least two types of apoptosis-suppressing genes, encoding p35 and IAPs. The p35 protein is a broad-specificity caspase inhibitor, which binds and inhibits multiple vertebrate and invertebrate caspases (21, 33). In this report we provide direct evidence that at least one baculoviral IAP, CpIAP, also can function as a caspase inhibitor, albeit with far more limited specificity than p35 for particular caspases. Whereas the origin of the p35 gene remains enigmatic, our cloning and functional characterization of a cellular IAP homolog from S. frugiperda provides strong evidence that baculovirus IAPs were pirated from the genomes of cells they infect. This argument is supported both by the strong sequence similarity of SfIAP with baculoviral IAPs and by structure–function comparisons of SfIAP with CpIAP, revealing identical domain requirements for suppression of apoptosis (e.g., BIR plus RING) and similar mechanisms with respect to caspase specificity (e.g., caspase-9).
SfIAP and CpIAP inhibit Bax-induced but not Fas-induced apoptosis in mammalian cells, unlike XIAP, which suppresses both pathways (10). Moreover, SfIAP and CpIAP suppress caspase activation in cytosolic extracts induced by the caspase-9 activator, Cyt c, but not by caspase-8, whereas XIAP blocked in both instances. These observations mapped the caspase-inhibitory functions of SfIAP and CpIAP to caspase-9. Direct confirmation of an inhibitory effect of SfIAP and CpIAP on caspase-9 then was obtained by using purified components, demonstrating that these IAPs directly bind and inhibit mammalian caspase-9. Recently, we reported that XIAP contains two independent caspase-inhibitory domains (9). The BIR2 domain of XIAP binds and potently inhibits the downstream caspase-3 and caspase-7 (13), whereas a XIAP fragment comprising the BIR3 and RING domains binds and inhibits caspase-9 (9). Analogous to XIAP, both the BIR and RING regions of SfIAP and CpIAP are required for suppression of mammalian caspase-9. We speculate, therefore, that the adaptation of BIR2 of XIAP for suppression of caspase-3 and caspase-7 represents a more recent evolutionary development, which is perhaps lacking in insects and possibly other invertebrates.
SfIAP and CpIAP can inhibit human caspase-9, but what is the endogenous caspase target of these IAPs in insect cells? Though preventing Sf-pro-caspase-1 from becoming activated, baculoviral IAPs do not directly inhibit active Sf-caspase-1 (16). We speculate, therefore, that Sf-caspase-1 is analogous to the downstream caspases in mammalian species, such as caspase-3 and caspase-7, and that the direct target of SfIAP and CpIAP in Sf-cells is an upstream caspase analogous to mammalian caspase-9. By inhibiting an upstream caspase-9-like protease, SfIAP and CpIAP would prevent processing and activation of the downstream caspase, Sf-caspase-1.
Though the BIR domain represents the minimal structural unit required for construction of a caspase inhibitory domain within IAP-family proteins (13), the role of the RING domain remains unclear. Interestingly, overexpression of the RING domains of SfIAP and CpIAP enhanced apoptosis, suggesting that this domain operates as a trans-dominant inhibitor of endogenous proteins involved in apoptosis suppression.
The proteins of Drosophila melanogaster, Reaper, Hid, and Grim, bind cellular IAPs and induce apoptosis in both insect and human cells (18, 19, 34), implying an evolutionarily conserved mechanism. The conserved N-terminal regions of these proteins are necessary and sufficient for binding IAPs and for inducing apoptosis (18, 19), arguing that IAP binding is critical for their proapoptotic function. However, until recently (20), it has been unclear whether death proteins such as Grim act as repressors of IAPs or, conversely, whether survival proteins such as IAPs operate as repressors of Grim (reviewed in ref. 8). Our data support models envisioning Drosophila death proteins as repressors of IAPs, which prevent IAPs from suppressing caspases. We also have observed suppression in vitro of human XIAP by peptides derived from the N-terminal IAP-binding domains of Drosophila apoptosis proteins, suggesting conservation of the mechanisms by which these peptides promote apoptosis (unpublished observations). These findings suggest opportunities for abrogating apoptosis inhibition by IAPs through peptide or peptiomimetic compounds, which might be useful for treatment of diseases such as cancer.
Acknowledgments
We thank Dr. Lois K. Miller for the vP35del virus. This work was supported by grants from the National Institutes of Health (ES02710, AG15402, ES04699), the U.S. Department of Agriculture (97-35302-4406), Binational Agricultural Research and Development (96-34339-3532), IDUN Pharmaceuticals, Center grants from the National Institute on Environmental Health Sciences (ES05707) and National Cancer Institute (CA30199), and a fellowship from the Leukemia Society of America (Q.L.D.). We dedicate this paper to Professor Susumu Maeda, who passed away during the completion of the project.
Abbreviations
- IAP
inhibitor of apoptosis protein
- BIR
baculovirus IAP repeat
- AcMNPV
Autographa californica nuclearpolyhedrovirus
- SfIAP
IAP from Spodoptera frugiperda
- GST
glutathione S-transferase
- AFC
7-amino-4-trifluoromethyl-coumarin
- Cyt c
cytochrome c
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF186378).
References
- 1.Wyllie A H, Kerr J F R, Currie A R. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 2.Salvesen G S, Dixit V M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 3.Clem R J, Fechheimer M, Miller L K. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 4.Ray C, Black R, Kronheim S, Greenstreet T, Sleath P, Salvesen G, Pickup D. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum M, Clem R, Miller L. J Virol. 1994;68:2521–2525. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crook N E, Clem R J, Miller L K. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller L. Trends Cell Biol. 1999;9:323–328. doi: 10.1016/s0962-8924(99)01609-8. [DOI] [PubMed] [Google Scholar]
- 8.Deveraux Q, Reed J C. Genes Dev. 1998;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 9.Deveraux Q, Leo E, Stennicke H, Welsh K, Salvesen G, Reed J. EMBO J. 1999;18:5242–5252. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deveraux Q L, Roy N, Stennicke H R, Van Arsdale T, Zhou Q, Srinivasula M, Alnemri E S, Salvesen G S, Reed J C. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deveraux Q, Takahashi R, Salvesen G S, Reed J C. Nature (London) 1997;388:300–303. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 12.Roy N, Deveraux Q L, Takashashi R, Salvesen G S, Reed J C. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen G S, Reed J C. J Biol Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser W, Vucic D, Miller L. FEBS Lett. 1998;440:243–248. doi: 10.1016/s0014-5793(98)01465-3. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins C, Wang S, Hay B. Proc Natl Acad Sci USA. 1999;96:2885–2890. doi: 10.1073/pnas.96.6.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seshagiri S, Miller L K. Proc Natl Acad Sci USA. 1997;94:13606–13611. doi: 10.1073/pnas.94.25.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White E, Cipriani R. Proc Natl Acad Sci USA. 1989;86:9886–9890. doi: 10.1073/pnas.86.24.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vucic D, Kaiser W J, Harvey A J, Miller L K. Proc Natl Acad Sci USA. 1997;94:10183–10188. doi: 10.1073/pnas.94.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vucic D, Kaiser W J, Miller L K. Mol Cell Biol. 1998;18:3300–3309. doi: 10.1128/mcb.18.6.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Hawkins C, Yoo S, Muller H, Hay B. Cell. 1999;98:453–463. doi: 10.1016/s0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad M, Srinivasula S, Wang L, Litwack G, Fernandes-Alnemri T, Alnemri E. J Biol Chem. 1997;272:1421–1424. doi: 10.1074/jbc.272.3.1421. [DOI] [PubMed] [Google Scholar]
- 22.Deveraux, Q., Welsh, K. & Reed, J. (2000) Methods Enzymol., in press. [DOI] [PubMed]
- 23.Stennicke H, Deveraux Q, Humke E, Reed J, Dixit V, Salvesen G. J Biol Chem. 1999;274:8359–8362. doi: 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- 24.Stennicke H R, Salvesen G S. J Biol Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 25.Stennicke H, Jürgensmeier J, Shin H, Deveraux Q, Wolf B, Yang X, Zhou Q, Ellerby H, Ellerby L, Bredesen D, et al. J Biol Chem. 1998;273:27084–27090. doi: 10.1074/jbc.273.42.27084. [DOI] [PubMed] [Google Scholar]
- 26.Quan L T, Caputo A, Bleackley R C, Pickup D J, Salvesen G S. J Biol Chem. 1995;270:10377–10379. doi: 10.1074/jbc.270.18.10377. [DOI] [PubMed] [Google Scholar]
- 27.Kozak M. Mamm Genome. 1996;7:563–574. doi: 10.1007/s003359900171. [DOI] [PubMed] [Google Scholar]
- 28.Clem R J, Miller L K. Mol Cell Biol. 1994;14:5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawkins C J, Uren A G, Hacker G, Medcalf R L, Vaux D L. Proc Natl Acad Sci USA. 1996;93:13786–13790. doi: 10.1073/pnas.93.24.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkins C, Ekert P, Uren A, Holmgreen S, Vaux D. Cell Death Differ. 1998;5:569–576. doi: 10.1038/sj.cdd.4400389. [DOI] [PubMed] [Google Scholar]
- 31.Uren A G, Pakusch M, Hawkins C J, Puls K L, Vaux D L. Proc Natl Acad Sci USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 33.Bump N, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, et al. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 34.Justin V, Dixit V. J Biol Chem. 1998;273:24009–24015. doi: 10.1074/jbc.273.37.24009. [DOI] [PubMed] [Google Scholar]