Abstract
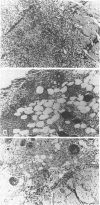
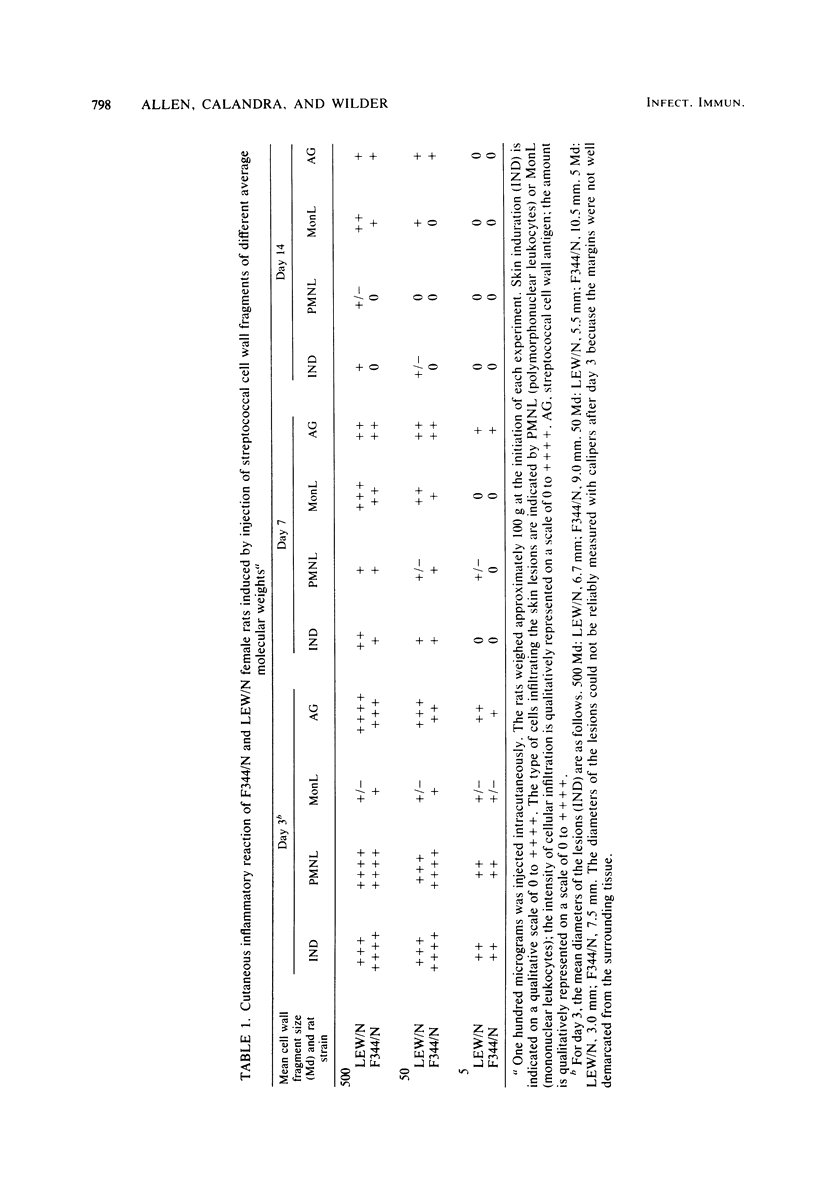
Systemic administration of an aqueous suspension of group A streptococcal cell wall fragments induces severe, chronic erosive polyarthritis in LEW/N female rats, but rarely in F344/N female rats. In the present study, we attempted to exclude unresponsiveness to the cell walls as a mechanism for arthritis resistance in F344/N females. Cutaneous inflammatory reactions were assessed in both strains at various time points after direct injection of cell wall fragments of three different average molecular weights. Fragments of all sizes induced an acute inflammatory reaction, with infiltration of polymorphonuclear leukocytes and a few mononuclear cells. Small fragments (approximately 5 megadaltons) induced a transient response which resolved by day 14. Large fragments (approximately 500 megadaltons) induced severe inflammation characterized by prominent mononuclear leukocyte infiltration, whereas the intermediate-sized fragments (approximately 50 megadaltons) induced inflammation of intermediate intensity and duration. The intensity and severity of the lesions paralleled the persistence of cell wall antigens at the site of deposition. F344/N female rats responded acutely to the cell walls, with an intensity equal to or greater than that of LEW/N female rats, but the lesions tended to resolve more rapidly. These findings indicate that severity and chronicity of streptococcal cell wall-induced inflammation are dependent on the size of the fragment and provide evidence that arthritis resistance in F344/N female rats does not result from a completely unresponsive state to the proinflammatory effects of the cell walls.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Blatter D., Calandra G. B., Wilder R. L. Sex hormonal effects on the severity of streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1983 Apr;26(4):560–563. doi: 10.1002/art.1780260418. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis H. A., Miller S. E., Wheat R. W. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun. 1976 May;13(5):1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., McCune W. J., Susman P., David J. R. Autoimmunity to collagen in adjuvant arthritis of rats. J Clin Invest. 1980 Nov;66(5):1109–1117. doi: 10.1172/JCI109940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]