Abstract
Recent studies suggest that adipose tissue hypoxia (ATH) may contribute to endocrine dysfunction in adipose tissue of obese mice. In this study, we examined hypoxia's effects on metabolism in adipocytes. We determined the dynamic relationship of ATH and adiposity in ob/ob mice. The interstitial oxygen pressure (Po2) was monitored in the epididymal fat pads for ATH. During weight gain from 39.5 to 55.5 g, Po2 declined from 34.8 to 20.1 mmHg, which are 40–60% lower than those in the lean mice. Insulin receptor-β (IRβ) and insulin receptor substrate-1 (IRS-1) were decreased in the adipose tissue of obese mice, and the alteration was observed in 3T3-L1 adipocytes after hypoxia (1% oxygen) treatment. Insulin-induced glucose uptake and Akt Ser473 phosphorylation was blocked by hypoxia in the adipocytes. This effect of hypoxia exhibited cell type specificity, as it was not observed in L6 myotubes and βTC6 cells. In response to hypoxia, free fatty acid (FFA) uptake was reduced and lipolysis was increased in 3T3-L1 adipocytes. The molecular mechanism of decreased fatty acid uptake may be related to inhibition of fatty acid transporters (FATP1 and CD36) and transcription factors (PPARγ and C/EBPα) by hypoxia. The hypoxia-induced lipolysis was observed in vivo after femoral arterial clamp. Necrosis and apoptosis were induced by hypoxia in 3T3-L1 adipocytes. These data suggest that ATH may promote FFA release and inhibit glucose uptake in adipocytes by inhibition of the insulin-signaling pathway and induction of cell death.
Keywords: abdominal obesity, insulin sensitivity, high-fat diet, hypoxia-inducible factor 1α, glucose transporter 1
in obesity, metabolic disorders in white adipose tissue contribute to pathogenesis of insulin resistance (1, 40). The decrease in triglycerides (TAG) biosynthesis and increase in lipolysis of adipose tissue lead to elevation in free fatty acids (FFA) in plasma and contribute to ectopic fat deposition in liver and skeletal muscle. The lipid disorder may promote systemic insulin resistance through several mechanisms (56), such as activation of PKCs/JNK through intermediate products (diacylglyceride or ceramide), induction of oxidative stress through incomplete β-oxidation, and induction of inflammatory responses through activation of Toll-like receptor 4 (27, 30, 42). FFA may contribute to hyperinsulinemia in obesity by a direct effect in β-cells (54). Impairment of insulin action in adipocytes may contribute to metabolic disorders in adipose tissue, since insulin stimulates synthesis and storage of TAG and inhibits lipolysis. Given the role of insulin in adipose tissue, impairment of insulin action in adipose tissue may represent an early event in systemic insulin resistance in obesity. However, the cause of the adipocyte malfunction remains to be identified in obesity.
The role of hypoxia in chronic inflammation in adipose tissue was first proposed in a review article in 2004 (50). Recent studies from three different laboratories have provided consistent evidence that adipose tissue hypoxia (ATH) exists in obese mice and that it contributes to initiation of chronic inflammation and inhibition of adiponectin expression in the white adipose tissue (21, 37, 55). These studies have nicely addressed the impact of hypoxia in the endocrine functions, but not metabolism of adipose tissue. Hypoxia was shown to enhance insulin-independent glucose uptake in human adipocytes through induction of GLUT1 expression in mRNA and protein (53). However, the effect of hypoxia on insulin-dependent glucose uptake in adipocytes was not reported in the study. Additionally, the dynamic relationship of ATH and adiposity has not been characterized.
In the current study, we examined dynamic change in ATH in ob/ob mice and investigated the hypoxia effects on insulin-stimulated glucose uptake. The results suggest that ATH went up with body weight and that hypoxia powerfully inhibited insulin action in adipocytes. The inhibition led to an increase in lipolysis and cell death in adipocytes. The results suggest that hypoxia may be a cause of metabolic disorders in the adipose tissue of obese mice.
RESEARCH DESIGN AND METHODS
Obese mice.
Male ob/ob mice (B6.V-Lepob/Lepob, stock no. 000632) and C57BL/6 mice were purchased from the Jackson Laboratory at age of 4–5 wk and used at 6–12 wk in this study for genetic and dietary obesity. The ob/ob mice were fed normal chow diet (12. 8% kcal in fat), and the sex-matched wild-type littermates of ob/ob mice were used as the lean control. In diet-induced obesity (DIO), male C57BL/6 mice at 5 wk of age were fed a high-fat diet (HFD, D12331; Research Diets, New Brunswick, NJ), which contained 58% calories in fat. In terms of weight, the total fat was 35.8% (wt/wt) in the diet (33.3% hydrogenated coconut oil and 2.5% soybean oil). There was 33.4% saturated, 0.86% monounsaturated, and 1.54% polyunsaturated fatty acids in the diet. In the control, mice (age- and sex-matched C57BL/6) were fed a normal chow diet. After 8 wk on HFD, the mice were used as dietary obese mice in this study. The fat composition was determined with nuclear magnetic resonance (model mq10; Brucker, Milton, ON, Canada). All of the mice were housed in the animal facility at the Pennington Biomedical Research Center on a 12:12-h light-dark cycle and constant temperature (22–24°C). The mice had free access to water and diet. All procedures were performed in accordance with National Institute of Health guidelines for the care and use of animals and approved by the Institute's Animal Care and Use Committee at the Pennington Biomedical Research Center.
Cells and differentiation.
Mouse 3T3-L1 preadipocytes, rat skeletal L6 myoblasts, and mouse βTC6 insulinoma cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in DMEM culture medium supplemented with 10% fetal bovine serum (15% for βTC6), 4 mM glutamine, and 50 mg/l gentamicin in an atmosphere of 5% CO2 at 37°C. The differentiations of 3T3-L1 into adipocytes and L6 myoblasts into myotubes were induced as described elsewhere (57).
Hypoxia treatment.
Ambient hypoxia was generated by filling in a sealed metal chamber (self-designed) with low-oxygen air that contained 1% oxygen, 5% carbon dioxide, and 94% nitrogen. In hypoxia treatments, 3T3-L1 adipocytes were maintained in DMEM supplemented with 0.25% bovine serum albumin (BSA) and 25 μM HEPES. The hypoxia was monitored with an oxygen meter. To keep the humidity and temperature at 37°C in the chamber, 200 ml of water was kept in the chamber, and the chamber was maintained in a 37°C water bath with a constant temperature of 37°C.
Interstitial Po2.
An oxygen meter with a needle type optic-fiber oxygen sensor (OXY-MICRO-AOT, World Precision Instruments) was used to determine interstitial Po2 in the epididymal fat pads, as described previously (55). The Po2 reading was taken from both sides of the fat pads, normalized with temperature and presented in millimeters of mercury. The mean value of three mice was used to represent Po2 in obese or lean mice.
Quantitative RT-PCR.
Total RNA was extracted from homogenized epididymal fat or cultured cells using Tri reagent (T9424, Sigma) according to the manufacturer's instructions. The epididymal fat pads were collected from mice and frozen in liquid nitrogen. Quantitative (q)RT-PCR was conducted using an ABI 7900HT fast real-time PCR system (Applied Biosystems, Foster City, CA). The primer and probe were ordered from Applied Biosystems: hif1a (Mm00468869_m1), vegf (Mm00437304_m1), glut1 (Mm00441473_m1), HO-1 (Mm00516004_m1), and pdk1 (Mm00554306_m1). The mRNA signal was normalized over the 18S ribosomal RNA (rRNA) signal. A mean value of triplicates was used for relative mRNA level or calculation of fold induction.
Western blot.
Whole cell lysate was made with homogenization and sonication in a lysis buffer, and Western blotting was conducted as described elsewhere (14). Lipids were removed from the lysate before the protein assay was conducted. Antibodies to hypoxia-inducible factor-1α (HIF1α), glucose transporter 1 (GLUT1), glucose transporter 4 (GLUT4), actin, and tubulin were from Abcam (Cambridge, MA). Antibodies to IRβ, IRS-1, Akt1/2, CCAAT/enhancer-binding protein α (C/EBPα), and fatty acid transport protein-1 (FATP1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody to Phospho-Akt (Ser473) was purchased from Calbiochem (Gibbstown, NJ). Rabbit antiserums to peroxisome proliferator-activated receptor-γ (PPARγ) and CD36 were made in our laboratory. Horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG were purchased from GE Healthcare UK (Buckinghamshire, UK). ImageJ 1.37V was used to quantify the Western signals.
Glucose uptake.
3T3-L1 adipocytes, L6 myotubes, or βTC6 cells were cultured in 12-well plates and treated with hypoxia in serum-free medium (DMEM with 0.25% BSA). After hypoxia treatment, 2-deoxy-d-[3H]glucose uptake was performed immediately in normoxic condition in PBS buffer. The glucose uptake assay has been described elsewhere (57).
Glycerol and TAG assays.
3T3-L1 adipocytes were cultured in a 24-well plate. In the hypoxia treatment, the cells were maintained in DMEM supplemented with 0.25% BSA and 25 μM HEPES. Tumor necrosis factor-α (TNF-α, final concentration 20 ng/ml) was added to cells. After hypoxia treatment for 24 h, the medium was collected and tested for glycerol and TAG with the Serum TAG Determination Kit (Sigma, St. Louis, MO). In the preparation of conditioned medium, the adipocytes were treated with normoxia or hypoxia for 24 h, and the supernatant was collected immediately after the treatment. The conditioned medium was used to treat cells for 24 h, and glycerol concentration was determined in the culture. The net change in glycerol was obtained by comparing glycerol before and after the treatment to determine lipolysis.
Necrosis and apoptosis assay.
3T3-L1 adipocytes were cultured in six-well plates. After hypoxia treatment for 24 h, both detached and attached cells were collected and used in analysis of necrosis and apoptosis with Vybrant Apoptosis Assay Kit #2 (Molecular Probes, Eugene, OR). Flow cytometric analysis was performed using the FACSCalibur cytometer (BD Biosciences, San Jose, CA). A total of 20,000 cells were examined in each sample, and the data were analyzed using Cellquest Pro software (BD Biosciences) and a Macintosh G5 computer (Apple, Cupertino, CA).
Fatty acid uptake.
3T3-L1 adipocytes were cultured in a 12-well plate. After hypoxia treatment in serum-free DMEM with 0.25% BSA for 24 h, [14C]palmitic acid uptake was examined as described elsewhere (34).
Nonviable cell assay.
3T3-L1 adipocytes were cultured in normal DMEM supplemented with serum and 25 μM HEPES in a six-well plate. After hypoxia treatment for 8 or 16 h, the medium was collected for counting the detached dead cells, which were collected after centrifugation and stained with trypan blue. The attached cells were trypsinized, diluted in serum-free DMEM, and stained with trypan blue for dead cells. The stained cells in the supernatant and on the plate were combined together to represent the nonviable cells in each well.
Plasma FFA after femoral artery clamp.
The effect of hypoxia on lipolysis was assessed in rats by determining the changes in plasma FFA level in femoral vein after occlusion of the femoral artery for 15 min in lean rats (400 g body wt; Harlan Sprague Dawley, Indianapolis, IN). The rats fed on regular rat chow were anesthetized by pentobarbital sodium (50 mg/kg iv), and the abdominal cavity was surgically opened to expose the femoral artery and vein. A basal blood sample was taken first from the femoral vein. Then, a small clamp was placed at the corresponding femoral artery to block the blood flow. Fifteen minutes later, the clamp was removed, and two blood samples were taken at 3-min intervals from the same vein. Plasma FFA concentration was determined enzymatically (19).
Statistical analysis.
In this study, all of the in vitro experiments were conducted three times with consistent results. The data of representative experiments are presented. In the bar figures, a mean value and standard error of multiple data points or samples were used to represent the final result. Student's t-test or two-way ANOVA was used in statistical analysis of the data with significance P ≤ 0.05.
RESULTS
Hypoxia in adipose tissue of ob/ob mice.
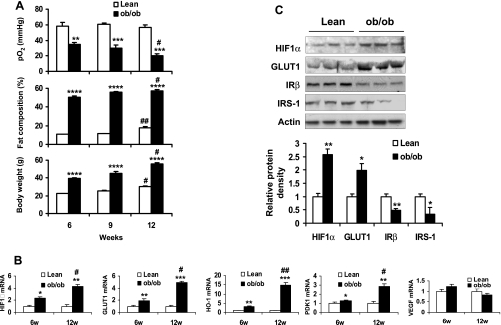
If hypoxia is a risk factor for adipocyte insulin resistance in vivo, it should increase with adiposity in the obese condition. To test this possibility, we conducted three experiments in ob/ob mice. In the first, interstitial Po2 was measured in the epididymal fat pads of ob/ob mice during weight gain between 6 and 12 wk in age. In the lean controls, Po2 was in the range of 56.8 to 60.4 mmHg at the same age range (Fig. 1A). Although their body fat content was increased from 10 to 18%, Po2 did not decrease in the fat tissue when the body fat content reached 18% at 12 wk of age. In the ob/ob mice, Po2 was 34.8 mmHg (40.4% lower than that in lean controls) at 6 wk of age. It further dropped to 20.1 mmHg (64.8% lower than that in lean mice) at 12 wk of age with a gain in adiposity (Fig. 1A). At 6 wk, the low Po2 was associated with 50% of body fat content in the obese mice. This adiposity was 400% higher than that of lean mice. The data suggest that ATH may occur when the body fat content is over 20% (Fig. 1A) and becomes more severe with gain in fat content thereafter.
Fig. 1.
Hypoxia in adipose tissue of ob/ob mice. A: Po2 in epididymal fat pads of lean and ob/ob mice was determined with an oxygen meter equipped with a fiber optic oxygen sensor. The assay was conducted in mice at 6, 9, and 12 wk in age. Fat composition and body weight of the mice were determined (n = 3). B: hypoxia response gene expression in epididymal fat of ob/ob mice was determined in quantitative RT-PCR. The assay was conducted in mice at 6 and 12 wk in age (n = 3). C: protein levels in epididymal fat of lean and ob/ob mice were examined in a Western blot (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.001 vs. lean mice; #P < 0.05, ##P < 0.01 vs. 6-wk-old (6w) mice.
In the second test, expression of hypoxia-responsive genes was examined by qRT-PCR to determine the hypoxia response. The genes include HIF1α, GLUT1, Heme oxygenase-1 (HO-1), pyruvate dehydrogenase kinase-1 (PDK1), and vascular endothelial growth factor (VEGF). Among the five genes, HIF1α is a transcription factor that controls expression of the other four genes (41). In the ob/ob mice, four of the five genes (except VEGF) increased in the epididymal fat pads compared with the control mice (Fig. 1B). The fold induction in these genes was positively associated with the degree in ATH and fat content in the ob/ob mice. Expression of these genes suggests a hypoxia response in the adipose tissue of obese mice.
In the third test, protein levels of hypoxia-responsive molecules including HIF1α and GLUT1 were examined in a Western blot. These proteins increased significantly in the adipose tissue of the ob/ob mice (Fig. 1C). In contrast, proteins of IRβ and IRS-1 decreased significantly in the obese mice (Fig. 1C). These data suggest that hypoxia exists in the visceral fat tissue of obese mice and that adipose tissue hypoxia may lead to impairment in the insulin signaling pathway.
Gene expression in diet-induced obesity.
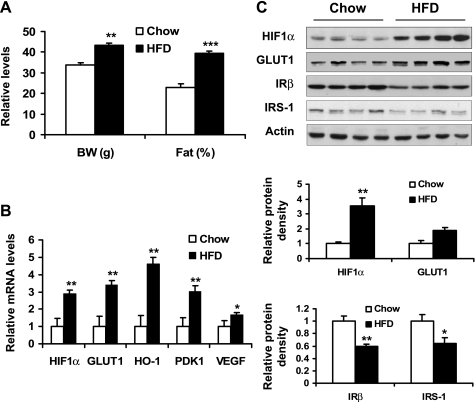
We hypothesized that the hypoxia response is present in adipose tissue in obesity regardless of model system. The above data suggest the hypoxia response in ob/ob mouse, a genetic model of obesity. To test the hypothesis, we examined the hypoxia response in dietary obese mice, which also suffer adipose tissue hypoxia (37). The dietary obese mice were used at 8 wk on HFD. Their body weight was increased by 27.7% and fat content by 71.6% at that time (Fig. 2A). mRNA of hypoxia-responsive genes, including HIF1α, GLUT1, HO-1, PDK-1, and VEGF, was increased significantly in epididymal fat pads (Fig. 2B). Proteins for HIF1α and GLUT1 were upregulated (Fig. 2C). Proteins for IRβ and IRS-1 were downregulated (Fig. 2C). These data suggest that hypoxia reaction is present in diet-induced obesity. The hypoxia may impair the insulin-signaling pathway in adipocytes or adipose tissue similar to what was observed in the ob/ob mice.
Fig. 2.
Hypoxia-induced gene expression in diet-induced obesity. A: after 8 wk on a high-fat diet (HFD), mice were examined for body weight (BW) and fat composition. B: hypoxia-induced gene expression in epididymal fat of lean and obese mice was determined using quantitative RT-PCR. C: protein levels in epididymal fat of HFD mice were examined by Western blot. *P < 0.05, **P < 0.01, ***P < 0.001 vs. chow diet-fed control mice.
Inhibition of insulin-induced glucose uptake by hypoxia.
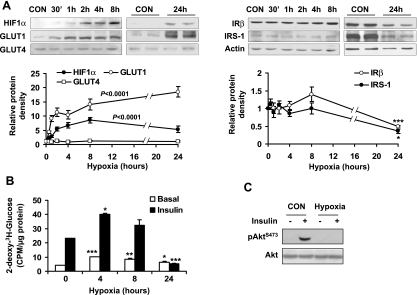
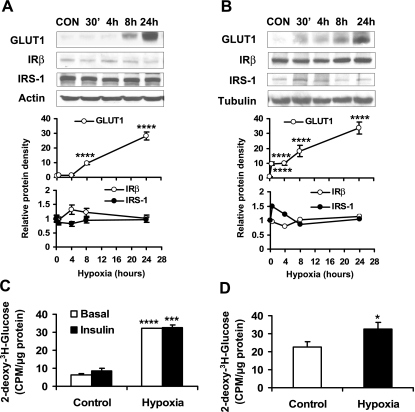
To understand the effect of hypoxia on insulin-dependent glucose metabolism, we examined hypoxia regulation of glucose uptake in 3T3-L1 adipocytes. Insulin-independent glucose uptake is mediated by GLUT1, and insulin-induced glucose uptake is mediated by both GLUT1 and GLUT4 (31, 32). In the study, expression of GLUT1 and GLUT4 proteins was determined in adipocytes after hypoxia treatment. In a time course study, a 4.3-fold elevation in GLUT1 protein level was observed at 30 min under hypoxia (Fig. 3A). The proteins for GLUT1 and HIF1α were both upregulated and maintained at high levels throughout the time of 24 h in the study. GLUT1 expression peaked at 24 h with an 18.5-fold increase, and HIF1α peaked at 8 h with an 8.6 fold increase. In this system, GLUT4 expression was not significantly changed. In this time course study, a reduction in IRβ (50%) and IRS-1 (64%) was observed at 24 h (Fig. 3A).
Fig. 3.
Inhibition of insulin-induced glucose uptake by hypoxia. A: 3T3-L1 adipocytes were exposed to hypoxia (1% oxygen), and proteins were determined by Western blot in a time course study (n = 3 or 6). B: 2-deoxy-d-[3H]glucose uptake. Uptake was examined in 3T3-L1 adipocytes after hypoxia treatment for different times. The assay was conducted in normoxic condition immediately after hypoxia treatment (n = 3). C: 15 min prior to cell collection, 100 nM insulin was added to normoxia- or hypoxia-treated 3T3-L1 adipocytes. Western blot was conducted to detect the phosphorylation of Akt in whole cell lysate. 0 represents normoxic condition. *P < 0.05, **P < 0.01, ***P < 0.001 vs. normoxic control (CON or 0 h).
Glucose uptake was examined in 3T3-L1 adipocytes by use of 2-deoxy-[3H]glucose. After 4 h of hypoxia treatment, insulin-independent (basal) and insulin-stimulated glucose uptake was enhanced by hypoxia for 142 and 72%, respectively (Fig. 3B). Subsequently, the glucose uptake decreased in a time-dependent manner. At 24 h, only 50.8% of the increase was observed in the basal glucose uptake. Under hypoxia for 24 h, the insulin-stimulated glucose uptake was completely blocked by hypoxia (Fig. 3B). The loss of insulin action was associated with a reduction in IRβ and IRS-1 proteins at the 24-h time point, suggesting that a long-term hypoxia response may lead to insulin resistance in adipocytes.
Insulin signaling was investigated in 3T3-L1 adipocytes after hypoxia treatment for 24 h. Insulin treatment for 15 min induced Akt phosphorylation (Ser473) in the control cells. However, the Akt phosphorylation was absent in the hypoxia-treated cells (Fig. 3C), suggesting that the insulin signaling pathway was impaired by hypoxia in 3T3-L1 adipocytes.
Regulation of IRβ and IRS-1 mRNA expression.
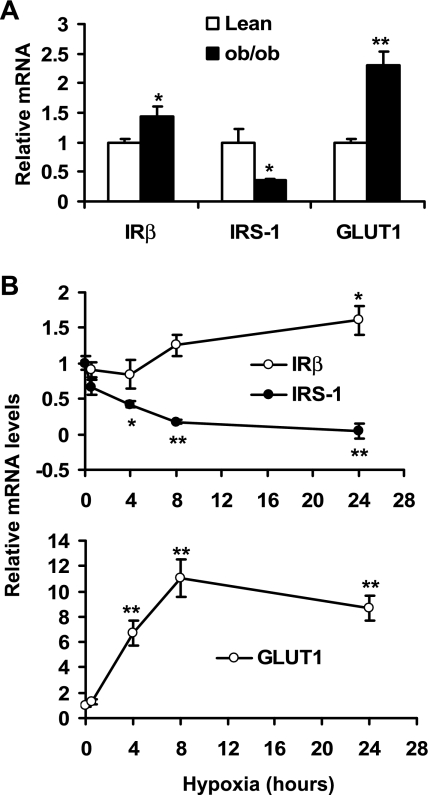
To understand the mechanism of protein change in hypoxia, we examined mRNA for IRβ, IRS-1, and GLUT1 in adipose tissue and 3T3-L1 adipocytes. In the epididymal fat of ob/ob mice, mRNA was increased significantly for IRβ and GLUT1 (Fig. 4A). In contrast, mRNA was decreased markedly for IRS-1 (Fig. 4A). In 3T3-L1 adipocytes, IRβ mRNA was induced and IRS-1 mRNA was reduced by hypoxia in a time-dependent manner (Fig. 4B). In the positive control, GLUT1 mRNA was increased as much as 10-fold in the hypoxia-treated 3T3-L1 adipocytes (Fig. 4B). These data suggest that the mRNA changes in IRβ, IRS-1, and GLUT1 in the adipose tissue of ob/ob mice may be a result of hypoxia response in obesity.
Fig. 4.
IRβ, IRS-1 and GLUT1 gene expression in adipose tissue and hypoxia-treated 3T3-L1 adipocytes. A: mRNA in epididymal fat pads of lean and ob/ob mice were determined with real-time quantitative RT-PCR. B: mRNA levels in 3T3-L1 adipocytes were determined with real-time quantitative RT-PCR after hypoxia treatment. *P < 0.05, **P < 0.01 vs. lean mice or untreated cells (0 h).
Hypoxia effects on L6 myotubes and βTC6 cells.
To test hypoxia effects on skeletal muscle cells and pancreatic β-cells, we repeated the experiments with L6 myotubes and βTC6 cells. In these two cell lines, GLUT1 protein was induced markedly by hypoxia (Fig. 5, A and B). However, no significant reduction was observed in IRβ and IRS-1 proteins. In the glucose uptake assay, a 50% increase was observed under insulin treatment in the L6 myotubes in the normoxia condition. In contrast, a fivefold induction was observed under hypoxia in the absence of insulin (Fig. 5C). This activity of hypoxia was so strong that insulin-induced glucose uptake was not detectable in the presence of hypoxia. In the βTC6 cells, insulin-independent glucose uptake increased 45% under hypoxia (Fig. 5D). Insulin-induced glucose uptake was not examined, as the βTC6 cells do not express GLUT4. These data suggest that hypoxia is able to stimulate GLUT1-mediated glucose uptake in muscle cells and β-cells. In these two types of cells, hypoxia did not inhibit the IRβ and IRS-1 proteins.
Fig. 5.
Hypoxia effects on L6 myotubes and βTC6 cells. A: effect of hypoxia on protein levels in L6 myotubes was investigated in a time course study in a Western blot (n = 3). B: hypoxia effect on protein levels in βTC6 cells (n = 3). C: 2-deoxy-d-[3H]glucose uptake was performed in L6 myotubes after hypoxia exposure for 24 h (n = 3). D: glucose uptake was performed in βTC6 cells after hypoxia treatment for 24 h (n = 3). *P < 0.05, ***P < 0.001, ****P < 0.0001 vs. control (CON) cells or 0 h.
Hypoxia regulation of lipid metabolism.
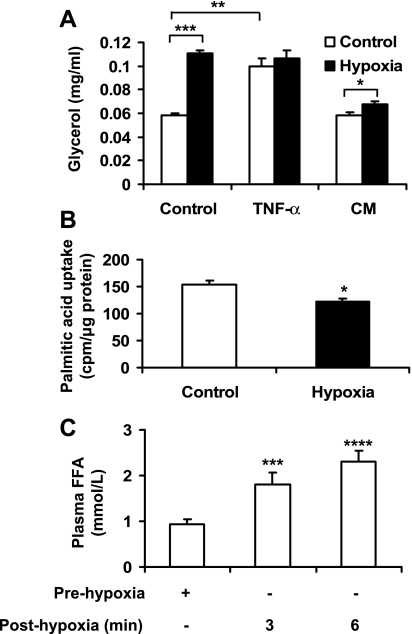
Plasma FFA is increased in obesity and is involved in pathogenesis of systemic insulin resistance. An imbalance in biosynthesis and lipolysis of TAG in adipocytes may contribute to the elevation of FFA in the circulation. To test hypoxia regulation of TAG metabolism, we examined TAG lipolysis by analysis of glycerol concentration in the culture medium of 3T3-L1 adipocytes. In response to hypoxia, glycerol level was doubled in the cell culture (Fig. 6A). This effect of hypoxia is comparable to that of TNF-α (20 ng/ml), which was used as a positive control in the stimulation of lipolysis. Both hypoxia and TNF-α generated the maximal level of lipolysis, as no additive effects were observed in combination of the two factors (Fig. 6A). To determine the role of autocrine in the stimulation of lipolysis by hypoxia, conditioned medium was prepared from 3T3-L1 adipocytes culture after 24 h of hypoxia treatment. The supernatant was used to treat the fresh 3T3-L1 adipocytes for 24 h in the normoxic condition. Glycerol concentration was determined in the culture medium after the treatment. The net increase in the glycerol level was obtained by comparing glycerol concentrations before and after 24-h culture in the conditioned medium. The glycerol concentration was increased significantly by the treatment. In addition to lipolysis, fatty acid uptake was investigated in 3T3-L1 adipocytes with radiolabeled palmitic acid. After 24 h in hypoxia, the uptake was decreased by 21% (Fig. 6B).
Fig. 6.
Hypoxia regulation of lipid metabolism. A: lipolysis was evaluated with glycerol release into culture medium of 3T3-L1 adipocytes. The assay was conducted after exposure to hypoxia or TNF-α (20 ng/ml) for 24 h (n = 4). Fresh 3T3-L1 adipocytes were cultured in conditioned medium (CM) made from supernatant of 3T3-L1 adipocytes treated by normoxia or hypoxia for 24 h. The net increase in glycerol level was obtained by comparing glycerol concentrations before and after 24-h culture in CM. B: [1-14C]palmitic acid uptake was performed in 3T3-L1 adipocytes after hypoxia treatment for 24 h (n = 3). C: FFA in femoral artery of lean rats. The femoral artery was clamped to block blood flow for 15 min to generate a hypoxia response in vivo. Blood samples were collected from the femoral vein before surgery and after clamp to determine the change in FFA (n = 11). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To test whether hypoxia is able to induce lipolysis in vivo, we generated hypoxia in the hindleg in rats by clamping the femoral artery for 15 min. After reestablishment of the circulation by removal of the clamp, blood samples were collected from the femoral vein and used in the test for plasma FFA. The FFA level was significantly increased by clamp-induced hypoxia. Compared with the FFA level in the vein blood before the clamp, FFA was increased ∼100% (P < 0.001) at 3 min and 150% (P < 0.0001) at 6 min after clamp (Fig. 6C). Adipocytes are the source of FFA. These data suggest that hypoxia is able to induce strong lipolysis in adipose tissue in vivo. These data support the idea that hypoxia may contribute to elevation of plasma FFA in obesity.
Hypoxia regulation of lipid metabolism-related protein levels.
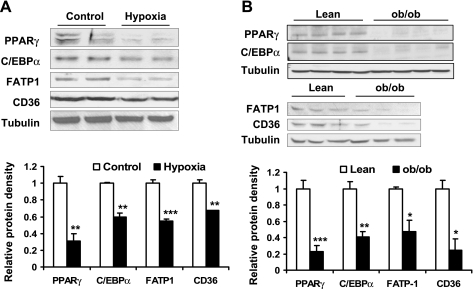
Since lipolysis was induced and fatty acid uptake was reduced by hypoxia, we wanted to investigate the mechanism of these reactions. We hypothesized that these effects might be related to changes in lipid metabolism-related protein levels. To test this possibility, expression of transcription factors (such as PPARγ and C/EBPα) and fatty acid transporters (such as FATP1 and CD36) were examined. In the adipocytes, all of these proteins were decreased significantly by 24-h hypoxia treatment (Fig. 7A). In adipose tissue, these proteins were reduced in the ob/ob mice as indicated by the Western blot, proteins for PPARγ, C/EBPα, FATP1, and CD36 (Fig. 7B). These data suggest that the reduction may be a result of chronic hypoxia response.
Fig. 7.
Hypoxia regulation of lipid metabolism-related proteins. A: protein levels in 3T3-L1 adipocytes were examined by Western blot after hypoxia treatment for 24 h (n = 6). B: protein levels in epididymal fat pad of ob/ob mice were examined by Western blot (n = 4 or 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Hypoxia induction of necrosis and apoptosis.
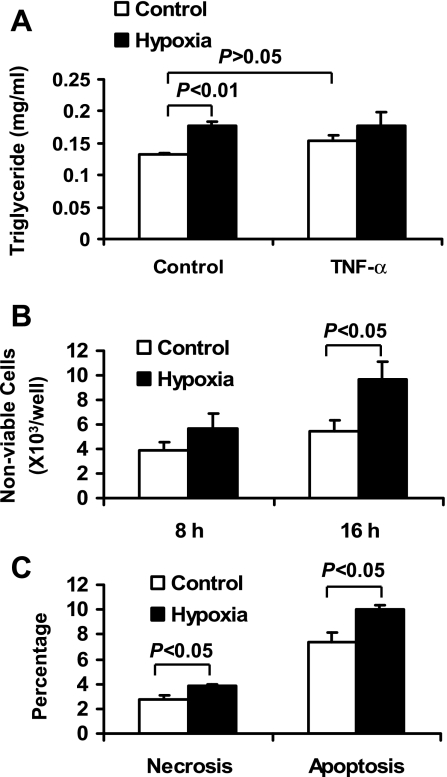
It was reported that adipocyte necrosis increased in adipose tissue of human and mice with obesity (9). The cell death may contribute to macrophage infiltration into adipose tissue. However, the cause of adipocyte death remains to be identified (46). In our study, we observed that total protein was reduced in the whole cell lysate of hypoxia-treated cells. This observation suggests that hypoxia may induce cell death, which in turn leads to protein reduction by reducing viable cell number. To test this possibility, cell death was determined by measuring TAG in the culture medium. TAG is released into the cell culture supernatant after adipocyte death. In 24-h hypoxia exposure, the TAG level was increased by 35% in the culture medium. TAG release was not significantly induced by TNF-α (Fig. 8A). These data suggest that hypoxia is stronger than TNF-α in the induction of TAG release.
Fig. 8.
Hypoxia induction of adipocyte death. A: adipocyte death was examined indirectly with triglyceride release into CM of 3T3-L1 adipocytes exposed to hypoxia and/or TNF-α (20 ng/ml) for 24 h (n = 4). B: nonviable adipocyte number was examined with trypan blue staining after hypoxia treatment for 8 and 16 h (n = 4). C: the necrosis and apoptosis ratios in 3T3-L1 adipocytes were determined using flow cytometry after cells were treated with normoxia or hypoxia for 24 h.
The cell death was examined with trypan blue staining and flow cytometry. The results showed that adipocyte death was increased by hypoxia in a time-dependent manner under hypoxia (Fig. 8B). With 16-h hypoxia treatment, the number of nonviable cells was increased by 75% (Fig. 8B). To determine necrosis and apoptosis for cell death, we used flow cytometry after the cells were stained with fluorescent dye. The results showed that necrosis and apoptosis of the adipocytes were increased by 40% and 35%, respectively (Fig. 8C). The data suggest that hypoxia was able to induce adipocyte death through necrosis and apoptosis.
DISCUSSION
Results from the current study suggest that hypoxia may lead to insulin resistance in adipocytes in obesity. It has been known for more than 30 years that large adipocytes isolated from adipose tissue of obese subject are more resistant to insulin than small adipocytes (38). This fact was used to explain the dysfunction of adipose tissue and insulin resistance in obesity, as the average size of adipocytes is increased in obesity (39). We tested the effects of mechanical pressure and vacuum on insulin-signaling activity in adipocytes. An increase in mechanical pressure to 20 psi for 72 h did not lead to any reduction in molecules or function of the insulin-signaling pathway (data not shown). In contrast, treatment of cells with vacuum for 24 h blocked the insulin action in the adipocytes. That led us to study the hypoxia effects on adipose tissue.
The current study suggests a relationship between ATH and body fat content. ATH may occur when body fat content is above 20%. This is supported by the data from lean mice, whose Po2 remained unchanged at ∼60 mmHg when the body fat content increased from 10 to 18%. In the ob/ob mice, the body fat content was 50% at 6 wk of age, and Po2 was at 39 mmHg. The reduction in Po2 continued in the ob/ob mice when the fat content gained further with age. The hypoxia was supported by the increased expression of hypoxia-responsive genes in the fat of ob/ob mice. The exact threshold of body fat content for ATH remains to be identified. We were unable to identify the threshold in this study, as the body fat content was 50% in ob/ob at the start of this study. The reason(s) for ATH remains to be investigated. An increase in adipocyte size may be a factor. In obesity, adipocyte size increases up to 140–180 μm in diameter (7). This size is bigger than the diffusion distance of oxygen, which is ∼100–120 μm at most (20). A large adipocyte may block oxygen diffusion. Additionally, a reduction in adipose tissue blood flow may be another factor (48, 52).
In this study, we observed that all of the hypoxia response genes were increased in the two obesity models except for VEGF. VEGF expression was increased in the dietary obese mice but not in the ob/ob mice. In the cell culture, VEGF expression was upregulated by hypoxia in all of the cells tested in this study. These data suggest that the nonresponsiveness of VEGF in ob/ob mice might be related to a factor that is unique in ob/ob mice. In the two obese models, a major difference is the absence of leptin in ob/ob mice. Leptin was reported to upregulate VEGF transcription through activation of the JAK/STAT3-signaling pathway (47). The leptin signal may be required for the VEGF response to hypoxia in vivo. The lack of VEGF response may lead to relatively poor vascularization, which has been reported in muscular arterioles of ob/ob mice (33).
Our data suggest that reduction of IRβ and IRS-1 by hypoxia may account for insulin resistance in adipose tissue of obese subjects. In this study, we examined many signaling molecules (IR, IRS-1, Akt, GSK-3, mTOR, p70S6K, and GLUT4) in the insulin-signaling pathway. IRβ and IRS-1 were decreased by hypoxia in 3T3-L1 adipocytes. The reduction was observed at 24 h in hypoxia. This effect of hypoxia suggests that IRβ and IRS-1 reduction in the adipose tissue of ob/ob mice may be a result of chronic hypoxia response. This study provides a new explanation for the reduction of IR protein in the adipose tissue of obese subject that has been known for 30 years (2, 13, 35). It was believed that the reduction was a consequence of hyperinsulinemia, which reduces IRβ protein level through ligand-mediated receptor degradation in adipocytes. Although TNF-α is able to reduce IRS-1 protein (22–24, 45), it does not reduce IRβ up to 24 h (45). Inflammation seems not to be able to explain the reduction in both IRβ and IRS-1.
The current study provides evidence that hypoxia regulates glucose metabolism in adipocytes by modifying both insulin-dependent and -independent pathways. Hypoxia is known to enhance insulin-independent glucose uptake in muscle cells (3, 11, 25). However, the effects of hypoxia on adipocytes and β-cells have been less studied. Our data suggest that hypoxia may increase glucose uptake through both insulin-dependent and -independent pathways in a short-term (∼4 h) exposure. Glucose uptake declined in both pathways after the long-term (∼24 h) hypoxia treatment. The insulin-induced glucose uptake was completely blocked after 24-h hypoxia exposure. The inhibition was associated with loss of IRβ and IRS-1 proteins. Since insulin action is critical in the maintenance of adipose tissue function, loss of insulin sensitivity may lead to adipocyte dysfunction in the storage of TAG as observed in this study. This effect of hypoxia seems specific to adipocytes, as the insulin-signaling pathway was not impaired by hypoxia in β-cells and muscle cells.
In this study, we observed that the basal glucose uptake was increased by hypoxia treatment in 3T3-L1 adipocytes. This result is likely a consequence of upregulation of GLUT1 expression by hypoxia. In response to hypoxia, the basal glucose uptake was increased by 142% at 4 h and then by 73 and 50.8% at 8 and 24 h, respectively. The reduction in glucose uptake after 8-h hypoxia treatment may be a result of reduced demand for energy in response to persistent hypoxia. ATP production in mitochondria is reduced by hypoxia. The loss of ATP supply may be responsible for cell necrosis and apoptosis in the current study. The cell death may contribute to the reduction in glucose uptake as well. Another possibility is inhibition of glucose uptake by FFA that is derived from the hypoxia-induced lipolysis. The current study indicates that a 24h hypoxia treatment was able to induce lipolysis in 3T3-L1 adipocytes. A high level of FFA in the medium may interfere with the glucose uptake via lipotoxicity (15). However, in these conditions, the basal glucose uptake remains higher than that in normoxia condition. In the βTC6 cells, the glucose uptake was increased by hypoxia as well, although the response was not as strong as that in 3T3-L1 adipocytes. There are two possibilities for the cell type-associated difference. First, the basal glucose uptake in β-cells is controlled by GLUT2, which is downregulated by hypoxia (36). The reduction in GLUT2 will prevent a huge increase in glucose uptake under hypoxia. Second, many β-cells may have died after hypoxia treatment. The cell death will lead to reduction in glucose uptake in the culture. β-Cells do not tolerate hypoxia well compared with muscle cells (29). Thus, the glucose uptake was not dramatically changed by hypoxia in the β-cells. The GLUT4 protein in 3T3-L1 adipocytes was unchanged by hypoxia treatment in this study. It was reported that, in matured 3T3-L1 adipocytes, the half-life of GLUT4 protein was longer than 16 h (43). In the current study, the test was conducted for 24 h. The literature suggests that hypoxia may reduce GLUT4 mRNA through downregulation of C/EBPα activity (26). A study with a longer exposure time may be required to test the hypoxia effect on GLUT4 expression.
The current study provides direct evidence that hypoxia is able to induce lipolysis and inhibit fatty acid uptake at the same time. Increased release of FFA from adipose tissue is a risk factor for FFA elevation in the circulation that contributes to systemic insulin resistance in obesity (5, 10, 54). Although the FFA release is related to insulin resistance in adipocytes that reduces insulin activity in the inhibition of lipolysis (12), other causes of lipolysis remain to be identified. The current study demonstrated that ATH may be a potential signal to induce FFA release from adipocytes through induction of lipolysis. This possibility is supported by FFA data from the supernatant of hypoxia-treated adipocytes and FFA in the vein blood samples of clamped leg in rats. In vivo, inhibition of adipogenesis by hypoxia may also contribute to the FFA elevation. Generation of new adipocytes from adipogenesis can make more space for storage of FFA in the form of TAG. Hypoxia was reported to inhibit adipocyte differentiation and adipogenesis (8, 16, 28, 58, 59). Downregulation of PPARγ gene expression was proposed as a mechanism of hypoxia action (28, 58, 59). However, the effects of hypoxia on fatty acid uptake and lipolysis in adipocytes were not reported in those earlier studies. In the current study, we have demonstrated that FFA uptake by adipocytes is decreased by hypoxia. The inhibition is associated with a decrease in FATP and CD36 proteins. It is likely that hypoxia reduces FFA uptake by suppression of FFA transporter expression.
Our previous study (55) suggests a potential role of hypoxia in the induction of chronic inflammation and inhibition of adiponectin in adipose tissue in obesity. Hypoxia was able to stimulate the gene expression of proinflammatory cytokines like TNF-α directly in primary adipocytes and macrophages (55). In this study, we observed that hypoxia led to insulin resistance and lipolysis in the adipocytes. However, it was not certain whether the insulin resistance was due to increased proinflammatory cytokines and/or decreased adiponectin secretion. Our conditioned medium experiments revealed that the autocrine effect of adipocytes plays a role in the hypoxia inhibition of insulin signaling.
Induction of adipocyte death may be another mechanism by which hypoxia contributes to chronic inflammation in obesity. Hypoxia was reported to induce an inflammatory response in adipocytes and macrophages (51, 55). Activation of transcription factors such as NF-kB and HIF1α plays an important role in the induction of inflammatory cytokines and chemokines by hypoxia. These cytokines are likely involved in macrophage infiltration into adipose tissue (4, 6, 17). The present study suggests that adipocyte death may be another mechanism by which hypoxia contributes to chronic inflammation. Macrophage infiltration was proposed to remove dead cells in adipose tissue in obesity (9). However, the cause of cell death is not clear in adipose tissue in obesity. We show here that hypoxia induces necrosis and apoptosis in 3T3-L1 adipocytes. In adipose tissue, macrophages were predominantly found in the hypoxic areas in adipose tissue of obese mice (37). The results provide a new mechanism for obesity-associated cell death in adipose tissue. Necrosis and apoptosis are two different types of cell death that are dependent on ATP supply. A complete loss of ATP leads to cell necrosis and a partial loss induces apoptosis. In response to hypoxia, ATP production is reduced as a result of inhibition of mitochondrial respiration. In some cells, this change makes the ATP supply unable to meet the minimal demand for cell survival; then, necrosis occurs. If the cells are able to maintain the minimal ATP supply, they will either survive or become apoptotic. Apoptosis is a programmed cell death that requires ATP. Inhibition of protein synthesis by hypoxia may be involved in the mechanism of mitochondrial inhibition and ATP reduction. An increase in permeability in the inner mitochondrial membrane is observed in hypoxia response (18). In this condition, cell apoptosis is associated with release of cytochrome c. In ischemia research, hypoxia-induced cell death has been well established in cardiac injury and brain damage (44, 49).
In summary, insulin resistance in large adipocytes in adipose tissue is likely a result of hypoxia in obesity. Hypoxia is found in adipose tissue of obese mice and is a potential risk factor for metabolic disorders in the adipose tissue. Hypoxia may inhibit insulin-induced glucose uptake by reducing insulin-signaling molecules IRβ and IRS-1. Hypoxia may stimulate lipolysis and inhibit uptake of FFA in adipocytes leading to FFA elevation in the plasma of obese subjects. Induction of adipocyte necrosis and apoptosis provides a mechanism for cell death observed in adipose tissue of obesity and may serve as a new mechanism for hypoxia-induced macrophage infiltration into adipose tissue. These data suggest that adipose tissue hypoxia may play an important role in adipose dysfunction and the development of insulin resistance in obesity.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-68036 and an American Diabetes Association research award (7-07-RA-189) to J Ye.
Acknowledgments
We thank Marilyn A. Dietrich for excellent technical support on flow cytometry measurement.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bano KA, Batool A. Metabolic syndrome, cardiovascular disease and type-2 diabetes. J Pak Med Assoc 57: 511–515, 2007. [PubMed] [Google Scholar]
- 2.Bar RS, Gorden P, Roth J, Kahn CR, De Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest 58: 1123–1135, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashan N, Burdett E, Hundal HS, Klip A. Regulation of glucose transport and GLUT1 glucose transporter expression by O2 in muscle cells in culture. Am J Physiol Cell Physiol 262: C682–C690, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17: 4–12, 2006. [PubMed] [Google Scholar]
- 5.Boden G Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3–10, 1997. [PubMed] [Google Scholar]
- 6.Bouloumie A, Curat CA, Sengenes C, Lolmede K, Miranville A, Busse R. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care 8: 347–354, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Brook CG, Lloyd JK, Wolf OH. Relation between age of onset of obesity and size and number of adipose cells. Br Med J 2: 25–27, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B, Lam KS, Wang Y, Wu D, Lam MC, Shen J, Wong L, Hoo RL, Zhang J, Xu A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun 341: 549–556, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46: 2347–2355, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care 10: 142–148, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Derave W, Hespel P. Role of adenosine in regulating glucose uptake during contractions and hypoxia in rat skeletal muscle. J Physiol 515: 255–263, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling HJ, Fried SK, Pi-Sunyer FX. Insulin resistance in adipocytes of obese women: effects of body fat distribution and race. Metabolism 44: 987–995, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Freychet P, Forgue E, Le Marchand Y, Laudat MH. Decreased number of insulin receptors in obesity: studies in the obese hyperglycemic mouse. Ann Endocrinol (Paris) 37: 87–88, 1976. [PubMed] [Google Scholar]
- 14.Gao Z, He Q, Peng B, Chiao PJ, Ye J. Regulation of nuclear translocation of HDAC3 by IkappaBalpha is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor gamma function. J Biol Chem 281: 4540–4547, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 18: 2024–2034, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gentil C, Le Jan S, Philippe J, Leibowitch J, Sonigo P, Germain S, Pietri-Rouxel F. Is oxygen a key factor in the lipodystrophy phenotype? Lipids Health Dis 5: 27, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goossens GH The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav : xx–xx, 2007. [DOI] [PubMed]
- 18.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol 57: 1009–1014, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Z, Zhou L. Muscle type-dependent responses to insulin in intramyocellular triglyceride turnover in obese rats. Obes Res 13: 2081–2087, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and Po2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 3: 177–182, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest 94: 1543–1549, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA 91: 4854–4858, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes 43: 1271–1278, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Idstrom JP, Rennie MJ, Schersten T, Bylund-Fellenius AC. Membrane transport in relation to net uptake of glucose in the perfused rat hindlimb Stimulatory effect of insulin, hypoxia and contractile activity. Biochem J 233: 131–137, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaestner KH, Christy RJ, Lane MD. Mouse insulin-responsive glucose transporter gene: characterization of the gene and trans-activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci USA 87: 251–255, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100: 1589–1596, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Kim KH, Song MJ, Chung J, Park H, Kim JB. Hypoxia inhibits adipocyte differentiation in a HDAC-independent manner. Biochem Biophys Res Commun 333: 1178–1184, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Ko SH, Ryu GR, Kim S, Ahn YB, Yoon KH, Kaneto H, Ha H, Kim YS, Song KH. Inducible nitric oxide synthase-nitric oxide plays an important role in acute and severe hypoxic injury to pancreatic beta cells. Transplantation 85: 323–330, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinases/AKT by saturated and polyunsaturated fatty acids. J Biol Chem 278: 37041–37051, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Liao W, Nguyen MT, Imamura T, Singer O, Verma IM, Olefsky JM. Lentiviral short hairpin ribonucleic acid-mediated knockdown of GLUT4 in 3T3-L1 adipocytes. Endocrinology 147: 2245–2252, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Liao W, Nguyen MT, Yoshizaki T, Favelyukis S, Patsouris D, Imamura T, Verma IM, Olefsky JM. Suppression of PPARγ attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 293: E219–E227, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Lindstrom P The physiology of obese-hyperglycemic mice [ob/ob mice]. ScientificWorldJournal 7: 666–685, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lobo S, Wiczer BM, Smith AJ, Hall AM, Bernlohr DA. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J Lipid Res 48: 609–620, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Olefsky JM, Reaven GM. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology 96: 1486–1498, 1975. [DOI] [PubMed] [Google Scholar]
- 36.Park SK, Haase VH, Johnson RS. von Hippel Lindau tumor suppressor regulates hepatic glucose metabolism by controlling expression of glucose transporter 2 and glucose 6-phosphatase. Int J Oncol 30: 341–348, 2007. [PubMed] [Google Scholar]
- 37.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32: 451–463, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Salans LB, Dougherty JW. The effect of insulin upon glucose metabolism by adipose cells of different size. Influence of cell lipid and protein content, age, and nutritional state. J Clin Invest 50: 1399–1410, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest 47: 153–165, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segura J, Ruilope LM. Obesity, essential hypertension and renin-angiotensin system. Public Health Nutr 10: 1151–1155, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Semenza G Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 64: 993–998, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell 9: 99–108, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Shohet RV, Garcia JA. Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. J Mol Med 85: 1309–1315, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem 272: 971–976, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56: 2910–2918, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Suganami E, Takagi H, Ohashi H, Suzuma K, Suzuma I, Oh H, Watanabe D, Ojima T, Suganami T, Fujio Y, Nakao K, Ogawa Y, Yoshimura N. Leptin stimulates ischemia-induced retinal neovascularization: possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes 53: 2443–2448, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Summers LK, Samra JS, Humphreys SM, Morris RJ, Frayn KN. Subcutaneous abdominal adipose tissue blood flow: variation within and between subjects and relationship to obesity. Clin Sci (Lond) 91: 679–683, 1996. [DOI] [PubMed] [Google Scholar]
- 49.Talhouk RS, Zeinieh MP, Mikati MA, El-Sabban ME. Gap junctional intercellular communication in hypoxia-ischemia-induced neuronal injury. Prog Neurobiol 84: 57–76, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92: 347–355, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflügers Arch 455: 479–492, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West DB, Prinz WA, Francendese AA, Greenwood MR. Adipocyte blood flow is decreased in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 253: R228–R233, 1987. [DOI] [PubMed] [Google Scholar]
- 53.Wood IS, Wang B, Lorente-Cebrian S, Trayhurn P. Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-d-glucose uptake in human adipocytes. Biochem Biophys Res Commun 361: 468–473, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye J Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle. Endocr Metab Immune Disord Drug Targets 7: 65–74, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Ye J, Kraegen T. Insulin resistance: central and peripheral mechanisms. The 2007 Stock Conference Report. Obes Rev 9: 30–34, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab 294: E148–E156, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell 2: 331–341, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-beta/Smad3 signaling. J Biol Chem 280: 22688–22696, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]