Abstract
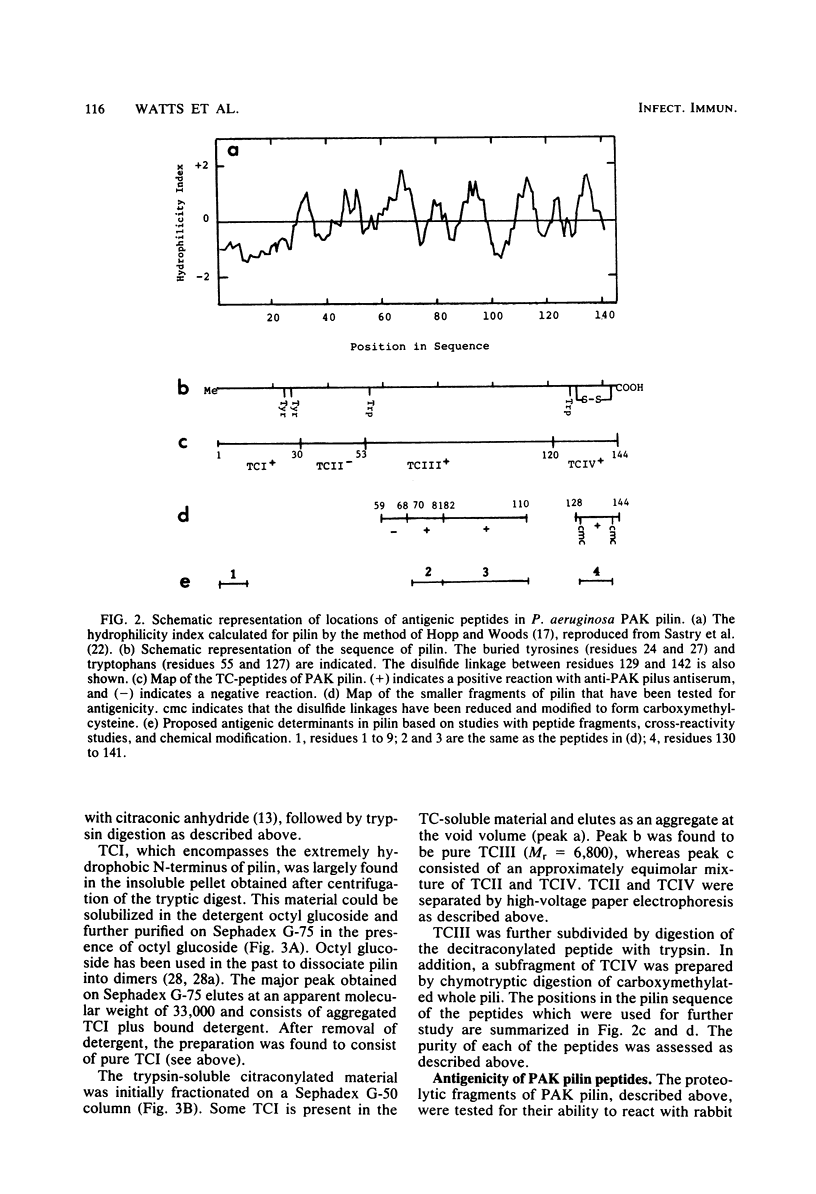
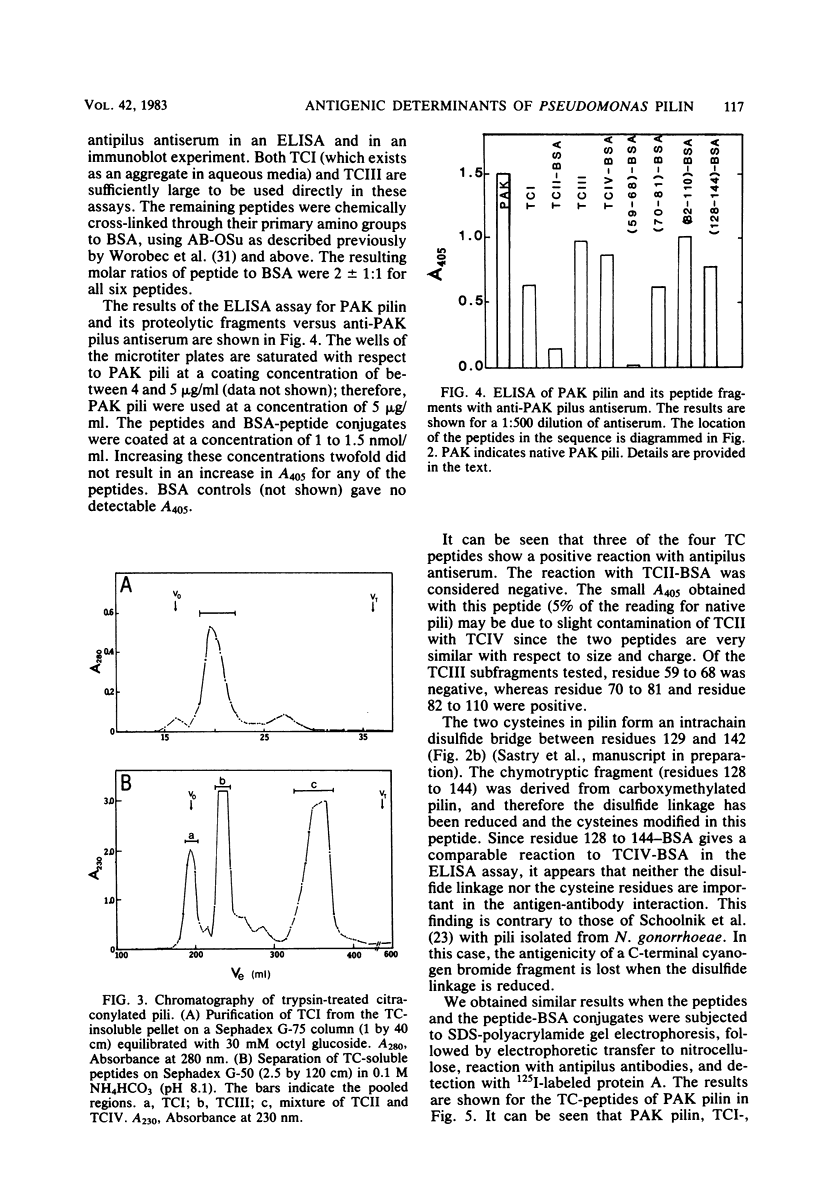
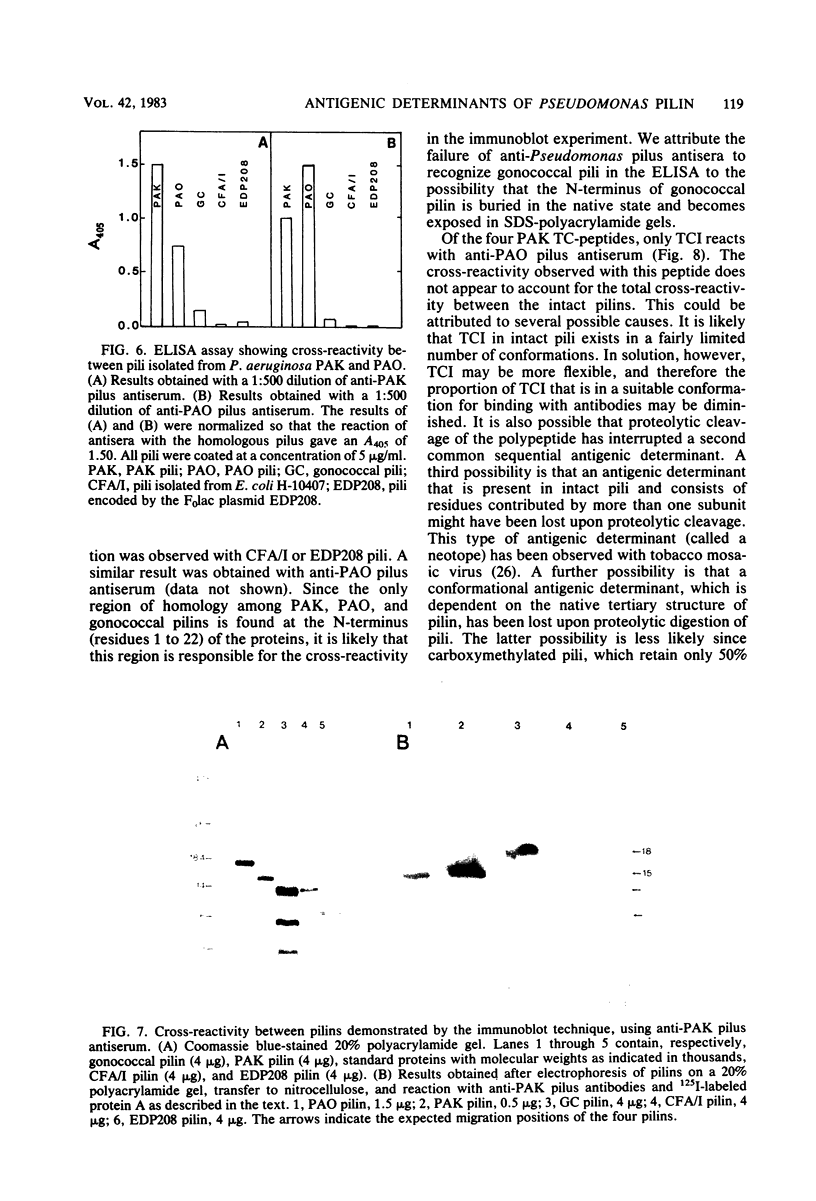
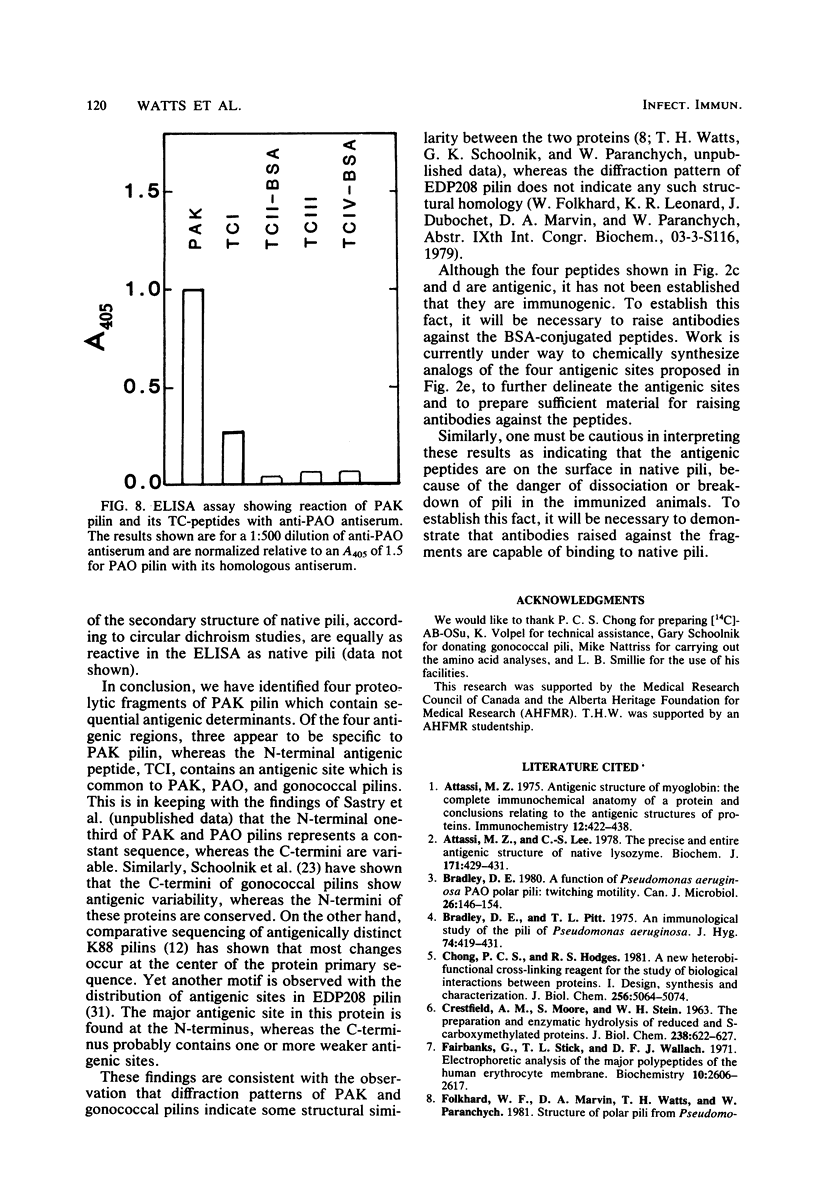
The polar pili of Pseudomonas aeruginosa are flexible filaments 5.2 nm in diameter and 2.5 microns in average length. They consist of a single subunit, pilin, which is a 144-residue polypeptide containing a hydrophobic N-terminal region (residues 1 to 30) and eight hydrophilic regions distributed throughout the remainder of the molecule. To delineate the antigenic regions of pilin, we cleaved the protein at Arg30, Arg53, and Arg120 to produce peptides TCI (residues 1 to 30), TCII (31 to 53), TCIII (54 to 120), and TCIV (121 to 144). TCIII and TCIV were further cleaved into several subfragments. The purified peptides were coupled to bovine serum albumin by using the N-hydroxysuccinimide ester of 4-azidobenzoic acid and were then subjected to immunological analysis, using the enzyme-linked immunosorbent assay and immunoblot procedures with polyclonal antiserum. Four antigenic regions were identified; one in TCI was found to be common to both PAK and PAO pilin. The remaining three were found to be specific to PAK pilin. Two of these were subfragments of TCIII, whereas the third was located close to the C-terminus of the molecule, most likely between Cys129 and Cys142. Modification of these cysteines by reduction and carboxymethylation of the disulfide linkage did not abolish the antigenicity of the C-terminal type-specific antigenic determinant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z., Lee C. L. The precise and entire antigenic structure of native lysozyme. Biochem J. 1978 May 1;171(2):429–434. doi: 10.1042/bj1710429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can J Microbiol. 1980 Feb;26(2):146–154. doi: 10.1139/m80-022. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. An immunological study of the pili of Pseudomonas aeruginosa. J Hyg (Lond) 1975 Jun;74(3):419–430. doi: 10.1017/s0022172400046933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chong P. C., Hodges R. S. A new heterobifunctional cross-linking reagent for the study of biological interactions between proteins. I. Design, synthesis, and characterization. J Biol Chem. 1981 May 25;256(10):5064–5070. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Folkhard W., Marvin D. A., Watts T. H., Paranchych W. Structure of polar pili from Pseudomonas aeruginosa strains K and O. J Mol Biol. 1981 Jun 15;149(1):79–93. doi: 10.1016/0022-2836(81)90261-8. [DOI] [PubMed] [Google Scholar]
- Froholm L. O., Sletten K. Purification and N-terminal sequence of a fimbrial protein from Moraxella nonliquefaciens. FEBS Lett. 1977 Jan 15;73(1):29–32. doi: 10.1016/0014-5793(77)80008-2. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Carpenter M., Paranchych W. N-methylphenylalanine at the N-terminus of pilin isolated from Pseudomonas aeruginosa K. Nature. 1978 Jan 5;271(5640):87–89. doi: 10.1038/271087a0. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W. Composition and molecular weight of pili purified from Pseudomonas aeruginosa K. J Bacteriol. 1977 Jul;131(1):259–269. doi: 10.1128/jb.131.1.259-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote J. G., Haworth C. An improved technique for the analysis of amino acids and related compounds on thin layers of cellulose. II. The quantitative determination of amino acids in protein hydrolysates. J Chromatogr. 1969 Aug 5;43(1):84–92. doi: 10.1016/s0021-9673(00)99169-6. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Oudin J. Immunochemical analysis by antigen-antibody precipitation in gels. Methods Enzymol. 1980;70(A):166–198. doi: 10.1016/s0076-6879(80)70048-4. [DOI] [PubMed] [Google Scholar]
- Paranchych W., Frost L. S., Carpenter M. N-Terminal amino acid sequence of pilin isolated from Pseudomonas aeruginosa. J Bacteriol. 1978 Jun;134(3):1179–1180. doi: 10.1128/jb.134.3.1179-1180.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Frost L. S., Carpenter M., Armstrong G. D., Watts T. H. Biochemical studies on pili isolated from Pseudomonas aeruginosa strain PAO. Can J Microbiol. 1979 Oct;25(10):1175–1181. doi: 10.1139/m79-182. [DOI] [PubMed] [Google Scholar]
- Sastry P. A., Pearlstone J. R., Smillie L. B., Paranchych W. Amino acid sequence of pilin isolated from pseudomonas aeruginosa PAK. FEBS Lett. 1983 Jan 24;151(2):253–256. doi: 10.1016/0014-5793(83)80080-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang L. W., Marusyk R. G. Purification and partial characterization of a 33 000 molecular weight endonuclease associated with human adenovirus type 5. Can J Microbiol. 1980 Oct;26(10):1224–1231. doi: 10.1139/m80-204. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D., Huldt G., Engvall E. A microplate method of enzyme-linked immunosorbent assay and its application to malaria. Bull World Health Organ. 1974;51(2):209–211. [PMC free article] [PubMed] [Google Scholar]
- Watts T. H., Kay C. M., Paranchych W. Dissociation and characterization of pilin isolated from Pseudomonas aeruginosa strains PAK and PAO. Can J Biochem. 1982 Sep;60(9):867–872. doi: 10.1139/o82-110. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Kay C. M., Paranchych W. Spectral properties of three quaternary arrangements of Pseudomonas pilin. Biochemistry. 1983 Jul 19;22(15):3640–3646. doi: 10.1021/bi00284a016. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Worobec E. A., Paranchych W. Identification of pilin pools in the membranes of Pseudomonas aeruginosa. J Bacteriol. 1982 Nov;152(2):687–691. doi: 10.1128/jb.152.2.687-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobec E. A., Taneja A. K., Hodges R. S., Paranchych W. Localization of the major antigenic determinant of EDP208 pili at the N-terminus of the pilus protein. J Bacteriol. 1983 Feb;153(2):955–961. doi: 10.1128/jb.153.2.955-961.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]