Abstract
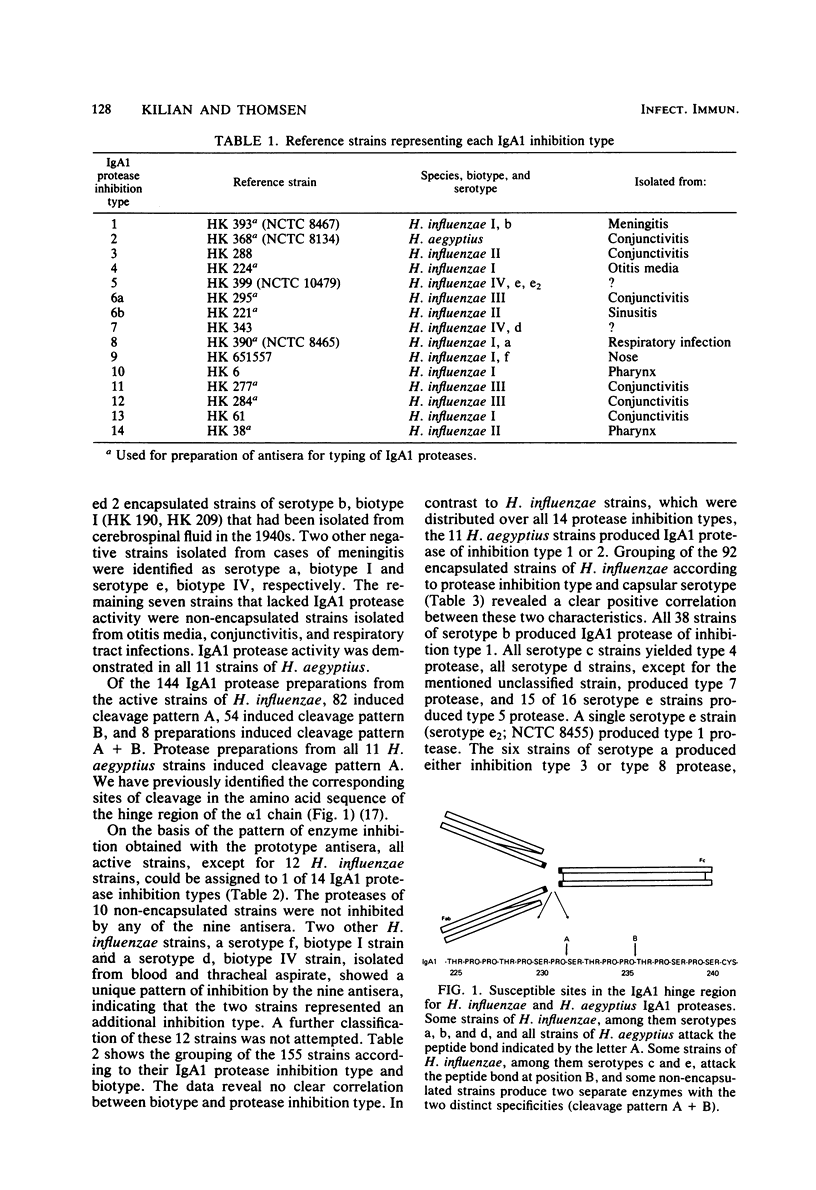
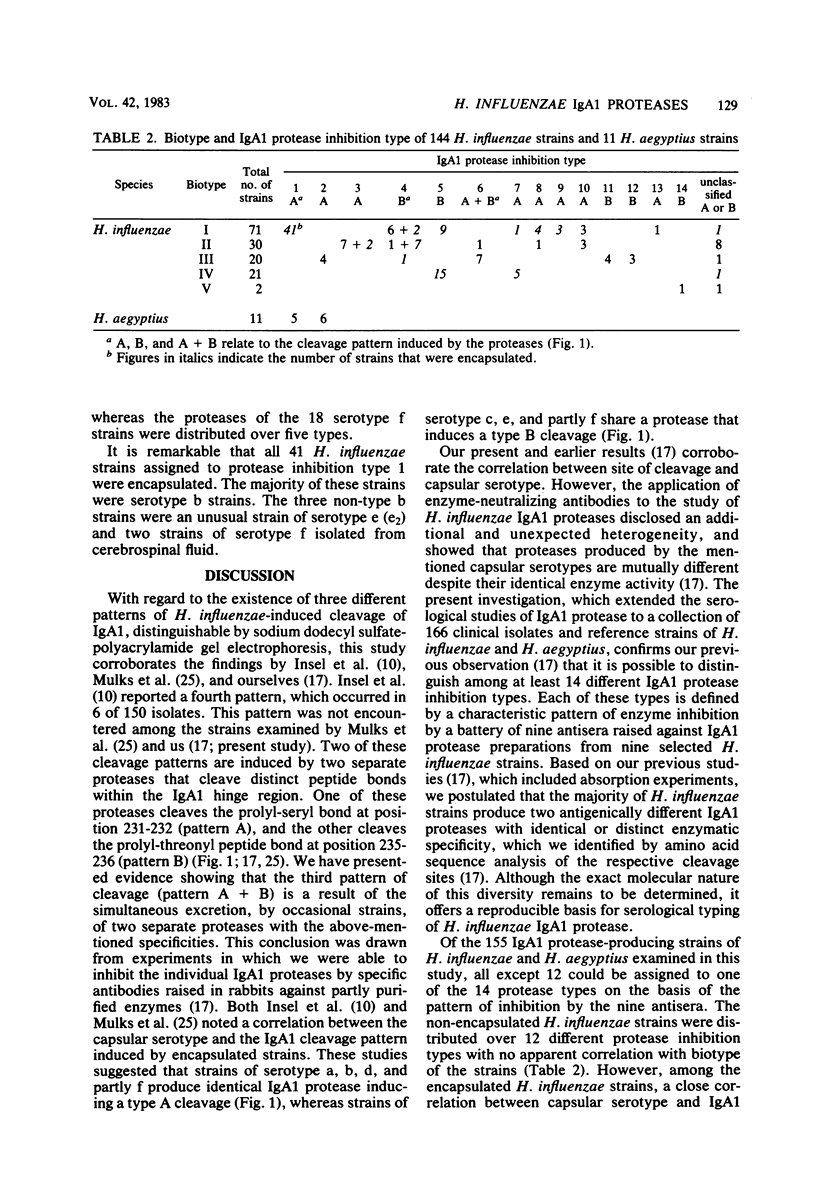
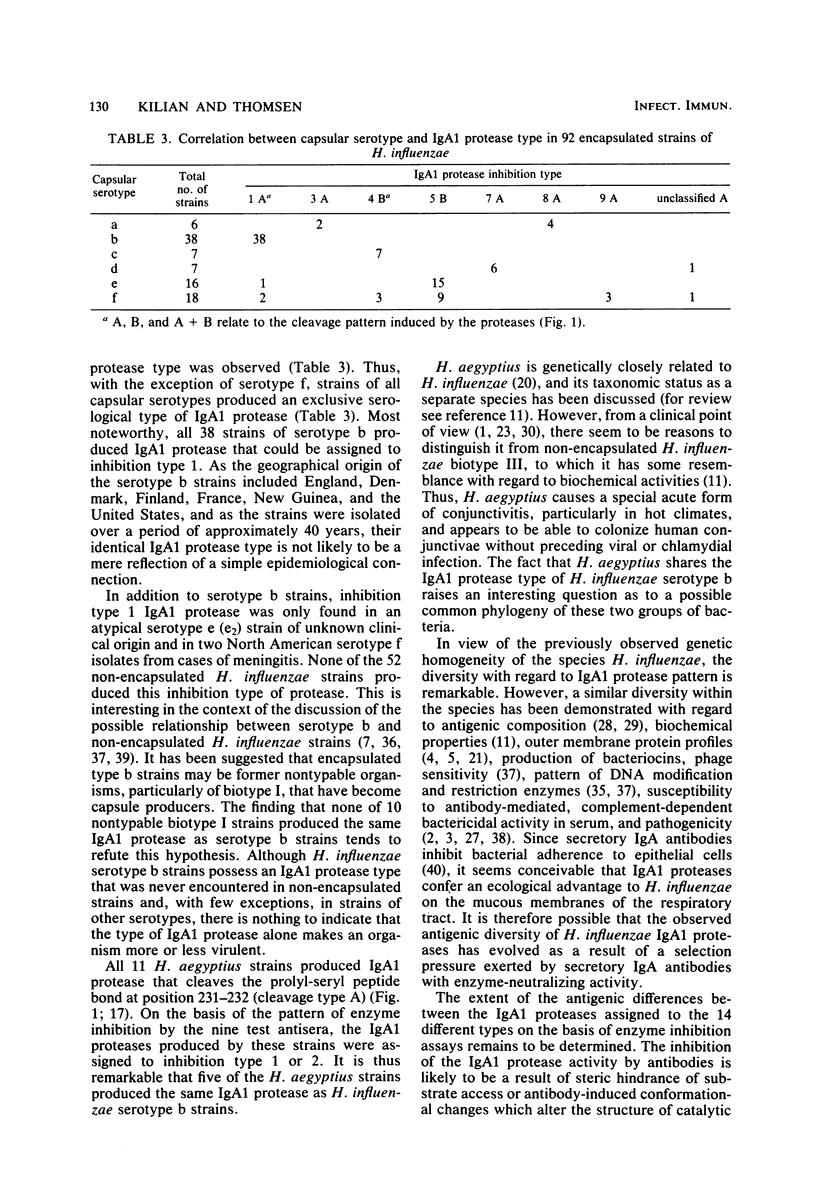
Indirect evidence suggests that immunoglobulin A1 (IgA1) proteases may be factors in the pathogenesis of certain infectious diseases, including meningitis, gonorrhoea, and destructive periodontitis. Bacterial IgA1 proteases are therefore potential candidates as vaccines. In this study, IgA1 proteases from 166 clinical isolates and reference strains of Haemophilus influenzae and Haemophilus aegyptius were compared with regard to specific activity and pattern of enzyme inhibition by antisera raised against IgA1 protease from nine selected strains of H. influenzae. A total of 93% of H. influenzae strains and all H. aegyptius strains had detectable IgA1 protease activity. The majority of strains cleaved a prolyl-seryl or a prolyl-threonyl peptide bond in the alpha 1 hinge region, whereas occasional H. influenzae strains possessed two separate IgA1 proteases with these two specific activities. Of the 155 IgA1 protease-producing strains, all except 12 could be assigned to one of 14 IgA1 protease "inhibition types," each defined by a characteristic pattern of inhibition by the nine antisera. There was no correlation between IgA1 protease type and biotype of the strains. However, among 92 encapsulated H. influenzae strains, a close correlation between capsular serotype and IgA1 protease type was observed. With the exception of serotype f, strains of all capsular serotypes produced an exclusive antigenic type of IgA1 protease. All 38 strains of serotype b produced IgA1 protease of inhibition type 1, which was never demonstrated in non-encapsulated H. influenzae strains. These results facilitate the detection of an antibody response against specific IgA1 proteases and are of practical value for a possible future vaccine against H. influenzae serotype b infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L. Infections due to Haemophilus species other than H. influenzae. Annu Rev Microbiol. 1982;36:199–216. doi: 10.1146/annurev.mi.36.100182.001215. [DOI] [PubMed] [Google Scholar]
- Anderson P., Flesher A., Shaw S., Harding A. L., Smith D. H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Invest. 1980 Apr;65(4):885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982 May;36(2):535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Swanson J. Studies on gonococcus infection. XVI. Purification of Neisseria gonorrhoeae immunoglobulin A1 protease. Infect Immun. 1978 Nov;22(2):350–358. doi: 10.1128/iai.22.2.350-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R. J., Plaut A. G., Moellering R. C., Jr Evaluation of human oral organisms and pathogenic Streptococcus for production of IgA protease. J Infect Dis. 1975 May;131 (Suppl):S17–S21. doi: 10.1093/infdis/131.supplement.s17. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Virella G., Cardenas R., Koistinen J., Fett J. W. New method for obtaining IgA-specific protease. J Immunol Methods. 1977;18(3-4):245–249. doi: 10.1016/0022-1759(77)90178-8. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Kilian M. Degradation of immunoglobulins A2, A2, and G by suspected principal periodontal pathogens. Infect Immun. 1981 Dec;34(3):757–765. doi: 10.1128/iai.34.3.757-765.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Holmgren K. Ecology and nature of immunoglobulin A1 protease-producing streptococci in the human oral cavity and pharynx. Infect Immun. 1981 Mar;31(3):868–873. doi: 10.1128/iai.31.3.868-873.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Schrohenloher R. E. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979 Oct;26(1):143–149. doi: 10.1128/iai.26.1.143-149.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Sørensen I., Frederiksen W. Biochemical characteristics of 130 recent isolates from Haemophilus influenzae meningitis. J Clin Microbiol. 1979 Mar;9(3):409–412. doi: 10.1128/jcm.9.3.409-412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Thomsen B., Petersen T. E., Bleeg H. S. Occurrence and nature of bacterial IgA proteases. Ann N Y Acad Sci. 1983 Jun 30;409:612–624. doi: 10.1111/j.1749-6632.1983.tb26903.x. [DOI] [PubMed] [Google Scholar]
- Kilian M., Thomsen B., Petersen T. E., Bleeg H. Molecular biology of Haemophilus influenzae IgA1 proteases. Mol Immunol. 1983 Sep;20(9):1051–1058. doi: 10.1016/0161-5890(83)90046-9. [DOI] [PubMed] [Google Scholar]
- LEIDY G., JAFFEE I., ALEXANDER H. E. FURTHER EVIDENCE OF A HIGH DEGREE OF GENETIC HOMOLOGY BETWEEN H. INFLUENZAE AND H. AEGYPTIUS. Proc Soc Exp Biol Med. 1965 Mar;118:671–679. doi: 10.3181/00379727-118-29935. [DOI] [PubMed] [Google Scholar]
- Labib R. S., Calvanico N. J., Tomasi T. B., Jr Studies on extracellular proteases of Streptococcus sanguis. Purification and characterization of a human IgA1 specific protease. Biochim Biophys Acta. 1978 Oct 12;526(2):547–559. doi: 10.1016/0005-2744(78)90145-6. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male C. J. Immunoglobulin A1 protease production by Haemophilus influenzae and Streptococcus pneumoniae. Infect Immun. 1979 Oct;26(1):254–261. doi: 10.1128/iai.26.1.254-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazloum H. A., Kilian M., Mohamed Z. M., Said M. D. Differentiation of Haemophilus aegyptius and Haemophilus influenzae. Acta Pathol Microbiol Immunol Scand B. 1982 Apr;90(2):109–112. doi: 10.1111/j.1699-0463.1982.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Hammack W. J., Kulhavy R., Wright G. P., Tomana M. Properties of IgA myeloma proteins isolated rom sera of patients with the hyperviscosity syndrome. J Lab Clin Med. 1977 May;89(5):919–927. [PubMed] [Google Scholar]
- Mulks M. H., Kornfeld S. J., Frangione B., Plaut A. G. Relationship between the specificity of IgA proteases and serotypes in Haemophilus influenzae. J Infect Dis. 1982 Aug;146(2):266–274. doi: 10.1093/infdis/146.2.266. [DOI] [PubMed] [Google Scholar]
- Mulks M. H., Plaut A. G., Feldman H. A., Frangione B. IgA proteases of two distinct specificities are released by Neisseria meningitidis. J Exp Med. 1980 Nov 1;152(5):1442–1447. doi: 10.1084/jem.152.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden C. W. Variable susceptibility of Hemophilus influenzae, type B strains to serum bactericidal activity. Proc Soc Exp Biol Med. 1972 Jan;139(1):59–61. doi: 10.3181/00379727-139-36076. [DOI] [PubMed] [Google Scholar]
- PITTMAN M., DAVIS D. J. Identification of the Koch-Weeks bacillus (Hemophilus aegyptius). J Bacteriol. 1950 Mar;59(3):413–426. doi: 10.1128/jb.59.3.413-426.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. VARIATION AND TYPE SPECIFICITY IN THE BACTERIAL SPECIES HEMOPHILUS INFLUENZAE. J Exp Med. 1931 Mar 31;53(4):471–492. doi: 10.1084/jem.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Artenstein M. S., Capra J. D. Neisseria gonorrhoeae and neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975 Dec 12;190(4219):1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Gilbert J. V., Heller I. Assay and properties of IgA protease of Streptococcus sanguis. Adv Exp Med Biol. 1978;107:489–495. doi: 10.1007/978-1-4684-3369-2_55. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. Microbial IgA proteases. N Engl J Med. 1978 Jun 29;298(26):1459–1463. doi: 10.1056/NEJM197806292982608. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. On the nature of nontypable Haemophilus influenzae. Antonie Van Leeuwenhoek. 1978;44(3-4):367–376. doi: 10.1007/BF00394313. [DOI] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]


