Abstract
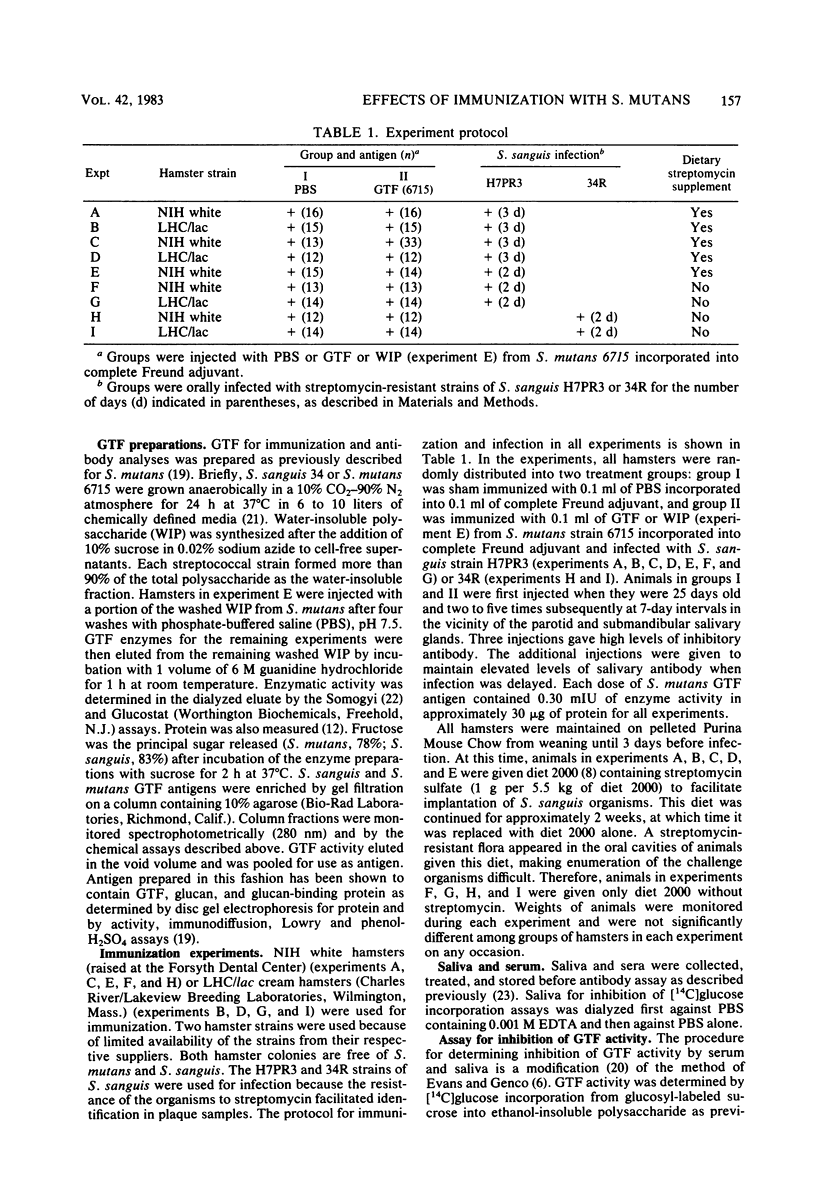
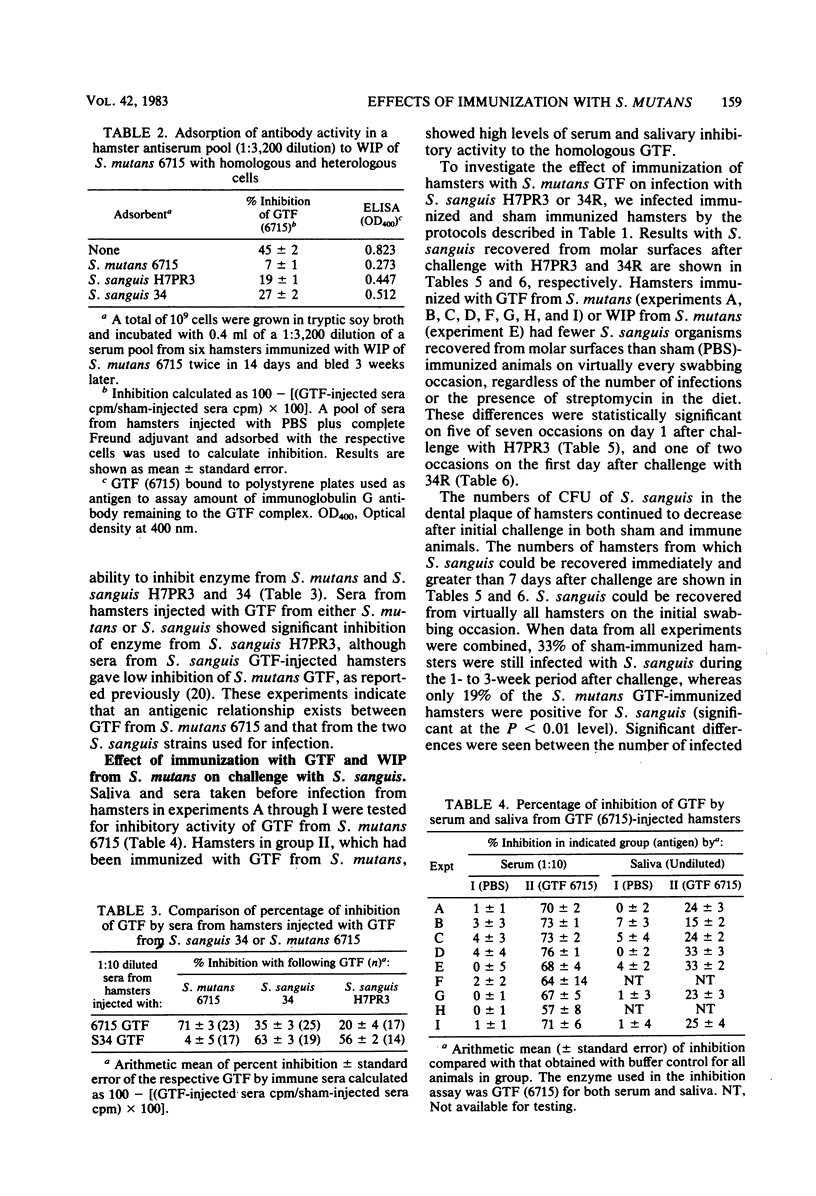
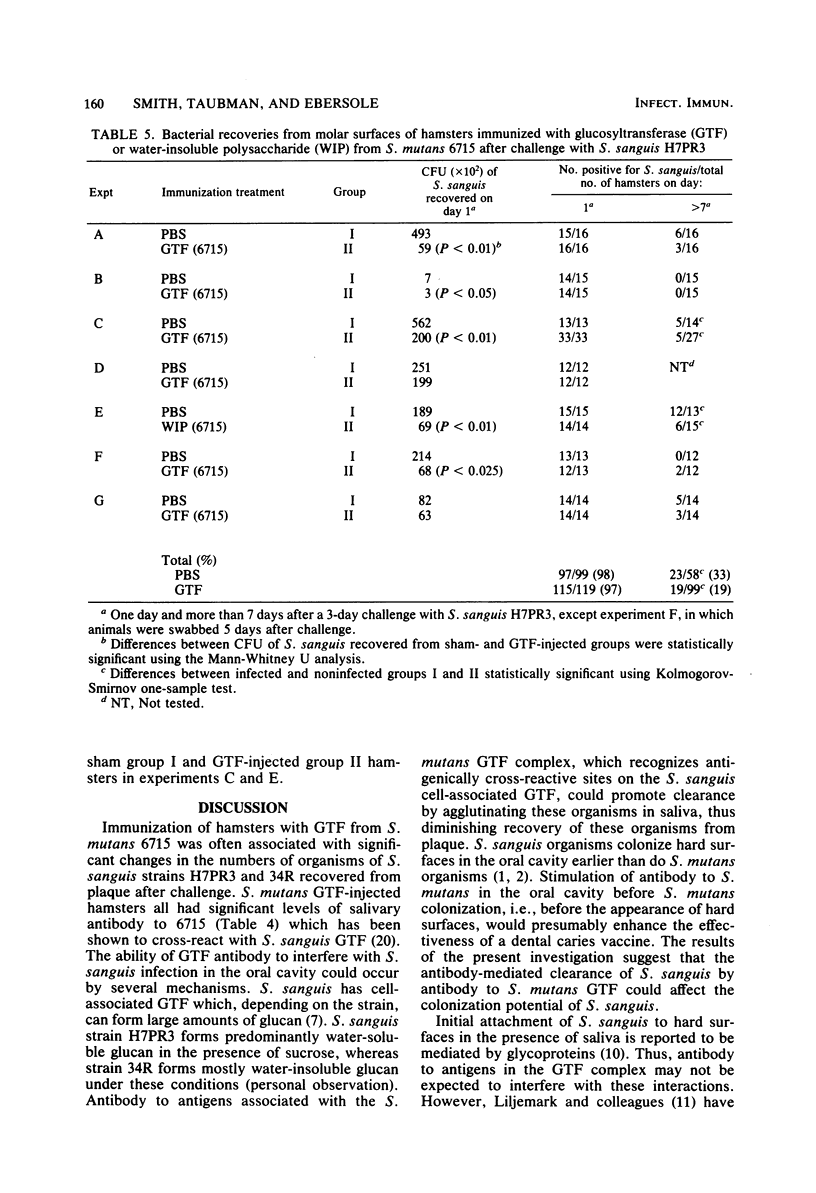
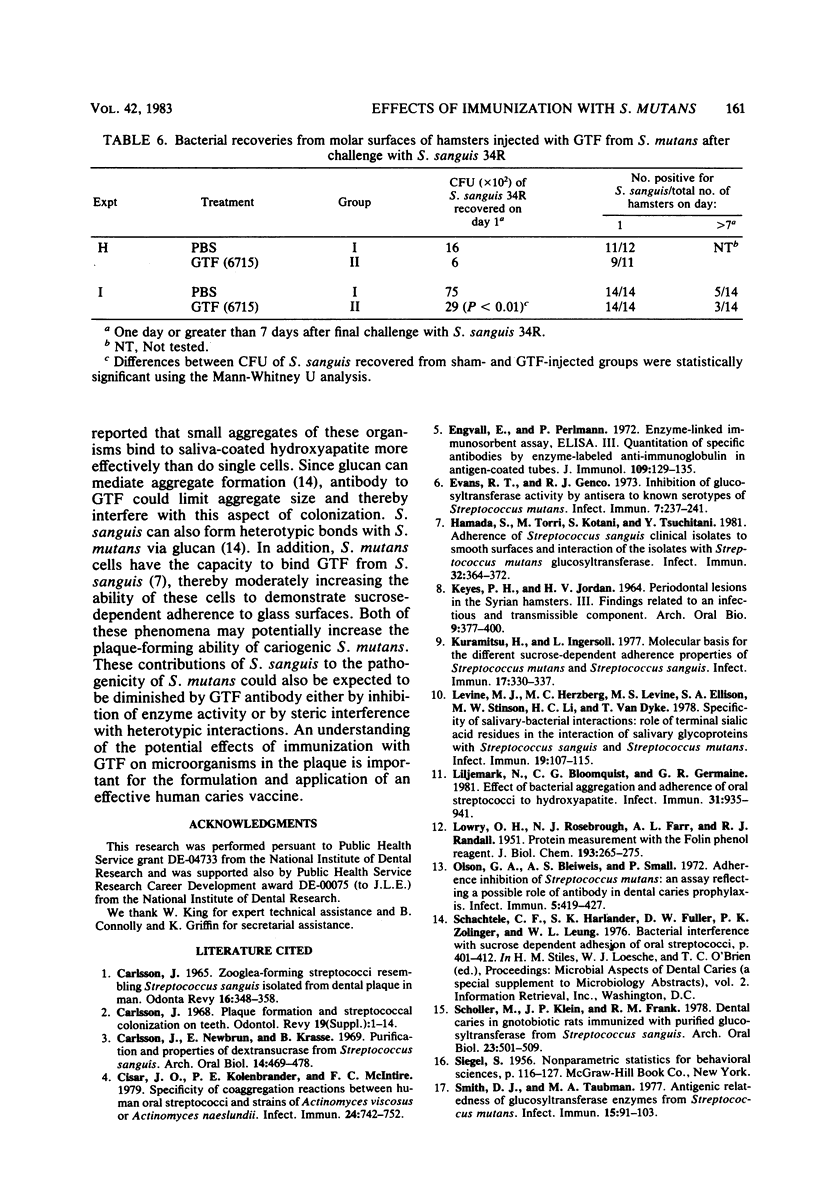
The effects of immunization with antigens of the Streptococcus mutans glucosyltransferase (GTF) complex on oral challenge with two Streptococcus sanguis strains (H7PR3 and 34) in hamsters were studied. Antisera to S. mutans GTF complex were able to inhibit one-third (strain H7PR3) to one-half (strain 34) of the S. sanguis GTF activity which could be inhibited when these S. sanguis GTFs were incubated with antisera to S. sanguis GTF. Washed, intact cells of strains H7PR3 and 34 were able to remove a significant amount of enzyme inhibitory activity and immunoglobulin G antibody activity from antisera to S. mutans GTF. These results established the existence of an antigenic relationship between S. sanguis and S. mutans GTFs. The effect of injection of S. mutans strain 6715 GTF or phosphate-buffered saline, incorporated into complete Freund adjuvant, on oral challenge with S. sanguis was compared in 243 hamsters in nine experiments. Salivary and serum GTF inhibitory activity was present in all GTF-injected animals before challenge. After a 2- or 3-day challenge with S. sanguis H7PR3 (seven experiments) or 34 (two experiments), fewer bacteria were recovered from GTF-injected hamsters in every experiment. Significant differences were observed in six of the nine experiments. During the 7- to 21-day period after challenge, 33% of the phosphate-buffered saline-injected sham group (group I) still had S. sanguis recoverable from the molar surfaces, whereas only 19% of the S. mutans GTF-injected group (group II) remained infected with S. sanguis (P less than 0.01). These results suggest that immunization with GTF from S. mutans may influence the colonization potential of S. sanguis in the oral cavity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson J., Newbrun E., Krasse B. Purification and properties of dextransucrase from Streptococcus sanguis. Arch Oral Biol. 1969 May;14(5):469–478. doi: 10.1016/0003-9969(69)90140-x. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Zooglea-forming streptococci, resembling Streptococcus sanguis, isolated from dental plaque in man. Odontol Revy. 1965;16(4):348–358. [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Torii M., Kotani S., Tsuchitani Y. Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interactions of the isolates with Streptococcus mutans glucosyltransferase. Infect Immun. 1981 Apr;32(1):364–372. doi: 10.1128/iai.32.1.364-372.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYES P. H., JORDAN H. V. PERIODONTAL LESIONS IN THE SYRIAN HAMSTER. III. FINDINGS RELATED TO AN INFECTIOUS AND TRANSMISSIBLE COMPONENT. Arch Oral Biol. 1964 Jul-Aug;9:377–400. doi: 10.1016/0003-9969(64)90024-x. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Molecular basis for the different sucrose-dependent adherence properties of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1977 Aug;17(2):330–337. doi: 10.1128/iai.17.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Germaine G. R. Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect Immun. 1981 Mar;31(3):935–941. doi: 10.1128/iai.31.3.935-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöller M., Klein J. P., Frank R. M. Dental caries in gnotobiotic rats immunized with purified glucosyltransferase from Streptococcus sanguis. Arch Oral Biol. 1978;23(6):501–504. doi: 10.1016/0003-9969(78)90084-5. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A. Antigenic relatedness of glucosyltransferase enzymes from streptococcus mutans. Infect Immun. 1977 Jan;15(1):91–103. doi: 10.1128/iai.15.1.91-103.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Effects of local immunization of hamsters with glucosyltransferase antigens from Streptococcus sanguis on dental caries caused by Streptococcus mutans. Arch Oral Biol. 1981;26(11):871–878. doi: 10.1016/0003-9969(81)90145-x. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in hamsters caused by homologous and heterologous serotypes of Streptococcus mutans. Infect Immun. 1978 Sep;21(3):843–851. doi: 10.1128/iai.21.3.843-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Preparation of glucosyltransferase from Streptococcus mutans by elution from water-insoluble polysaccharide with a dissociating solvent. Infect Immun. 1979 Feb;23(2):446–452. doi: 10.1128/iai.23.2.446-452.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1977 Feb;118(2):710–720. [PubMed] [Google Scholar]
- Wood J. M. A dextransucrase activity from Streptococcus FA-1. Arch Oral Biol. 1967 Dec;12(12):1659–1660. doi: 10.1016/0003-9969(67)90202-6. [DOI] [PubMed] [Google Scholar]
- Zachrisson B. U. Mast cells of the human gingiva. I. Investigations concerning the preservation and demonstration of mast cells in the gingival area. Odontol Revy. 1968;19(1):1–22. [PubMed] [Google Scholar]


