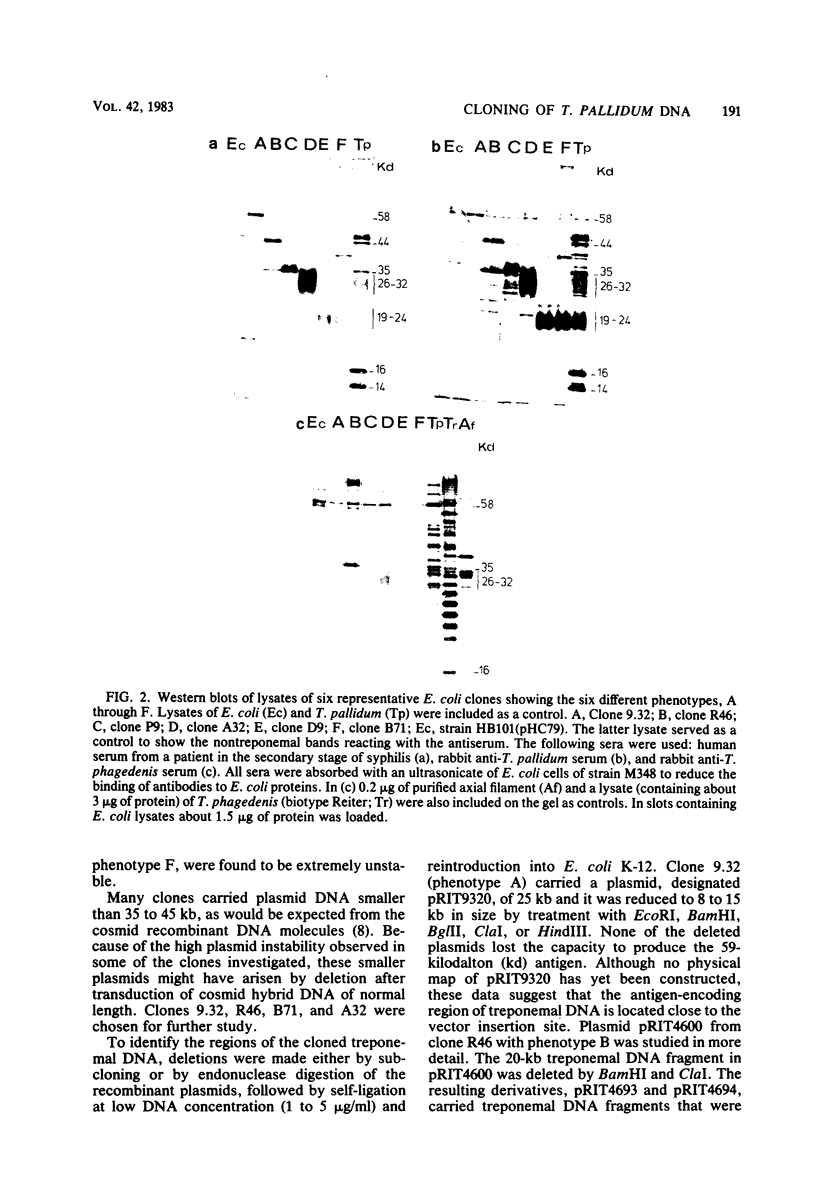
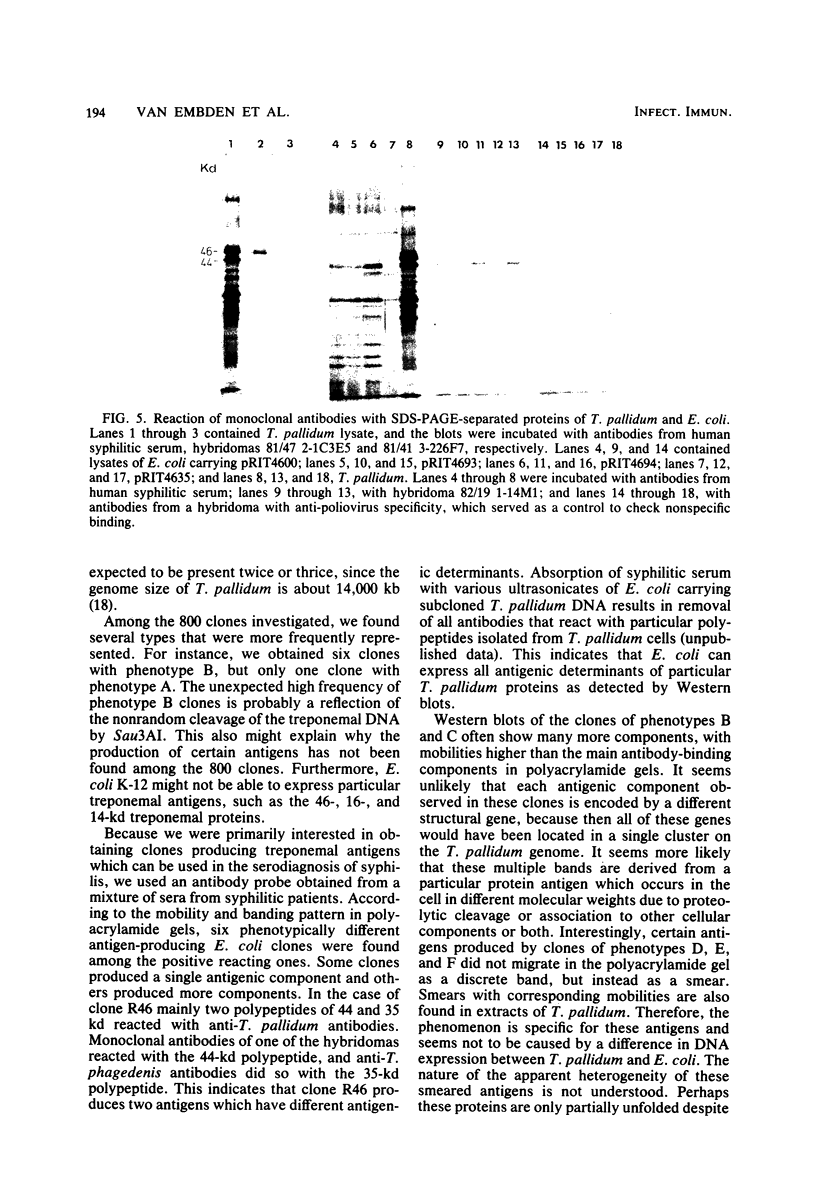
Abstract
A gene bank of Treponema pallidum DNA in Escherichia coli K-12 was constructed by cloning SauI-cleaved T. pallidum DNA into the cosmid pHC79. Sixteen of 800 clones investigated produced one or more antigens that reacted with antibodies from syphilitic patients. According to the separation pattern of the antigens produced on sodium dodecyl sulfate-polyacrylamide gels, six different phenotypes were distinguished among these 16 clones. These antigens reacted also with anti-T. pallidum rabbit serum. No antibodies against the cloned antigens were found in normal rabbit serum and in nonsyphilitic human serum. The antigens produced by the E. coli K-12 recombinant DNA clones comigrated in sodium dodecyl sulfate-polyacrylamide gels with antigens extracted from T. pallidum bacteria, suggesting that the treponemal DNA is well expressed in E. coli K-12. Several of the cosmid recombinant plasmids have been subcloned, resulting in smaller T. pallidum recombinant plasmids which are more stably maintained in the cell and produce more treponemal antigen. Monoclonal antibodies were raised against T. pallidum, and one hybridoma produced antibodies that reacted not only with an antigen from T. pallidum but also with the antigen produced by one of the E. coli clones.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Analysis of serum IgG against Treponema pallidum protein antigens in experimentally infected rabbits. Br J Vener Dis. 1981 Oct;57(5):302–308. doi: 10.1136/sti.57.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface characterization of virulent Treponema pallidum. Infect Immun. 1980 Dec;30(3):814–823. doi: 10.1128/iai.30.3.814-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Rumpp J. W., Hayes N. S. Purification of Treponema pallidum from Infected Rabbit Tissue: Resolution into Two Treponemal Populations. Infect Immun. 1974 Nov;10(5):1062–1067. doi: 10.1128/iai.10.5.1062-1067.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckel P., Zehelein E. Expression of Pseudomonas fluorescens D-galactose dehydrogenase in E. coli. Gene. 1981 Dec;16(1-3):149–159. doi: 10.1016/0378-1119(81)90071-8. [DOI] [PubMed] [Google Scholar]
- Collins J., Brüning H. J. Plasmids useable as gene-cloning vectors in an in vitro packaging by coliphage lambda: "cosmids". Gene. 1978 Oct;4(2):85–107. doi: 10.1016/0378-1119(78)90023-9. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H., Cox D. L., Moeckli R. A. Further studies on replication of virulent Treponema pallidum in tissue cultures of Sf1Ep cells. Infect Immun. 1982 Feb;35(2):449–455. doi: 10.1128/iai.35.2.449-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaev M. M., Bobkov A. F., Bobkova A. F., Kalinin V. N., Smirnov V. D., Khudakov YuE, Tikchonenko T. I. The site-specific deletion in plasmid pBR322. Gene. 1982 Apr;18(1):21–28. doi: 10.1016/0378-1119(82)90052-x. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Fredericks W. R., Nell E. E. Isolation and antigenic characteristics of axial filaments from the Reiter Treponeme. Infect Immun. 1975 Feb;11(2):380–386. doi: 10.1128/iai.11.2.380-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Nell E. E. Isolation and purification of Treponema pallidum from syphilitic lesions in rabbits. Infect Immun. 1975 Jun;11(6):1296–1299. doi: 10.1128/iai.11.6.1296-1299.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Osterhaus A. D., van Wezel A. L., van Steenis B., Drost G. A., Hazendonk T. G. Monoclonal antibodies to polioviruses. Production of specific monoclonal antibodies to the Sabin vaccine strains. Intervirology. 1981;16(4):218–224. doi: 10.1159/000149270. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Kettman J. R., Miller J. N., Norgard M. V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect Immun. 1982 Jun;36(3):1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Folds J. D., Bassford P. J., Jr Expression of Treponema pallidum antigens in Escherichia coli K-12. Infect Immun. 1982 Jun;36(3):1238–1241. doi: 10.1128/iai.36.3.1238-1241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg Pedersen N., Sand Petersen C., Axelsen N. H., Birch-Andersen A., Hovind-Hougen K. Isolation of a heat-stable antigen from Treponema Reiter, using an immunoadsorbent with antibodies from syphilitic patients. Scand J Immunol. 1981 Aug;14(2):137–144. doi: 10.1111/j.1365-3083.1981.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Veldkamp J., Visser A. M. Application of the enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of syphilis. Br J Vener Dis. 1975 Aug;51(4):227–231. doi: 10.1136/sti.51.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A. M., Hanff P. A., Lovett M. A. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982 Apr 30;216(4545):522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]
- van Embden J. D., de Graaf F. K., Schouls L. M., Teppema J. S. Cloning and expression of a deoxyribonucleic acid fragment that encodes for the adhesive antigen K99. Infect Immun. 1980 Sep;29(3):1125–1133. doi: 10.1128/iai.29.3.1125-1133.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]