Abstract
Hypoxia exists widely in developing embryos where it may regulate blood vessel formation. VEGF and FGF2 produced in developing renal primordia (metanephroi) stimulate microvessel formation from embryonic thoracic aorta cultured under hypoxic conditions (HC) relative to room air (RA). The aim of the present study was to provide insight into the participation of hypoxia in a process that occurs concomitant with metanephros vascularization in vivo, ureteric bud (UB) branching. To this end, the arborization of the UB and growth of metanephroi were measured in metanephroi grown in serum-free organ culture for two days under RA or HC. When metanephroi were cultured under HC the arborization of UB was stimulated relative to RA. In the presence of anti-VEGF neutralizing antibody (αmVEGF), or anti-FGF2 neutralizing antibody (αhFGF2) UB branching was inhibited under both RA and HC. When both αmVEGF and αhFGF2 were added, the inhibition was enhanced. Addition of exogenous VEGF or FGF2 to cultures stimulated UB branching under RA and HC and addition of both stimulated it further. These findings provide evidence for roles of hypoxia and metanephric VEGF and FGF2, as regulators not only for vascularization but also for UB bud branching during renal organogenesis.
Key Words: metanephroi, embryogenesis, fibroblast growth factor, vascular endothelial growth factor
Introduction
Microenvironmental oxygen concentration is an important signal for regulation of vascular development in embryos.1 Previously, we developed a culture model, which allows us to observe in vitro, microvessel formation originating from mouse thoracic embryonic aorta2–4 from which the renal vasculature is derived.5 Using this system, we showed that microvessel formation is stimulated in aortas cultured under 5% O2 (designated hypoxic conditions; HC) relative to 5% CO2/20% O2 (designated room air; RA).4 Moreover, addition of supernatants obtained from mouse metanephroi grown in serum-free organ culture or extracts of the metanephroi stimulate microvessel formation in aortic explants grown under HC.4 We determined that immunoreactive VEGF and FGF2 are produced in metanephroi and present in metanephros culture supernatants (VEGF) or extracts (VEGF and FGF2) and that growth of metanephroi under HC relative to RA enhances VEGF production. Furthermore, agents that block biological activities of these growth factors inhibit supernatant or extract-induced microvessel formation.4 We concluded that microvessel formation from aorta is stimulated in vitro by secreted VEGF and FGF2 produced by metanephroi.4
Hypoxia is a stimulus not only for both for renal vascular development, but also for growth of metanephroi in vitro.6,7 The growth and differentiation of nephron components within the renal anlage occurs concomitant with its vascularization in vivo.8,9 The roles, if any, that hypoxia VEGF and FGF2 play in the concurrent processes of UB branching and renal growth are less well characterized than their roles in vascularization.
The aim of the present study was to provide insight into the roles of hypoxia, and VEGF and FGF2 as regulators of embryonic kidney growth in vitro. To this end we cultured isolated mouse metanephroi under HC and RA. Here we show that arborization of the ureteric bud (UB) and growth (protein content) of metanephroi in organ culture are enhanced if metanephroi are grown under HC relative to RA. Growth and UB arborization are inhibited by addition of anti-VEGF antibody and/or anti-FGF2 antibody and are stimulated by addition of exogenous VEGF and/or FGF2. Our findings provide evidence for roles for hypoxia, VEGF and FGF2 as regulators not only for vascularization but also for UB branching and growth of metanephroi.
Materials and Methods
Embryonic tissue culture
Metanephroi were surgically dissected from pregnant C57BL/6J mice (Nihon SLC, Inc., Hamamatsu, Japan) on day 12 of the pregnancy as previously described.4 The dissected mouse metanephroi were transferred onto 1.2 µm pore size and 13 mm diameter polycarbonate sterile filter membranes (Millipore, Burlington, MA) floating on a 300 µl of serum free culture medium (MCDB131) in 24 well culture plates, and incubated at 37°C in a humidified incubator under 5% CO2/20% O2 (designated room air; RA), or 5% O2 (designated hypoxic conditions; HC) for 2 days.4 The medium was supplemented with 1.18 g/l sodium bicarbonate, insulin 5 µg/ml, transferrin 5 µg/ml, sodium selenate 5 ng/ml, triiodothyronine 50 pg/ml, penicillin 100 U/ml, and streptomycin 100 U/ml. The following additions were made to cultures as indicated: 25 µg/ml anti-human FGF2 neutralizing antibody (αhFGF2) (R and D Systems, Minneapolis, MN), 1 µg/ml anti-mouse VEGF neutralizing antibody (αmVEGF) (R and D Systems); 20 ng/ml recombinant human FGF2 (rhFGF2) or recombinant human VEGF (rhVEGF); (Sigma Chemical, St.Louis, MO) or 4 µg/ml anti-human albumin antibody (Sigma #A1151). The concentrations of antibodies were chosen because, based on our previous studies using αhFGF2 and/or αmVEGF, we would have expected these concentrations to completely inhibit at least one biological activity of the growth factors, i.e., microvessel formation stimulated by rhVEGF or rhFGF2.4 Each culturing condition included at least four cultures. Experiments were repeated at least three times. Data are shown as mean ± SEM of 4 separate experiments (each including five cultures).
UB branching and protein content in metanephric organ culture
UB branching was determined using methodology described by Vilar et al.10 with some modification. Metanephroi were detached from the filter, permeabilized with 2% Triton X-100® in PBS, and labeled with Dolichos biflorus agglutinin11 that was FITC coupled, to analyze the ureteric bud arborization and to count the end buds. Fluorescent microscope images were viewed and captured by an OLYMPUS BX-50 microscope with an OLYMPUS DP-70 color digital camera (Olympus Optical Co., Ltd, Tokyo, Japan). Images were imported into Adobe Photoshop 5.0J for final processing and layout. Growth of the explanted metanephroi was then determined by measurement of their protein content. The labeled metanephroi were placed in individual tubes that contained 0.5 ml cell lysis buffer. After preparation of cell lysates, the protein content was measured by Lowry's method as before.10
Statistics
Data were analyzed by an analysis of variance combined with Fisher's protected least significant difference (Fisher's PLSD). Differences with p < 0.05 were considered to be significant.
Results
When metanephroi were cultured in control media under HC, UB branching was enhanced significantly relative to RA (Table 1). In a separate set of experiments (n = 5) we determined that addition of anti-human albumin antibody to cultures had no effect on UB branching. In RA cultures, the number (mean ± SD) of end buds was 13.8 ± 1.6 (no addition) and 14.2 ± 1.7 (anti-human albumin antibody added), and in HC cultures the number of end buds was 22.2 ± 1.3 (no addition) and 23.2 ± 2.3 (anti-human albumin antibody added).
Table 1.
Number of end buds in metanephroi
| Numbers of end buds | ||
| Conditions | RA | HC |
| End buds | 13.8 ± 0.7 | 22.8 ± 0.8** |
Metanephroi were cultured under RA or HC for two days. Data represent means ± SEM of n = 5 experiments. RA, room air; HC, hypoxic conditions. **p < 0.01 versus RA.
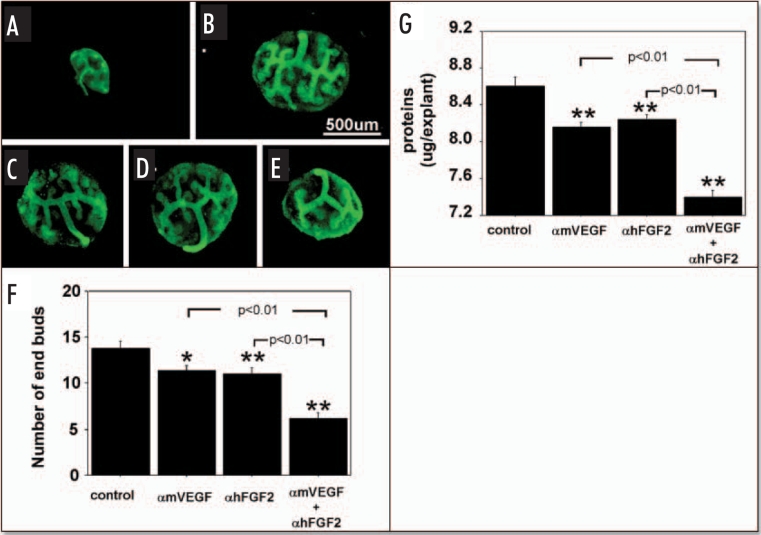
Since VEGF and FGF2 produced by metanephroi enhance hypoxia-stimulated microvessel formation in cocultured embryonic aorta, and growth of metanephroi occurs concurrently with vascularization, we characterized effects of blocking VEGF and FGF2 in and adding VEGF and FGF2 to metanephros cultures, on UB branching and metanephros growth (protein content). At the time of isolation for this study (E12), metanephroi had T-shaped ureteric buds (see Figs. 1A and 2A). After two days culture in control media (no growth factors or antibodies added) under RA, the buds branched several more times (Fig. 1B). In the presence of αmVEGF (Fig. 1C) or αhFGF2 (Fig. 1D), or both (Fig. 1E) branching of the UB was significantly inhibited relative to addition of no neutralizing antibodies (Fig. 1B). When both αmVEGF and αhFGF2 were added, UB branching was reduced further relative to addition of either antibody by itself (Fig. 1F). Metanephros protein content changed in parallel with enhanced UB branching (Fig. 1G).
Figure 1.
Effect of endogenous VEGF and FGF2 sequestering by specific antibodies on UB growth under RA conditions. UBs were stained using FITC-conjugated DBA. Metanephroi were freshly dissected from 12-day embryos (A), cultured for two days under RA in control media (B, control) or control media containing either 1 µg/ml αmVEGF (C), 25 µg/ml αhFGF2 (D), or a combination of the two (E). (F) Quantitative analysis of UB branching. (G) Protein content of metanephroi. Data shown in A–E are representative. Data shown in (F and G) are means ± SEM of four independent experiments. *p < 0.05 versus control, **p < 0.01 versus control.
Figure 2.
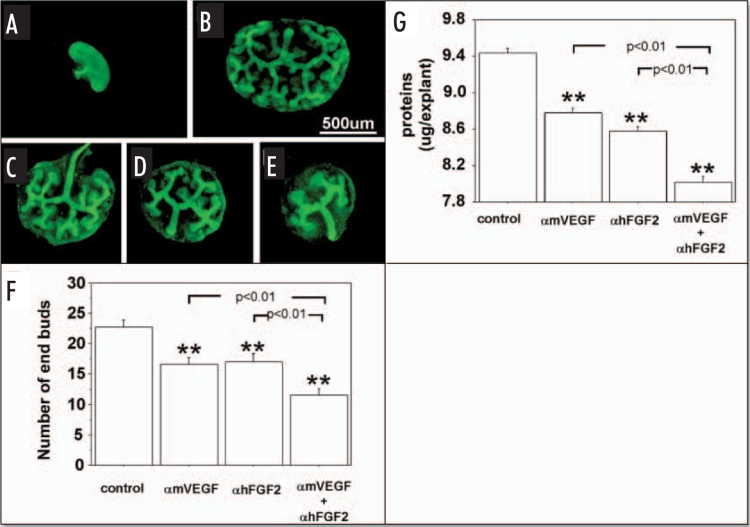
Effect of endogenous VEGF and FGF2 sequestering by specific antibodies on UB growth in metanephroi cultured under HC. UBs were stained using by FITC-conjugated DBA. (A) Metanephros freshly dissected from 12-day embryo. Metanephros cultured for two days in control media (B, control) or control media plus 1 µg/ml αmVEGF (C), 25 µg/ml αhFGF2 (D), or a combination of two (E). (F) Quantitative analysis of the inhibition of UB branching. (G) Metanephros protein content. Data shown in (A–E) are representative. Data shown in (F and G) are means ± SEM of four independent experiments. **p < 0.01 versus control.
Similar to RA conditions, UB branching (Fig. 2B) was inhibited significantly (Fig. 2F) by addition of αmVEGF (Fig. 2C), αhFGF2 (Fig. 2D) or both (Fig. 2E) to HC metanephros cultures, as was protein content of cultured metanephroi (Fig. 2G).
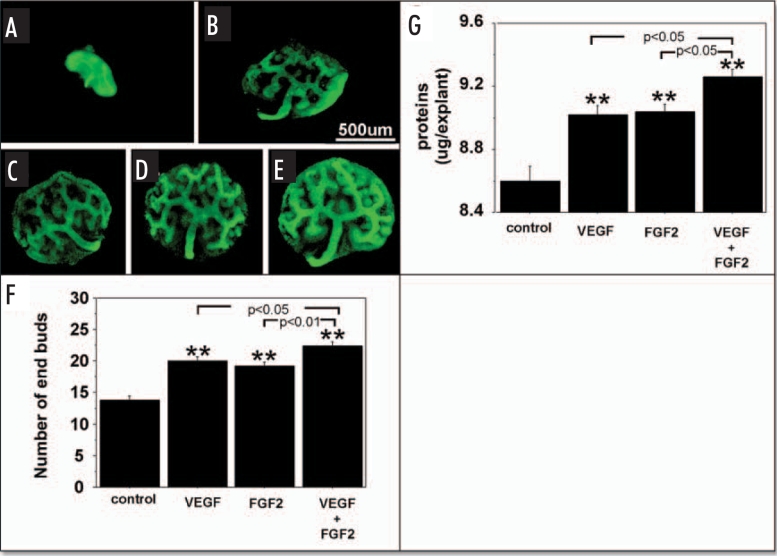
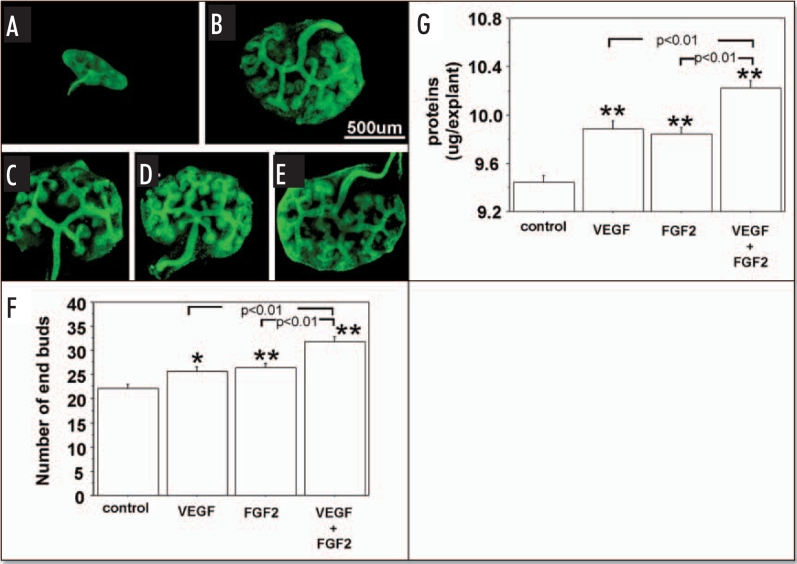
Figures 3 and 4, show the effect of exogenous VEGF and FGF2 added to metanephros cultures on UB branching and protein content during two days under RA and HC, respectively. Under both RA (Fig. 3F) and HC (Fig. 4F), addition of exogenous VEGF or FGF2 significantly stimulated the UB branching (Figs. 3F and 4F respectively) and increased metanephros protein content (Figs. 3G and 4G respectively). Addition of both growth factors enhanced UB branching and metanephros protein contents relative to addition of either growth factor alone (Figs. 3F and G; 4F and G).
Figure 3.
Effect of added exogenous VEGF and FGF2 on metanephroi cultured under RA. UBs were stained using FITC-conjugated DBA lectin. (A) Metanephroi freshly dissected from 12-day embryo. Metanephroi cultured for two days under RA in control media (B, control) or in the presence of 20 ng rhVEGF (C), 20 ng/ml rhFGF2 (D), or a combination of the two (E). (F) Quantitative analysis of UB branching. (G) Protein content. Data shown in A–E are representative. Data shown in (F and G) are means ± SEM of four independent experiments. **p < 0.01 versus control.
Figure 4.
Effect of exogenous VEGF and FGF2 on UB branching and growth when metanephroi were cultured under HC. UBs were stained using FITC-conjugated DBA. (A) Metanephros freshly dissected from 12-day embryo. Metanephroi cultured for two days under RA in control media(B, control) or in the presence of 20 ng rhVEGF (C), 20 ng/ml rhFGF2 (D), or a combination of two (E). (F) Quantitative analysis of UB branching. (G) Protein content determination. Data shown in (A–E) are representative. Data shown in (F and G) are means ± SEM of four independent experiments. *p < 0.05 versus control, **p < 0.01 versus control.
Discussion
Previously, we showed that organ cultured metanephroi produce growth factors including VEGF and FGF2 that stimulate microvessel formation from embryonic thoracic aorta in vitro and that microvessel formation is enhanced by HC.4 The results of the present study help to define the roles of low ambient O2, VEGF and FGF2 on UB branching and metanephros growth (protein content) during two days in organ culture. Here we show: (1) for the first time that hypoxia stimulates UB branching in vitro; (2) that sequestering metanephric VEGF and/ or FGF2 using antibodies has inhibitory effects on UB branching and metanephros protein content; and (3) that addition of exogenous VEGF and FGF2 or both enhances UB branching and metanephros protein content.
There are several reports documenting that both VEGF and FGF2 and their receptors are expressed within the developing embryonic kidney. VEGF mRNA and protein can be detected in glomerular epithelia and in proximal and distal tubules, while mRNA for VEGF receptors is present in not only endothelial cells of glomerular and peritubular capillaries, but also in renal tubular epithelial cells.12–14 Cancilla et al. detected the expression of mRNAs for FGFs1 through FGF5, and FGF7 through FGF10, and FGFR1 through FGFR4 in rat metanephroi from E14 to E21,15 and Qiao et al showed that FGF receptors are present in rat UB.16
Possible roles for VEGF and FGF2 on the process of kidney development are indicated by a number of studies. Kitamoto et al. showed that if endogenous VEGF activity is blocked by administration of anti-VEGF antibodies to newborn mice, blood vessel formation in the part of the kidney still undergoing development, the superficial cortex, is disturbed. Also, kidneys originating from antibody-treated mice had fewer nephrons than those from control animals.14 Data generated in other laboratories indicate that metanephric VEGF may act not only as an enhancer or inducer of vascularization but also as a promoter of tubulogenesis.6,7,12
Nigam and coworkers utilized isolated rat metanephros organ cultures and explants of isolated UBs to show that multiple growth factors including FGFs support UB growth and branching in vitro.16,17 Qiao et al. (this group) showed that addition of soluble FGF receptors or of FGF blocking antibodies to organ cultures inhibits UB branching.16 Several FGFs including FGF2 in combination with GDNF and BSN-CM regulated isolated UB growth and branching in vitro. FGF1 induced the formation of elongated UB branching stalks with distinct proliferative ampullary tips, while FGF7 induced amorphous buds displaying nonselective proliferation with little distinction between stalks and ampullae. FGF1 and FGF7 were at extreme ends of the spectrum, with FGF2 (more FGF7-like) falling in between.16 Our data do not speak to the distinctions between FGF actions reported by Qiao et al such as amorphous buds displaying nonselective proliferation and delineation between stalks and ampullae.16 However, these investigators employed isolated UB,16 while we use whole metanephros organ culture.
Microenvironmental oxygen concentration is an important signal for regulation of vascular development in embryos.1 Hypoxia stimulates not only endogenous VEGF production but also up regulation of Flt-1 and Flk-1, two forms of VEGF receptors.18,19 Hypoxia enhances metanephros VEGF production,4 in addition to VEGF3 and FGF22 binding in embryonic thoracic aorta from which the renal macrovasculature dervives.5
Here we show that hypoxia stimulates UB branching in organ culture, suggesting that low microenvironmental oxygen acts as to stimulate not only metanephros vascularization4 but concurrently UB growth in vivo. The data are consistent with the concept that renal vascular development and branching morphogenesis are coupled. While they do not define the nature of the link, our findings are consistent with participation by VEGF and FGF2 produced in metanephroi.
The action of FGF2 could be mediated directly via the FGF receptors present in UB.16 However, UB does not express receptors for VEGF in freshly isolated developing kidneys12 or in the setting of organ culture under normoxic or hypoxic conditions.6,7 Therefore, the action of VEGF on UB branching must be an indirect one, mediated by one or more UB-branching factors the production or action of which is stimulated by VEGF in organ culture.
Acknowledgements
T. Akimoto and E. Kusano were supported by in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, and Grants from Jin-Kenkyu-Kai. M.R. Hammerman was supported by grant DK 63379 from NIDDK.
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/abstract.php?id=1726
References
- 1.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: A possible signal for vessel development. Developmental Dynamics. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto T, Hammerman MR. Fibroblast growth factor 2 promotes microvessel formation from mouse embryonic aorta. Am J Physiol. 2003;284 :C378–C388. doi: 10.1152/ajpcell.00193.2002. [DOI] [PubMed] [Google Scholar]
- 3.Akimoto T, Liapis H, Hammerman MR. Microvessel formation from mouse embryonic aortic explants is oxygen and VEGF dependent. Am J Physiol. 2002;283:F487–F495. doi: 10.1152/ajpregu.00699.2001. [DOI] [PubMed] [Google Scholar]
- 4.Akimoto T, Hammerman MR. Microvessel formation from mouse aorta is stimulated in vitro by secreted VEGF and extracts from metanephroi. Am J Physiol. 2003;284:C1625–C1632. doi: 10.1152/ajpcell.00436.2002. [DOI] [PubMed] [Google Scholar]
- 5.Netter FH. Anatomy structure and embryology. In: Becker EL, Churg J, editors. The Netter Collection of Medical Illustrations. Vol. 6. Pittsburgh PA: Kidneys Ureter and Bladder Novartis; 1997. pp. 2–35. [Google Scholar]
- 6.Tufro-McReddie A, Norwood VF, Aylor KW, Botkin SJ, Carey RM, Gomez RA. Oxygen regulates vascular endothelial growth factor-mediated vasculogenesis and tubulogenesis. Developmental Biology. 1997;183:139–149. doi: 10.1006/dbio.1997.8513. [DOI] [PubMed] [Google Scholar]
- 7.Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999;10:2125–2134. doi: 10.1681/ASN.V10102125. [DOI] [PubMed] [Google Scholar]
- 8.Horster MF, Braun GS, Huber SM. Renal embryonic epithelial induction nephrogenesis and cell differentiation. Physiol Rev. 1999;70:1157–1191. doi: 10.1152/physrev.1999.79.4.1157. [DOI] [PubMed] [Google Scholar]
- 9.Woolf AS, Loughna S. Origin of glomerular capillaries: Is the verdict in? Experimental Nephrology. 1998;6:17–21. doi: 10.1159/000020500. [DOI] [PubMed] [Google Scholar]
- 10.Vilar J, Lalou C, Duong JP, Huyen V, Charrin S, Hardouin S, Raulais D, Merlet-Benichou C, LelievrePegorier M. Midkine is involved in kidney development and its regulation by retinoids. J Am Soc Nephrol. 2002;13:668–676. doi: 10.1681/ASN.V133668. [DOI] [PubMed] [Google Scholar]
- 11.Laitinen L, Virtanen I, Saxen L. Changes in the glucosylation pattern during embryonic development of mouse kidney as revealed with lectin conjugates. J Histochemistry & Cytochemistry. 1987;35:55–65. doi: 10.1177/35.1.3794309. [DOI] [PubMed] [Google Scholar]
- 12.Simon H, Gröne HJ, Jöhren O, Kullmer J, Plate KH, Risau W, Fuchs E. Expression of vascular endothelial growth factor and its receptor in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240–F250. doi: 10.1152/ajprenal.1995.268.2.F240. [DOI] [PubMed] [Google Scholar]
- 13.Kanellis J, Fraser S, Katerelos M, Power DA. Vascular endothelial growth factor is a survival factor for renal tubular epithelial cells. Am J Physiol. 2000;278:F905–F915. doi: 10.1152/ajprenal.2000.278.6.F905. [DOI] [PubMed] [Google Scholar]
- 14.Kitamoto Y, Tokunaga H, Tomita H. Vascular endothelial growth factor is an essential molecule for mouse kidney development: Glomerulogenesis and nephrogenesis. J Clinical Investigation. 1997;99:2351–2357. doi: 10.1172/JCI119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mechanisms in Development. 2001;109:123–135. doi: 10.1016/s0925-4773(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai H, Barros EJ, Tsukamoto T, Barasch J, Nigam SK. An in vitro tubulogenesis system using cell lines derived from the embryonic kidney shows dependence on multiple soluble growth factors. Proc Natl Acad Sci USA. 1997;94:6279–6284. doi: 10.1073/pnas.94.12.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuefeld G, Cohen T, Gengrinovitch S, Polrprak Z. Vascular endothelial growth factor and its receptors. FASEB Journal. 1999;113:9–22. [PubMed] [Google Scholar]
- 19.Shreeniwas R, Ogawa S, Cozzolino F, Torsia G, Braunstein N, Butura C, Brett J, Lieberman HB, Furie MB, Joseph-Silverstein J, Stern D. Macrovascular and microvascular endothelium during long-term hypoxia: Alteration in cell growth, monolayer permeability, and cell surface coagulant properties. J Cell Physiology. 1991;146:8–17. doi: 10.1002/jcp.1041460103. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki T, Yamada K, Otsuki H, Yuguchi T, Kohmura E, Hayakawa T. Brief exposure to hypoxia induces FGF2 mRNA and protein and protects rat cortical neurons from prolonged hypoxic stress. Neuroscience Research. 1995;23:289–296. doi: 10.1016/0168-0102(95)00954-x. [DOI] [PubMed] [Google Scholar]