Abstract
Neurogenin 3 (ngn3) is a basic helix loop helix transcription factor that is transiently expressed in the developing mouse pancreas with peak expression around E15. In mice lacking the ngn3 gene the endocrine cells of the pancreas fail to develop suggesting that the ngn3-positive cell may represent a progenitor cell for the endocrine pancreas. In order to purify and characterize this cell in detail we have generated a transgenic mouse, in which the ngn3 promoter drives expression of enhanced green fluorescent protein (EGFP). In the E15.5 embryo EGFP was expressed in the dorsal and ventral pancreas, the duodenum, and lower intestine as well as in the brain. This pattern of expression was in keeping with the known expression profile of the endogenous ngn3 gene. Within the pancreas EGFP was localized in close proximity to cells that stained positive for ngn3, insulin, and glucagon, but was absent from regions of the pancreas that stained positive for amylase. EGFP was also present in the pancreas at E18.5, although there was no detectable expression of ngn3. At this stage EGFP did not colocalize with any of the hormones or exocrine markers. EGFP+ cells were FACS purified (96%) from the E15 pancreas yielding ∼ 10,000 cells or 1.6% of the total pancreatic cells from one litter. RT/PCR analysis confirmed that the purified cells expressed EGFP, ngn3, insulin, glucagon, somatostatin and pancreatic polypeptide. The ability to purify ngn3+ cells provides an invaluable source of material for charactering in detail their properties.
Key Words: islets of Langerhans, diabetes mellitus, insulin gene
Introduction
During development, the pancreas arises from dorsal and ventral evaginations that form in regions of the duodenum that lie posterior to the stomach.1 These pancreatic buds grow and form a branching epithelial structure in response to signals generated by the surrounding mesenchyme.2 Some of these epithelial cells form acini and ductule structures that form the exocrine pancreas. Others differentiate into the four cell types of the endocrine pancreas, i.e., insulin secreting β cells, glucagon secreting α cells, somatostatin secreting δ cells and pancreatic polypeptide secreting PP cells. These cells migrate from the epithelium to aggregate and form islets of Langerhans within the interstitial tissue.
A number of transcription factors have been implicated in the development of the endocrine pancreas, including PDX-1,3 Nkx2.2,4 Nkx6.1,5 Pax4,6 Pax67 and the basic helix-loop-helix (bHLH) transcription factor neurogenin3 (ngn3).8 Targeted disruption of the ngn3 gene in mice results in mice that lack pancreatic endocrine cells and consequently die shortly after birth from diabetes,8 while overexpression of ngn3 in pancreatic buds leads to an increase in the number of pancreatic endocrine cells.9,10 The phenotype of the ngn3 knockout and overexpressing mice, along with direct lineage tracing experiments11 strongly supports the conclusion that ngn3+ cells are islet progenitor cells in the developing pancreas.
In order to purify and characterize ngn3+ cells we generated a transgenic mouse, in which expression of the green fluorescent protein EGFP was driven by regulatory regions of the human ngn3 gene.12 We describe here the expression pattern of EGFP in the developing pancreas of these mice and the characteristics of purified EGFP-expressing cells.
Materials and Methods
Generation of transgenic mice
The pngn3EGFP construct was generated by ligating a 5.7 kb ngn3 promoter fragment (12) upstream of the EGFP gene in pEGFP-1 (Clontech, Cowley, Oxford). The same ngn3 promoter fragment was also ligated upstream of the neomycin gene in pOCTNeolox (PPL, unpublished) to generate pngn3neo. The pngn3EGFP and pngn3neo plasmids were linearized, and transgenic mice generated from pronuclear injection into fertilized oocytes, followed by transfer into pseudopregnant mice.
Genotyping
Approximately 5 mm of tail tissue was removed from each of the offspring of the founder stock. Tail tips were subjected to Proteinase K (Sigma, Poole, Dorset) digestion overnight at 55°C. Resultant digest product was centrifuged in a microcentrifuge and the supernatant used to prepare DNA using ethanol precipitation. The subsequent DNA preparations were then used in PCR reactions with the following primer sets: HPRT, 5′-GAGTTCCGGAACTGCCTTTGGTG-3′ and 5′-CTGTGCCACCGGGCGCATGG-3′ to yield a 350 bp product; EGFP, 5′-ACCCTCGTGACCACCCTGACCTAC-3′ and 5′-GACCATGTGATCGCGCTTCTCGTT-3′ to yield a 483 bp product; and neomycin 5′-TGCATACGCTTGATCCG-3′ and 5′-GAAGGCGATAGAAGGCGA-3′ to yield a 410 bp product.
RT/PCR
Cells were lysed with TRIzol and total RNA purified following the manufacturer's protocol (Invitrogen, Groningen, The Netherlands). Total RNA was treated with DNaseI (Invitrogen) followed by reverse transcription with SuperScriptII (Invitrogen) using standard protocols. The cDNA product was then used to carry out 25 µl PCR reactions using the following primer sets: β-actin, 5′-TGACCCAGATCATGTTTGAGA-3′ and 5′-TCTCCAGGGAGGAAGAGGAT-3′ to yield a 357 bp product; EGFP, 5′-ACCCTCGTGACCACCCTGACCTAC-3′ and 5′-GACCATGTGATCGCGCTTCTCGTT-3′ to yield a 483 bp product; Ngn3, 5′-CAGCTCAGAAATCCCTCTGG-3′ and 5′-GAGGCGCCATCCTAGTTCTC-3′ to yield a 246 bp product; INS1, 5′-ACCATCAGCAAGCAGGTCAT-3′ and 5′-CACTT GTGGGTCCTCCACTT-3′ to yield a 218 bp product; INS2, 5′-CAGCAAGCAGGAAGCCTATC-3′ and 5′-TTGTGCCACTTGTGG-GTCCT-3′ to yield a 235 bp product; GLU, 5′-CCAGATCATTCCC -AGCTTCA-3′ and 5′-TGGTGCTCATCTCGTCAGAG-3′ to yield a 384 bp product; SOM, 5′-CCACCGGGAAACAGGAACTG-3′ and 5′-GGGCCAGGAGTTAAGGAAGA-3′ to yield a 303 bp product; PP, 5′-TAGCTCAGCACACAGGATGG-3′ and 5′-GCCTGGTCAGTGTGTTGATG-3′ to yield a 203 bp product.
Immunohistochemistry
Timed matings were set up and embryos dissected from the pregnant females in ice-cold PBS. The pancreas and gut were dissected from individual embryos in ice cold phosphate buffered saline (PBS) and visualized for EGFP fluorescence using a stereomicroscope. EGFP positive pancreas dissections were fixed in 4% paraformaldehyde in PBS for 1 h at RT, removed from the fixative and immersed in 30% sucrose in PBS overnight at 4°C to protect the tissue for freezing. Fixed and cryoprotected tissue was embedded in OCT medium then snap-frozen by immersion into iso-pentane frozen in liquid nitrogen. Samples were stored at −70°C until needed for cryosectioning. Cryostat sections (5 µm) were adhered to polylysine coated glass slides (BDH). The sections were allowed to dry fully and then stored at −70°C or used immediately for immunohistochemistry analysis. Frozen slides were allowed to thaw for 10 min at RT then immunohistochemistry was performed using standard immunohistochemistry techniques. Primary antibodies were incubated at 4°C overnight at the following concentrations: rabbit anti-ngn3 at 1:1000, mouse anti-insulin (Sigma) at 1:1000, mouse anti-glucagon (Sigma) at 1:1000, rabbit anti-pancreatic polypeptide (Zymed, San Francisco, California) at 1:50, rabbit anti-somatostatin (Zymed) at 1:50, rabbit anti-PDX-1 (Dr. Chris Wright, Vanderbilt University) at 1:1000, and rabbit anti-amylase (Sigma) at 1:500. Primary antibodies were detected with secondary antibodies: goat anti-rabbit conjugated to AlexaFluor 350 (Molecular Probes), and rabbit anti-mouse conjugated to Rhodamine (Sigma).
FACS analysis
Dissections were carried out on embryos as described for immunohistochemistry, with the addition that any residual duodenum or parenchyma was removed using tungsten needles in ice cold PBS. The dissected tissue was then pooled into EGFP positive and negative groups and the cells dissociated in 0.3 mg/ml collagenase in Hank's balanced salt solution (HBSS, Gibco, Paisley, Scotland) at 37°C for 30 min-1 h. The resultant cell suspension was then filtered through a 100 µm cell strainer and the cells washed 3 times with HBSS. Cells were then resuspended in HBSS. The cells were analysed and sorted with a Becton Dickson FACS Vantage SE machine.
Results
The ngn3/EGFP transgenic mice were generated by injecting two DNA constructs into oocytes: one containing a 5.7 kb fragment of the human ngn3 promoter upstream of the EGFP gene and the second containing a 5.7 kb fragment of the human ngn3 promoter upstream of a neomycin gene, allowing both visualisation and selection of ngn3 expressing cells. Viable offspring (n = 318) were genotyped for the presence of the EGFP and neo transgenes by PCR and Southern blot analysis of DNA extracted from tail tips. Twenty-three founders (13 male and 10 female) were identified, and bred with F1 (C57Bl6 X CBA) mice. From these timed matings, embryos at E15.5 stage from 3 of the 13 founders expressed EGFP in the pancreas, duodenum, and lower intestine (Fig. 1). This is in keeping with the endogenous ngn3 gene, which is expressed transiently at around E15.5 in these tissues. We also observed expression of EGFP in forebrain and hindbrain of E15.5 embryos (data not shown). These founders were used to generate three separate lines, of which 02/1.37 gave the strongest expression of EGFP and was used in the following studies. Further genotyping confirmed that this line retained both the EGFP and neo transgenes (Fig. 2).
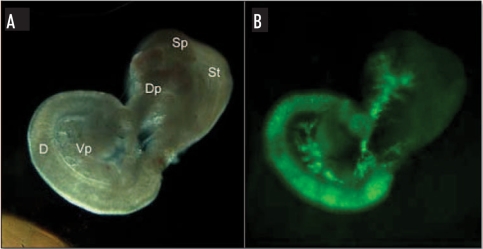
Figure 1.
EGFP expression in E15.5 pancreas. (A) Stereomicroscopic view of pancreas/gut dissection from an E15.5 ngn3-EGFP mouse embryo. (B) The same pancreas/gut dissection using EGFP fluorescent filters. D, duodenum; St, stomach; Sp, spleen; Dp, dorsal pancreas; Vp, ventral pancreas.
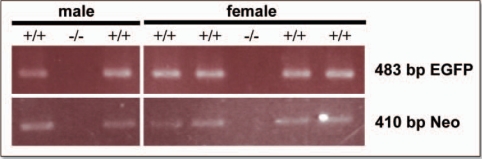
Figure 2.
Genotyping the ngn3-EGFP 1.37 line. Genotyping PCR was carried out on tail tip DNA to check for the presence of both EGFP (483 bp) and neomycin (neo) (410 bp) constructs in the offspring of the founder mice. +/+, positive for EGFP and Neomycin transgenes; −/−, negative for EGFP and Neomycin transgenes.
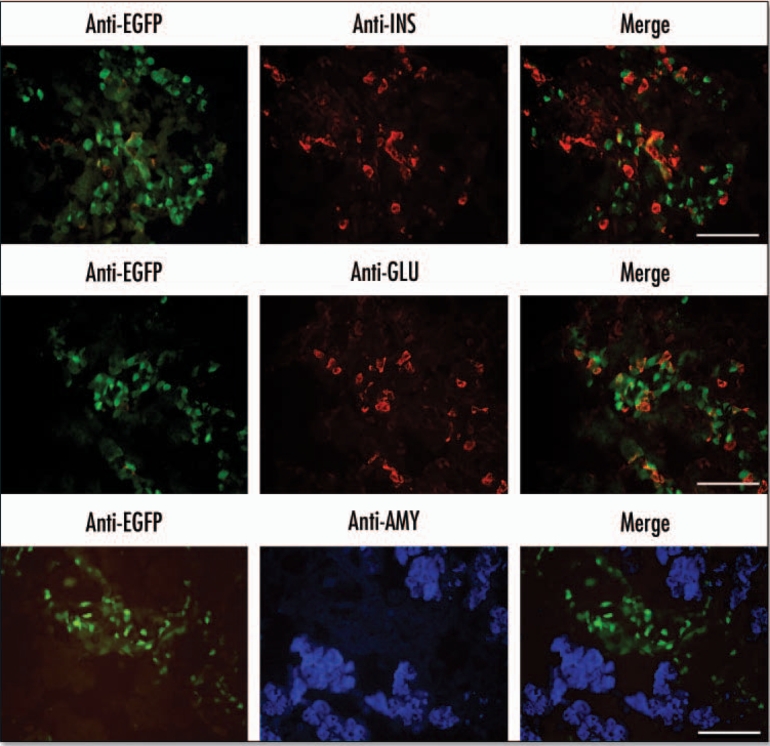
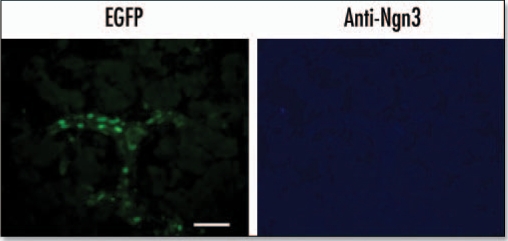
At E15.5 the EGFP fluorescence was localized in a broad track of cells present in the central region of the pancreas (Fig. 3). All the cells that were positive for EGFP fluorescence stained positive with an anti-EGFP antibody. EGFP fluorescence was also localized to regions of the pancreas that were enriched in ngn3-expressing cells (Fig. 4) and the endocrine markers insulin and glucagon (Fig. 5). About 30% of these cells were positive for both EGFP and ngn3, while 60% of the insulin and glucagon positive cells were also positive for EGFP. There was no colocalization of EGFP and amylase (Fig. 5).
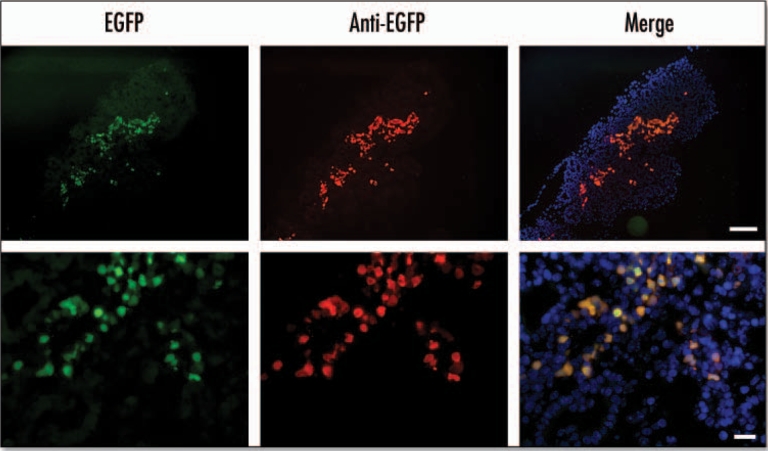
Figure 3.
EGFP fluorescence colocalizes with anti-EGFP staining in the E15.5 pancreas. In the panels marked EGFP the fluorescence was detected using EGFP filters. In panels marked anti-EGFP immunohistochemistry was performed on cryosections using an anti-EGFP antibody and a Texas Red conjugated secondary antibody with DAPI staining to identify nuclei. The merged image was obtained by merging images using Photoshop 6.0 software (Scale bar for: upper panels = 100 µm, lower panels = 20 µm).
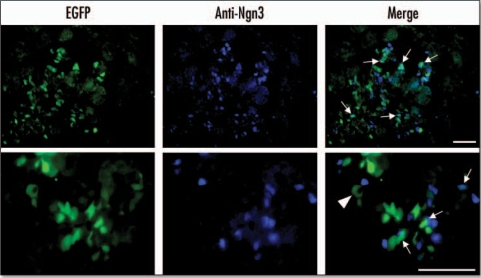
Figure 4.
EGFP colocalizes with ngn3 at E15.5. EGFP fluorescence was measured using an EGFP filter. Ngn3 was localized by immunohistochemistry of cryosections using an anti-ngn3 antibody and AlexaFluor 350 conjugated secondary antibody. Arrows indicate cells in which EGFP and ngn3 colocalize. The arrow-head indicates auotofluorescence associated with a blood cell (Scale bar = 50 µm).
Figure 5.
Localisation of EGFP in the E15.5 pancreas to regions enriched in endocrine markers. Immunohistochemistry was performed on cryosections of E15.5 embryonic pancreas dissections. Primary antibodies for insulin (INS) and glucagon (GLU) were visualized with an anti-mouse Rhodamine conjugated secondary antibody. Primary antibodies for amylase were visualized using anti-rabbit AlexaFluor 350 secondary antibody. EGFP was visualized directly using an EGFP filter (Scale bars = 50 µm).
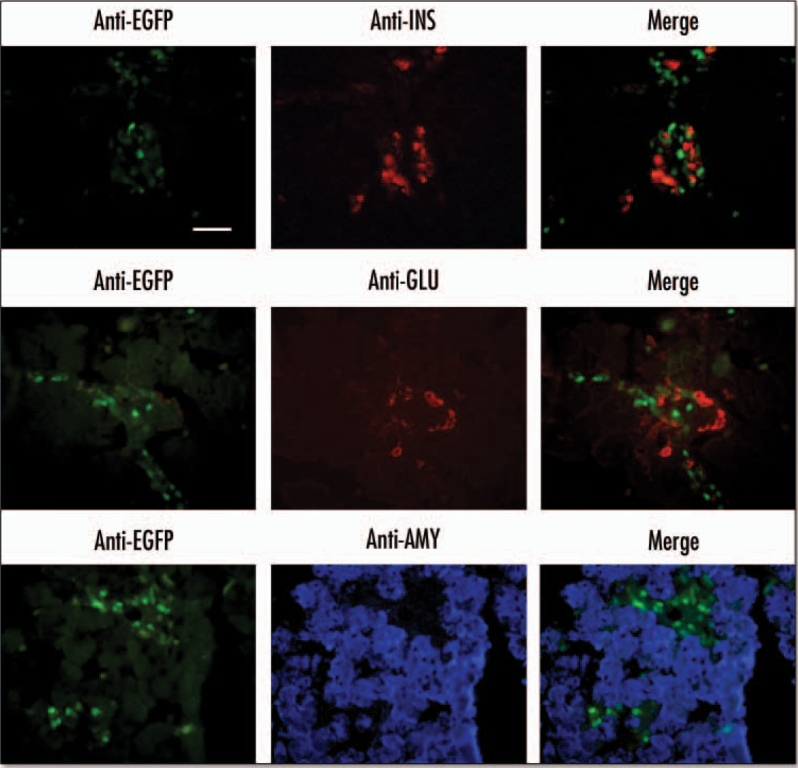
EGFP was also present in the E18.5 pancreas, where it was localized to ductal structures or interstitial cells surrounding the acini (Fig. 6). There was no expression of ngn3 in the E18.5 pancreas. EGFP was also present within islet-like structures but there was no colocalisation with insulin, glucagon, somatostatin or pancreatic polypeptide (Fig. 7) and also no colocalisation of EGFP with amylase.
Figure 6.
EGFP expression in the E18.5 pancreas. Immunohistochemistry was performed on cryosections from an E18.5 pancreas. Anti-ngn3 primary antibody and AlexaFluor 350 secondary antibody were used to detect Ngn3 expression. EGFP was visualized directly using an EGFP filter (Scale bar = 50 µm).
Figure 7.
EGFP expression in endocrine-enriched regions of the E18.5 pancreas. Immunohistochemistry was performed on cryosections from an E18.5 pancreas. Primary antibodies for insulin (INS) and glucagon (GLU) were visualized with an anti-mouse rhodamine conjugated secondary antibody. Antibodies against amylase (AMY) were visualized using anti-rabbit AlexaFluor 350 secondary antibody. EGFP was visualized directly using an EGFP filter (Scale bar = 50 µm).
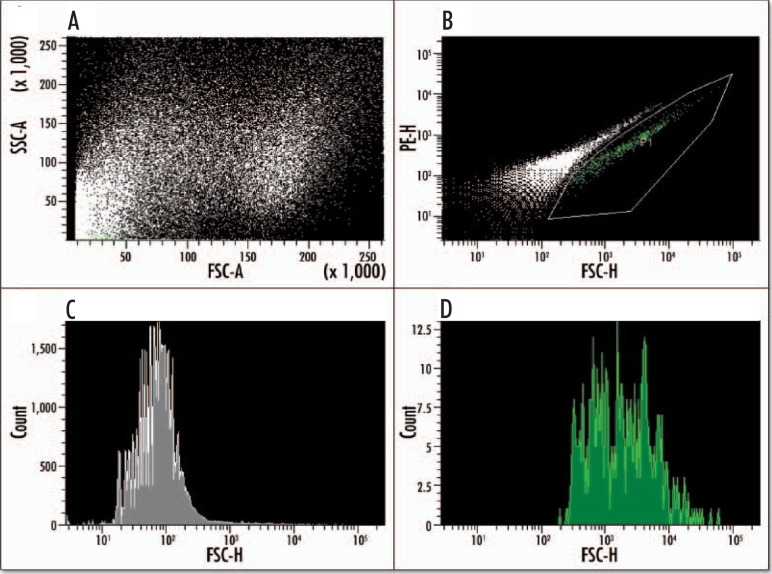
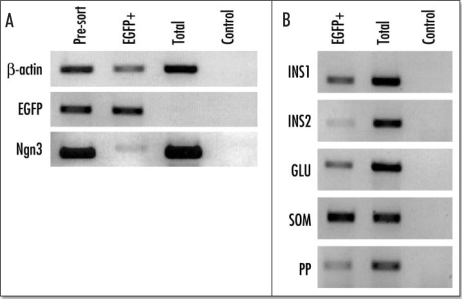
EGFP positive cells could be purified from the surrounding E15.5 pancreatic tissue using FACS. From the pooled EGFP-positive pancreases of one litter we were able to purify approximately 10,000 cells representing 1.6–2% of the total cell population (Fig. 8A and B). The purified population was achieved using a ‘purity sort’ to give a 96% pure population relative to start material (Fig. 8C and D). Reverse transcriptase PCR showed that the purified population expressed EGFP and ngn3, although the amount of ngn3 relative to that in the presorted population was much less than for EGFP (Fig. 9). The purified cells also expressed insulin 1 and insulin 2, as well as glucagon, somatostatin, and pancreatic polypeptide.
Figure 8.
Fluorescence activated cell sorting (FACS) of EGFP positive cells from an E15.5 pancreas. Dissected pancreases from a litter of transgenic mice were dispersed into a single cell suspension via enzyme digest and sorted based on their EGFP fluorescence. Analysis of the cells' scatter (A) shows two distinct and viable populations. The EGFP-positive population (gated green population) can be sorted (B) from the background population, which is shown as a white diagonal line of events. (C) A histogram analysis of the purified EGFP-negative population (gray). (D) A histogram of the purified EGFP-positive population (green).
Figure 9.
Reverse transcriptase PCR (RT/PCR) analysis of the purified EGFP-positive cells from an E15.5 pancreas. Ethidium bromide stained agarose gel of RT/PCR products from total RNA isolated from presorted FACS purified EGFP-positive cells, and the total population of enzyme dispersed cells from EGFP-negative embryos from the same litter. The control panel shows the reaction in the absence of reverse transcriptase. (A) b actin, EGFP and ngn3; (B) The results for insulin 1 (INS1), insulin 2 (INS2), glucagon (GLU), somatostatin (SOM) and pancreatic polypeptide (PP).
Discussion
We describe here a transgenic mouse, in which the ngn3 promoter drives EGFP. The fluorescent signal from the EGFP was sufficiently strong such that all fluorescing cells stained positively with an anti-EGFP antibody, i.e., the EGFP fluorescence was far in excess of any non-specific autofluorescence. In general terms the expression of EGFP was similar to that of endogenous ngn3. It peaked at around E15.5 in the dorsal and ventral lobes of the pancreas and, like ngn3, was also present in cells of the duodenum and nervous system. There was, however, no absolute correlation between EGFP fluorescence and ngn3 staining at the cellular level. Thus digital photographs of random stained fields showed that the frequency of cells expressing both EGFP and ngn3 was about 20–40%. This is in keeping with previous findings12 using similar promoter constructs (5.7 kb) and could be attributed to the random silencing of the transgene in some ngn3-expressing cells. We also observed that EGFP was present in regions of the pancreas that were enriched in insulin and glucagon expressing cells. These EGFP-expressing cells were located close to and within islet structures but excluded from ductal and acinar structures and may represent the descendents of ngn3+ progenitor cells. The EGFP+ cells from the E15.5 embryo were subjected to fluorescence activated cell sorting to provide a 96% pure population of cells that transcribed the hormone genes associated with all four endocrine cell types.
There are several possible reasons why coincident stimulation of the endogenous ngn3 gene and the ngn3 promoter-driven EGFP gene need not necessarily lead to readily observable coexpression. The half lives of transcription factors vary enormously, with those that induce expression of a broad range of genes often being subject to rapid degradation in order to maintain tight control over the transcriptome and to aid in the creation of gradients during development e.g., mammalian HIF-1α has a half life of only 5–8 minutes.13 This is in contrast to EGFP, which has a half-life of over 24 hours. This probable difference in protein half-lives would be compounded by potential differences in mRNA stabilities. The half life of transcription factor mRNAs is regulated and is generally short during development e.g., bicoid mRNA is stable in D. melanogaster oocytes but has a half life of only 30 minutes during embryo development.14 The mouse ngn3 gene has an AU-rich element (ARE) in the 3′-untranslated region (UTR) of the gene. This sequence (AAUAAAUAA or AUAAAUA) when present in mRNA causes the rapid degradation of that particular mRNA.15 The ARE is conserved in the mouse, rat and human ngn3 genes suggesting that it may be functionally active, and may underlie the observation that ngn3 expression is extinguished in the progeny of ngn3+ cells that were labelled using a CreER”/LoxP system.11 This is consistent with their role as islet progenitors but not self-renewing stem cells.16 Although the ngn3 mRNA in the mouse embryos contained the wild-type 3′-UTR, the corresponding region in the EGFP mRNA contained the efficient SV40 early polyadenylation signal derived from the plasmid during the creation of the transgenic mice. It has been shown that replacement of the AREcontaining 3′-UTR of mouse cyp7 mRNA with the SV40 early 3′-UTR in CMV promoter-driven chloramphenicol acetyltransferase (CAT) reporter constructs leads to a four-fold increase in CAT activity.17 Taken together, it is not surprising that colocalisation of EGFP and ngn3 was observed in only a portion of cells. Also, prolonged existence of EGFP mRNA and the protein itself in comparison to ngn3 would account for the former being present in E18.5 pancreas at a time when there was no detectable ngn3 immunoreactivity.
Consistent with their role as islet progenitors the purified EGFP+ cells also contained mRNA encoding insulin 1 and insulin 2 as well as glucagon, somatostatin and pancreatic polypeptide. Interestingly, the levels of insulin 2 were consistently lower than that of insulin 1 suggesting that the insulin 1 gene is expressed at a different stage than insulin 2. This is in keeping with reported findings that the two insulin genes are differentially expressed in developing mouse embryos. Their relative expression is subject to complex multistep control involving parental imprinting as well as tissue-and development stage-specific regulation.18
While this work was in progress a mouse was described in which ngn3+ cells were labelled with enhanced yellow fluorescent protein (EYFP) using a knock-add-on strategy.19 The expression pattern of EYFP in this mouse was very similar to the expression of EGFP in the present study. The EYFP cells were purified by FACS to yield about 1300 cells from a single E15.5 pancreas, while the effect of extrinsic factors on the appearance of EYFP in E12.5 pancreatic explant cultures could be monitored in real time. In our mice the presence of the neo gene may facilitate further studies on the culture and expansion of the purified EGFP+ cells.
Acknowledgements
J.B. was supported by an ACERO research studentship.
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/abstract.php?id=1727
References
- 1.Slack JMW. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 2.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111–127. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- 3.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes & Development. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- 5.Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 6.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 7.St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing a-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- 8.Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 10.Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha-and beta-cells from neurogenin3-expressing precursors: A role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- 11.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 12.Lee JC, Smith SB, Watada H, Lin J, Scheel D, Wang J, Mirmira RG, German MS. Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes. 2001;50:928–936. doi: 10.2337/diabetes.50.5.928. [DOI] [PubMed] [Google Scholar]
- 13.Berra E, Roux D, Richard DE, Pouyssegur J. Hypoxia-inducible factor-1 alpha (HIF-1 alpha) escapes O(2)-driven proteasomal degradation irrespective of its subcellular localization: Nucleus or cytoplasm. EMBO Rep. 2001;2:615–620. doi: 10.1093/embo-reports/kve130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surdej P, Jacobs-Lorena M. Developmental regulation of bicoid mRNA stability is mediated by the first 43 nucleotides of the 3′ untranslated region. Mol Cell Biol. 1998;18:2892–2900. doi: 10.1128/mcb.18.5.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan XC, Myer VE, Steitz JA. AU-rich elements target small nuclear RNAs as well as mRNAs for rapid degradation. Genes Dev. 1997;11:2557–2568. doi: 10.1101/gad.11.19.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 17.Agellon LB, Cheema SK. The 3′-untranslated region of the mouse cholesterol 7alpha-hydroxylase mRNA contains elements responsive to post-transcriptional regulation by bile acids. Biochem J. 1997;328:393–399. doi: 10.1042/bj3280393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deltour L, Montagutelli X, Guenet JL, Jami J, Paldi A. Tissue-and developmental stage-specific imprinting of the mouse proinsulin gene, Ins2. Dev Biol. 1995;168:686–688. doi: 10.1006/dbio.1995.1114. [DOI] [PubMed] [Google Scholar]
- 19.Mellitzer G, Martin M, Sidhoum-Jenny M, Orvain C, Barths J, Seymour PA, Sander M, Gradwohl G. Pancreatic islet progenitor cells in neurogenin 3-yellow fluorescent protein knock-add-on mice. Mol Endocrinol. 2004;18:2765–2776. doi: 10.1210/me.2004-0243. [DOI] [PubMed] [Google Scholar]