Abstract
Developmental exposure to organophosphates (OP) produces long-term changes in serotonin (5HT) synaptic function and associated behaviors, but there are disparities among the different OPs. We contrasted effects of chlorpyrifos and diazinon, as well as non-OP neurotoxicants (dieldrin, Ni2+) using undifferentiated and differentiating PC12 cells, a well-established neurodevelopmental model. Agents were introduced at 30 μM for 24 or 72 hr, treatments devoid of cytotoxicity, and we evaluated the mRNAs encoding the proteins for 5HT biosynthesis, storage and degradation, as well as 5HT receptors. Chlorpyrifos and diazinon both induced tryptophan hydroxylase, the rate-limiting enzyme for 5HT biosynthesis, but chlorpyrifos had a greater effect, and both agents suppressed expression of 5HT transporter genes, effects that would tend to augment extracellular 5HT. However, whereas chlorpyrifos enhanced the expression of most 5HT receptor subtypes, diazinon evoked overall suppression. Dieldrin evoked even stronger induction of tryptophan hydroxylase, and displayed a pattern of receptor effects similar to that of diazinon, even though they come from different pesticide classes. In contrast, Ni2+ had completely distinct actions, suppressing tryptophan hydroxylase and enhancing the vesicular monoamine transporter, while also reducing 5HT receptor gene expression, effects that would tend to lower net 5HT function. Our findings provide some of the first evidence connecting the direct, initial mechanisms of developmental neurotoxicant action on specific transmitter pathways with their long-term effects on synaptic function and behavior, while also providing support for in vitro test systems as tools for establishing mechanisms and outcomes of related and unrelated neurotoxicants.
Keywords: Chlorpyrifos, Diazinon, Dieldrin, Metal neurotoxicity, Microarrays, Neuronal differentiation, Neurotoxicity, Nickel, Organochlorine insecticides, Organophosphate insecticides, PC12 cells, Serotonin, Serotonin receptors
INTRODUCTION
Organophosphate pesticides are undergoing increased scrutiny because of their propensity to elicit developmental neurotoxicity at exposures devoid of any signs of systemic intoxication and below the threshold for cholinesterase inhibition, the standard exposure biomarker (Physicians for Social Responsibility, 1995; Landrigan et al., 1999; Pope, 1999; Slotkin, 1999, 2004, 2005; May, 2000; Landrigan, 2001; Casida and Quistad, 2004; Weiss et al., 2004; Perera et al., 2005; Costa, 2006). Indeed, it is now clear that disruption of brain development by these agents involves mechanisms unrelated to anticholinesterase actions, largely reflecting direct effects on neural cell replication and differentiation (Pope, 1999; Slotkin, 1999, 2004, 2005; Barone et al., 2000; Qiao et al., 2002, 2003a; Yanai et al., 2002; Betancourt and Carr, 2004; Casida and Quistad, 2004; Gupta, 2004; Betancourt et al., 2006; Slotkin et al., 2007c). Accordingly, native organophosphate chemicals can actually be more active as neurodevelopmental disruptors than are their oxon metabolites, which are the forms that produce irreversible cholinesterase inhibition (Buznikov et al., 2001; Qiao et al., 2001; Jameson et al., 2007). Nevertheless, most reports still focus on the impact of organophosphates on acetylcholine systems and related behaviors (Slotkin, 1999, 2004, 2005; Jett et al., 2001; Landrigan, 2001; Levin et al., 2001, 2002; Icenogle et al., 2004; Weiss et al., 2004; Rauh et al., 2006; Eskenazi et al., 2007, 2008). Recent studies show, however, that developing serotonin (5HT) systems may be even more sensitive, leading to abnormalities of emotional, appetitive and social behaviors (Aldridge et al., 2003, 2004, 2005a, 2005b, 2005c; Ricceri et al., 2003, 2006; Slotkin and Seidler, 2005, 2007b, 2007c; Slotkin et al., 2006b, 2008c, 2008e; Moreno et al., 2008; Timofeeva et al., 2008); indeed, organophosphate exposures are now being linked to depression and suicide (London et al., 2005; Beseler et al., 2006; Jaga and Dharmani, 2007; Lee et al., 2007).
If the organophosphates disrupt brain development through mechanisms other than their shared property as cholinesterase inhibitors, then there is no reason they should all produce exactly the same outcomes. In a series of reports, we found that exposure of neonatal rats to pharmacodynamically-equivalent doses of chlorpyrifos, diazinon and parathion, all elicit long-term alterations in 5HT synaptic function and in 5HT-related behaviors, but that the changes differ substantially among the three agents, even including alterations in opposite directions (Aldridge et al., 2003, 2004, 2005a; Slotkin and Seidler, 2005, 2007a; Slotkin et al., 2006b, 2008c; Timofeeva et al., 2008). In the current study, we compared the effects of chlorpyrifos and diazinon on the expression of genes involved in 5HT biosynthesis, storage and degradation, as well as genes for the 5HT receptor subtypes. We focused on the critical period in which developing neurons first express specific neurotransmitter phenotypes, so as to determine if the differences seen with in vivo exposures reflect disparities in the direct actions of these agents. We performed the studies in PC12 cells, a well-established neurodevelopmental model (Teng and Greene, 1994) that has already been validated to reproduce the mechanisms and outcomes underlying organophosphate developmental neurotoxicity (Tuler et al., 1989; Flaskos et al., 1994; Bagchi et al., 1995, 1996; Nagata et al., 1997; Li and Casida, 1998; Song et al., 1998; Das and Barone, 1999; Crumpton et al.,2000a, 2000b; Qiao et al., 2001, 2005; Jameson et al., 2006, 2007; Slotkin et al., 2007a, 2007b, 2008d). In the presence of nerve growth factor (NGF), PC12 cells differentiate to form neuritic projections and acquire neuronal excitability, as well as specific neurotransmitter characteristics. Although acetylcholine and dopamine are the primary phenotypes for this cell line (Fujita et al., 1989; Teng and Greene, 1994; Song et al., 1998), PC12 cells also manufacture, store and secrete 5HT, exhibit expression of the 5HT biosynthetic enzymes, transporters and receptors, display receptor-mediated responses to 5HT, and show enhancement of all these features during differentiation (Furukawa et al., 1992; King et al., 1992; Li and DePetrillo, 2002; Zhang et al., 2004, 2005; Reaney and Smith, 2005; Homma et al., 2006; Lee et al., 2006).
Besides the organophosphates, we also evaluated the response to two agents from different classes, the organochlorine pesticide, dieldrin, and a metal, divalent nickel. We previously found that chlorpyrifos, diazinon, dieldrin and Ni2+ all produce similar changes in the balance of acetylcholine vs. dopamine phenotypes (Jameson et al., 2006; Slotkin et al., 2007b). Here, our interest was to see whether diverse neurotoxicants, outside the organophosphate class, produce similar or dissimilar effects on development of 5HT neuronal characteristics. These agents also have intrinsic interest because of significant environmental concerns about human exposure and safety (U.S. National Library of Medicine, 2006). For dieldrin, this clearly includes known endpoints for developmental neurotoxicity (Uzoukwu and Sleight, 1972; Brannen et al., 1998; Liu et al., 1998; Kitazawa et al., 2001, 2003; Slotkin et al., 2007b). Nickel accumulates in the fetus at concentrations similar to those of lead (Casey and Robinson, 1978; Jacobsen et al., 1978), and the neural actions of this metal resemble those of lead and cadmium (Benters et al., 1996).
METHODS
Cell cultures
Because of the clonal instability of the PC12 cell line (Fujita et al., 1989), the experiments were performed on cells that had undergone fewer than five passages. As described previously (Song et al., 1998; Qiao et al., 2003b), PC12 cells (American Type Culture Collection, 1721-CRL, obtained from the Duke Comprehensive Cancer Center, Durham, NC) were seeded onto poly-D-lysine-coated plates in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% inactivated horse serum (Sigma Chemical Co., St. Louis, MO), 5% inactivated fetal bovine serum (Sigma), and 50 μg/ml penicillin streptomycin (Invitrogen). Incubations were carried out with 7.5% CO2 at 37°C, standard conditions for PC12 cells. To initiate neurodifferentiation (Teng and Greene, 1994; Jameson et al., 2006; Slotkin et al., 2007b) twenty-four hours after seeding, the medium was changed to include 50 ng/ml of 2.5 S murine NGF (Invitrogen). Along with the NGF, we added 30 μM of each of the test agents: chlorpyrifos (Chem Service, West Chester, PA), diazinon (Chem Service), dieldrin (Chem Service) or NiCl2 (Sigma). The concentration was chosen from earlier studies that demonstrated adverse effects on differentiation of PC12 cells without outright cytotoxicity (Qiao et al., 2001; Jameson et al., 2007;Slotkin et al., 2007b,2008d). Because of the limited water solubility of the three insecticides, these agents were dissolved in dimethylsulfoxide (final concentration 0.1%), which was also added to the control cultures and to cultures containing NiCl2; this concentration of dimethylsulfoxide has no effect on PC12 cell growth or differentiation (Song et al., 1998; Qiao et al., 2001, 2003b). Cultures were examined 24 and 72 hr after commencing exposure, with 5–8 independent cultures evaluated for each treatment at each time point. We used two time points so as to be able to evaluate changes in gene expression regardless of whether the mRNA for a given gene has a rapid turnover (and hence can rise rapidly) or a slower turnover that would require a longer period to show corresponding increases or decreases. For chlorpyrifos, we evaluated the effects both on undifferentiated cells and during NGF-induced differentiation, whereas for the other agents, we studied only the effects during differentiation.
Microarray determinations
Our earlier studies detailed all the techniques for mRNA isolation, preparation of cDNA, conversion to cRNA incorporating cyanine-3 (reference RNA) or cyanine-5 (sample RNA), verification of RNA purity and quality, hybridization to the microarrays, washing and scanning (Slotkin and Seidler, 2007a; Slotkin et al., 2007c, 2008d). These all involve commercial kits and procedures, and since the current studies were done identically, the techniques will not be described here. The mRNA used for the reference standard was created by pooling aliquots from each of the samples in the study so as to ensure measurable levels of all genes expressed over the background. Similarly, array normalizations and error detection were carried out by procedures described previously (Slotkin and Seidler, 2007a; Slotkin et al., 2007c, 2008d). We used Agilent Whole Rat Genome Arrays (Agilent Technologies, Palo Alto, CA), type G4131A for the studies of chlorpyrifos in undifferentiated and differentiating cells, and type G4131F for the studies of diazinon, dieldrin and nickel in differentiating cells. The two chips contain exactly the same sequences but the latter has a lower detection threshold; however, all the genes reported here passed the quality control filters with both arrays.
For many of the genes, the arrays contain multiple probes and/or replicates of the same probe in different locations on the chip, and these were used to verify the reliability of values and the validity of the measures on the chip. In these cases, to avoid artificially inflating the number of positive findings, we limited each gene to a single set of values, selecting those obtained for the probe showing the smallest intragroup variance. The other values for that gene were used only to corroborate direction and magnitude of change. We also validated the readings on the arrays through the use of duplicate arrays for selected samples (Slotkin and Seidler, 2007a;Slotkin et al., 2007c). Values reported for four of the genes presented here (maoa, maob, slc18a1, slc18a2) are also being used in a study of catecholamine phenotypes because of their relation to monoamines in general.
Statistical procedures
Because of the requirement to normalize the data across arrays and within each gene, the absolute values for a given gene are meaningless, so only the relative differences between treatments can be compared. Accordingly, results are presented as means and standard errors of the percentage change from control values to allow for visual comparison of the effects across families of genes. However, statistical comparisons were based on the actual ratios (log-transformed, since the data are in the form of ratios) rather than the percent change.
Our design involved multiple planned comparisons of four agents at two time points, as well as the effects of one agent (chlorpyrifos) in undifferentiated vs. differentiating states. It was therefore important to consider the false positive rate and to protect against the increased probability of type 1 errors engendered by repeated testing of the same data base. Accordingly, before looking at effects on individual genes, we performed a global ANOVA incorporating all the variables in a single comparison: treatment, time, and all genes. Lower-order ANOVAs on subdivisions of the data set were then carried out as permitted by the interactions of treatment with the other variables. Finally, differences for individual treatments for a specified gene at a single time point were evaluated with Fisher’s Protected Least Significant Difference. However, for a given gene where there was no interaction of treatment with other variables (time, differentiation state), only the main treatment effect was reported without subtesting of effects at a single time point. Treatment effects were considered significant at p < 0.05 (two-tailed, since we were interested in both increases and decreases in gene expression). In addition to these parametric tests of the direction and magnitude of changes in gene expression, we evaluated the incidence of significant differences as compared to the predicted false positive rate, using Fisher’s Exact Test, applying a one-tailed criterion of p < 0.05, since only an increase above the false positive rate would be predicted; at the criterion of p < 0.05, one gene out of every 20 tested can be expected to show a difference at random. Finding a significant decrease in the incidence of detected differences relative to the false positive rate would be biologically implausible and statistically meaningless.
RESULTS
Global statistical analysis
We evaluated effects in both undifferentiated and differentiating cells for chlorpyrifos, whereas for the other three agents, studies were confined to differentiating cells only. Accordingly, we conducted two initial global statistical tests. For the evaluations of chlorpyrifos, ANOVA incorporating all factors (treatment, differentiation state, time, gene) identified a main effect of treatment (p < 0.003, control < chlorpyrifos) and interactions of treatment × differentiation state (p < 0.04), treatment × gene (p < 0.0001), treatment × gene × time (p < 0.0002) and treatment × gene × state (p < 0.0003). For the entire set of 19 genes, we found 13 showing significant differences, as compared to a predicted false positive rate of 1 gene (p < 0.0005). For the study of diazinon, dieldrin and Ni2+ conducted in differentiating cells, global ANOVA (factors of treatment, gene, time) identified a main effect of treatment (p < 0.0001; control > dieldrin ≈ diazinon > Ni2+) and interactions of treatment × time (p < 0.03), treatment × gene (p < 0.0001) and treatment × gene × time (p < 0.0001). Out of 19 total genes, we found significant differences for 15 (p < 10−5 vs. the predicted false positive rate). In light of the interactions of treatment with the other variables, we divided the data into the separate treatments for presentation, with genes grouped by functional relationships. The first group contained the genes involved in 5HT synthesis, storage and degradation: tryptophan hydroxylase (tph), the high-affinity presynaptic 5HT transporter (slc6a4), the vesicular monoamine transporter genes (slc18a1, slc18a2), and both of the monoamine oxidases (maoa, maob). The second grouping contained the genes encoding all of the 5HT receptor subtypes that passed the quality control filters: htr1a, htr1b, htr1d, htr1f, htr2a, htr2b, htr2c, htr3a, htr3b, htr5a, htr5b, htr5 and htr7.
Chlorpyrifos. In both undifferentiated and differentiating cells, chlorpyrifos exposure evoked upregulation of tph, the gene encoding tryptophan hydroxylase, the rate-limiting enzyme in 5HT biosynthesis, and downregulation of slc6a4, encoding the presynaptic high-affinity 5HT transporter (Figure 1A). The effects on the vesicular monoamine transporter genes were highly dependent on differentiation state: there was a small reduction in slc18a1 expression restricted to differentiating cells, but more robust changes in slc18a2, with suppression in the undifferentiated state and stimulation during differentiation. Neither isoform of monoamine oxidase showed any significant effect of chlorpyrifos exposure.
Figure 1.

Effects of 30 μM chlorpyrifos exposure on expression of genes encoding proteins for: (A) 5HT synthesis, storage and degradation, (B) 5HT receptors. Results are shown for both undifferentiated cells (U 24h, U 72h) and cells undergoing NGF-induced differentiation (D 24h, D 72h). Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with the other variables (differentiation state, time) and show the individual values for which treatment effects were present. Multivariate ANOVA indicates a main effect of treatment (p < 0.003) and interactions of treatment × differentiation state (p < 0.04), treatment × gene (p < 0.0001), treatment × gene × time (p < 0.0002) and treatment × gene × state (p < 0.0003).
Chlorpyrifos had major effects on the expression of 5HT receptors (Figure 1B), predominantly representing a significant net increase: p < 0.009 for both differentiation states, p < 0.02 for differentiating cells. In both undifferentiated and differentiating cells, chlorpyrifos increased expression for htr1a, htr1d, htr2b and htr5a, and decreased expression for htr3b. More selective effects were seen for htr1b and htr6 (stimulated in differentiating cells only), htr5b (stimulated in undifferentiated cells only) and htr7 (small but significant decrease restricted to differentiating cells).
Diazinon
Although diazinon, like chlorpyrifos, increased tph expression, the effect was much smaller (Figure 2A). Diazinon also evoked significant decrements in expression of all three transporter genes (slc6a4, slc18a1, slc18a2), as well as maob. There were marked differences in the effects on 5HT receptors between the two organophosphates. Whereas chlorpyrifos evoked an overall increase in receptor expression, diazinon exposure evoked a significant (p < 0.03) net decrease (Figure 2B) Individually, significant reductions were seen for htr1d, htr2b and htr6, with htr2c showing a transient increase followed by a decrease, and htr3a showing a small but significant increase.
Figure 2.
Effects of 30 μM diazinon exposure on expression of genes encoding proteins for: (A) 5HT synthesis, storage and degradation, (B) 5HT receptors. Results are shown for cells undergoing NGF-induced differentiation. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with time and show the individual values for which treatment effects were present. Multivariate ANOVA indicates a main effect of treatment (p < 0.0002) and interactions of treatment × gene (p < 0.03) and treatment × gene × time (p < 0.004).
Dieldrin
Exposure to dieldrin during differentiation produced marked upregulation of tph, more than that seen with the other agents (Figure 3A). As with chlorpyrifos or diazinon, dieldrin reduced the expression of slc6a4 and slc18a1 but did not affect slc18a2, maoa or maob. For 5HT receptor expression, dieldrin evoked a significant (p < 0.002) overall decrease with much more widespread effects than those seen for diazinon. We found reduced expression for eight subtypes (htr1a, htr1d, htr2b, htr2c, htr3a, htr3b, htr4b, htr6), with only one subtype showing an increase (htr5a).
Figure 3.
Effects of 30 μM dieldrin exposure on expression of genes encoding proteins for: (A) 5HT synthesis, storage and degradation, (B) 5HT receptors. Results are shown for cells undergoing NGF-induced differentiation. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with time and show the individual values for which treatment effects were present. Multivariate ANOVA indicates a main effect of treatment (p < 0.0003) and interactions of treatment × gene (p < 0.0001) and treatment × gene × time (p < 0.0003).
Divalent nickel
The effects of Ni2+ were entirely distinct from those of the other three neurotoxicants. Uniquely, Ni2+ reduced expression of tph and enhanced that of slc18a1 (Figure 4A); however, like chlorpyrifos, diazinon and dieldrin, Ni2+ also evoked a suppression of slc6a4. Overall, Ni2+ exposure reduced the net expression of 5HT receptor genes (Figure 4B) even more strongly than did diazinon or dieldrin (p < 0.0001 vs. control, p < 0.01 vs. diazinon, p < 0.05 vs. dieldrin). Individually, significant decrements were seen for htr1d, htr2a and htr3b. The htr3a subtype showed a biphasic effect (initial suppression and a small rebound increase), whereas htr5b showed a transient elevation.
Figure 4.
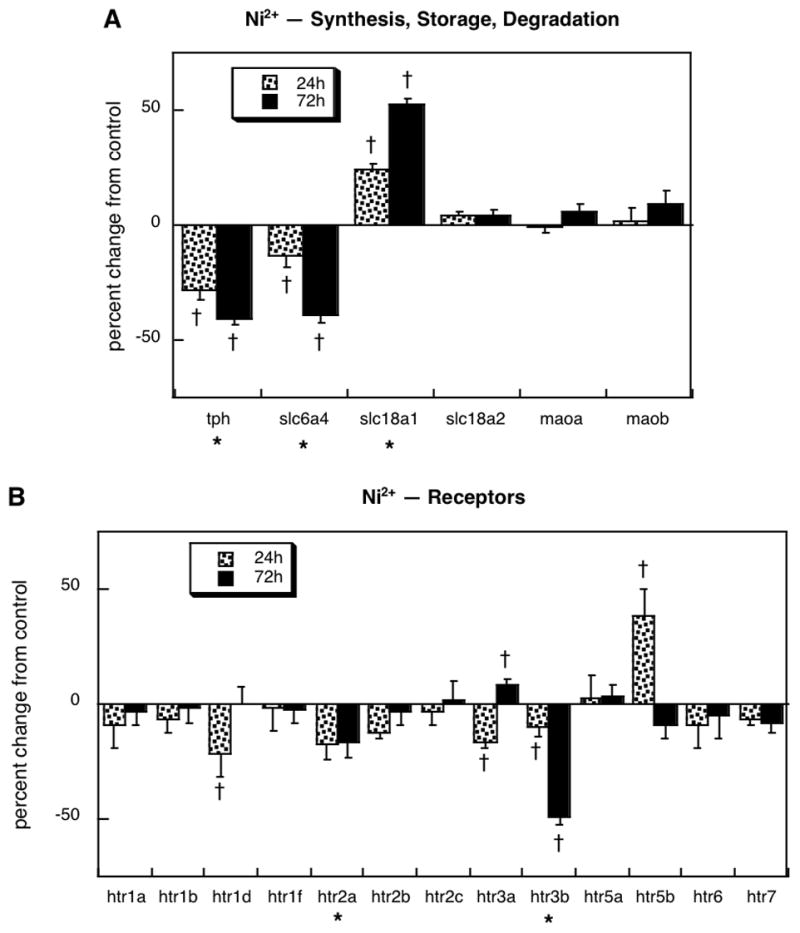
Effects of 30 μM Ni2+ exposure on expression of genes encoding proteins for: (A) 5HT synthesis, storage and degradation, (B) 5HT receptors. Results are shown for cells undergoing NGF-induced differentiation. Asterisks shown below each gene denote a significant main treatment effect. Daggers denote genes for which treatment interacted with time and show the individual values for which treatment effects were present. Multivariate ANOVA indicates a main effect of treatment (p < 0.0001) and interactions of treatment × gene (p < 0.0001) and treatment × gene × time (p < 0.0008).
DISCUSSION
In our earlier, in vivo work with chlorpyrifos administered to neonatal rats, we demonstrated permanent, profound and global upregulation of 5HT receptors, the 5HT transporter, and 5HT synaptic activity, associated with major alterations in 5HT-dependent behaviors (Aldridge et al., 2004, 2005a, 2005b; Slotkin and Seidler, 2005, 2007c). In contrast, equivalent neonatal exposure to diazinon produced a much more restricted spectrum of effects and a correspondingly lesser impact on 5HT-related behaviors (Roegge et al., 2008;Slotkin et al., 2008c; Timofeeva et al., 2008); in particular, whereas the largest promotional effect for chlorpyrifos was for 5HT1A receptors, diazinon produced downregulation of this subtype, and the two agents had opposite effects in anxiety tests. Here, we found that these disparities in long-term outcomes are likely to originate in differences in the direct actions of the two agents on neurodifferentiation into the 5HT phenotype. The main effect of chlorpyrifos represented an increase in the overall expression of 5HT-related genes, whereas the main effect of diazinon was to repress the same repertoire. These relationships hold up even for receptor subtypes, as chlorpyrifos produced its strongest induction for the 5HT1 receptor genes whereas diazinon produced downregulation of 5HT1d.
Within the set of 5HT-related genes, the patterns of up- and downregulation give important clues as to the immediate functional consequences of chlorpyrifos or diazinon exposure. With chlorpyrifos, we found upregulation of tph, the gene encoding the rate-limiting enzyme for 5HT biosynthesis, combined with downregulation of slc6a4, the high-affinity 5HT transporter responsible for terminating the actions of 5HT after its release (Cooper et al., 1996). An increase in 5HT synthesis, combined with a decrease in synaptic reuptake and superimposed on the induction of 5HT receptors, would clearly tend to enhance 5HT actions. The reason this is important is that 5HT plays a critical morphogenetic role in the developing brain (Lauder, 1985; Whitaker-Azmitia, 1991; Dreyfus, 1998). Thus, the fact that chlorpyrifos directly augments the actions of 5HT is likely to provide one of the key factors in more global disruption of brain development. Indeed, this may be one of the reasons that chlorpyrifos appears to elicit greater long-term disruption than diazinon or parathion (Qiao et al., 2001; Slotkin et al., 2006a, 2006b, 2007b, 2007c, 2008a, 2008b, 2008c, 2008d; Jameson et al., 2007; Roegge et al., 2008; Timofeeva et al., 2008). Here, unlike the impact on genes for the 5HT receptors, diazinon evoked changes in 5HT synthesis and uptake in the same direction as chlorpyrifos (increased tph, decreased slc6a4 and slc18a1), but with a smaller magnitude of effect for tph. Consequently, diazinon might be expected to elicit similar disruption of some neurotrophic events related to 5HT function, but to a lesser extent than for chlorpyrifos.
In addition to our evaluations of chlorpyrifos and diazinon, we performed studies comparing the effects of the organophosphates to two unrelated developmental neurotoxicants, dieldrin and Ni2+. Dieldrin produced some effects that were surprisingly similar to those of diazinon, with a significant overall decrease in the expression of 5HT-related genes, largely reflecting receptor downregulation. Like diazinon and chlorpyrifos, dieldrin also induced tph while suppressing the transporter genes, slc6a4 and slc18a1. We might therefore expect that the long-term synaptic and behavioral consequences of neonatal dieldrin exposure will resemble those of diazinon, but like chlorpyrifos, might be much greater because of the higher degree of tph induction; these predictions need to be tested in future work. In contrast, the effects of Ni2+ were totally distinct from those of the other three agents, with strong downregulation of tph and induction of slc18a1, combined with overall suppression of 5HT receptor gene expression, effects that would tend to reduce net 5HT function. Consequently, we would predict a very different spectrum of neurobehavioral abnormalities from developmental exposure to this metal.
Our combined use of cell cultures and microarrays introduce a number of interpretational limitations. First, since PC12 cells are a transformed cell line, they are typically less sensitive to toxicants in general, requiring higher concentrations than those experienced with typical in vivo exposures. Further, since treatments occur over a very short time-span, rather than throughout brain development as would occur in vivo, higher concentrations are required to produce corresponding changes. To offset some of these problems, two of the test compounds, chlorpyrifos and diazinon, have been thoroughly studied and found to elicit parallel outcomes in the PC12 model and in developing rat brain (Tuler et al., 1989; Flaskos et al., 1994; Bagchi et al., 1995, 1996; Nagata et al., 1997; Li and Casida, 1998; Song et al., 1998; Das and Barone, 1999;Crumpton et al., 2000a,2000b; Qiao et al., 2001, 2005; Jameson et al., 2006, 2007; Slotkin et al., 2007a, 2007b, 2008d). Indeed, although the concentration used here, 30 μM, is approximately an order of magnitude higher than those found in newborn babies after nonsymptomatic environmental exposures in agricultural communities (Ostrea et al., 2002), the cultures contain high concentrations of serum proteins; accordingly, less than 10% of the nominal concentration is actually available to diffuse into the cells (Qiao et al., 2001). As with all studies of gene expression, the fact that microarrays detect mRNA also produces some limitations in that the transcriptional changes may not correspond exactly to alterations at the protein level. Again, the main conclusions reached here for chlorpyrifos and diazinon have been verified with in vivo exposures (Aldridge et al., 2003, 2004, 2005a; Slotkin and Seidler, 2005, 2007a; Slotkin et al., 2006b, 2008c; Timofeeva et al., 2008). As just one example, chlorpyrifos exposure in neonatal rats enhances 5HT turnover, which obligates an increase in 5HT biosynthesis (Aldridge et al., 2005b; Slotkin and Seidler, 2007c), paralleling the upregulation of tryptophan hydroxylase observed in the present study. Nevertheless, it is important, as done here, to render interpretations based on changes in multiple genes within a given pathway, rather than relying on a single gene. Finally, a number of genes did not show monotonic effects but rather exhibited biphasic alterations (e.g. slc18a2 for chlorpyrifos in differentiating cells, htr2c for diazinon, htr1d for dieldrin, htr5b for Ni2+). This is not unexpected, since genes that have fast turnover times are likely to show equally rapid increases or decreases, followed by compensatory changes in the opposite direction from the initial effect. Indeed, this is the reason we chose to evaluate the temporal course of effects, rather than relying on a single time point, as is more often done in microarray studies. Similar factors operate for many of the genes that show statistically significant alterations despite a small magnitude of effect: genes that are tightly controlled will naturally show smaller coefficients of variation and correspondingly smaller degrees of change over a short time-span; yet, these smaller changes may be functionally just as important as larger changes in genes whose expression is more volatile. Although it may be difficult to ascertain the impact of biphasic mRNA changes or small-magnitude changes on protein levels, these patterns nevertheless provide a “signature” for comparison of agents acting on the same gene families. Because we have many of the parallel in vivo findings for two of the four agents evaluated here (chlorpyrifos, diazinon), we can now make corresponding predictions about the potential effects of dieldrin and Ni2+ than can focus and guide future in vivo work.
Our findings provide some of the first evidence connecting the direct, initial mechanisms of toxicant action on specific neurotransmitter pathways with their long-term effects on synaptic function and behavior. In this instance, we were able to distinguish clearly between the actions of two different organophosphates, and to make corresponding predictions about dieldrin and Ni2+ that can help focus future investigations on the most appropriate synaptic and behavioral endpoints. Equally important, the results with PC12 cells provide strong support for the view that in vitro test systems can give key indications of lasting neurodevelopmental changes, while at the same time providing a framework that enables comparisons of mechanisms and outcomes applicable to both related and unrelated neurotoxicants.
Acknowledgments
Research was supported by NIH ES10356. The sponsor had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Abbreviations
- 5HT
5-hydroxytryptamine serotonin
- ANOVA
analysis of variance
- NGF
nerve growth factor
Footnotes
Conflicts of Interest: The authors do not have any conflicts of interest, but have provided past expert witness testimony on behalf of government agencies, corporations and/or individuals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005a;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005b;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005c;203:134–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bhattacharya G, Stohs SJ. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology. 1996;112:57–68. doi: 10.1016/0300-483x(96)03350-1. [DOI] [PubMed] [Google Scholar]
- Barone S, Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology. 2000;21:15–36. [PubMed] [Google Scholar]
- Benters J, Schafer T, Beyersmann D, Hechtenberg S. Agonist-stimulated calcium transients in PC12 cells are affected differentially by cadmium and nickel. Cell Calcium. 1996;20:441–446. doi: 10.1016/s0143-4160(96)90007-x. [DOI] [PubMed] [Google Scholar]
- Beseler C, Stallones L, Hoppin JA, Alavanja MCR, Blair A, Keefe T, Kamel F. Depression and pesticide exposures in female spouses of licensed pesticide applicators in the Agricultural Health Study Cohort. J Occup Environ Med. 2006;48:1005–1013. doi: 10.1097/01.jom.0000235938.70212.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt AM, Burgess SC, Carr RL. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicol Sci. 2006;92:500–506. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- Betancourt AM, Carr RL. The effect of chlorpyrifos and chlorpyrifos-oxon on brain cholinesterase, muscarinic receptor binding, and neurotrophin levels in rats following early postnatal exposure. Toxicol Sci. 2004;77:63–71. doi: 10.1093/toxsci/kfh003. [DOI] [PubMed] [Google Scholar]
- Brannen KC, Devaud LL, Liu JP, Lauder JM. Prenatal exposure to neurotoxicants dieldrin or lindane alters tert-butylbicyclophosphorothionate binding to GABA(A) receptors in fetal rat brainstem. Dev Neurosci. 1998;20:34–41. doi: 10.1159/000017296. [DOI] [PubMed] [Google Scholar]
- Buznikov GA, Nikitina LA, Bezuglov VV, Lauder JM, Padilla S, Slotkin TA. An invertebrate model of the developmental neurotoxicity of insecticides: effects of chlorpyrifos and dieldrin in sea urchin embryos and larvae. Environ Health Perspect. 2001;109:651–661. doi: 10.1289/ehp.01109651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey CE, Robinson MF. Copper, manganese, zinc, nickel, cadmium and lead in human foetal tissues. Br J Nutrition. 1978;39:639–646. doi: 10.1079/bjn19780079. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 7. Oxford University Press; New York: 1996. [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factor involved in cell replication and differentiation. Brain Res. 2000a;857:87–98. doi: 10.1016/s0006-8993(99)02357-4. [DOI] [PubMed] [Google Scholar]
- Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res. 2000b;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- Das KP, Barone S. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: is acetylcholinesterase inhibition the site of action? Toxicol Appl Pharmacol. 1999;160:217–230. doi: 10.1006/taap.1999.8767. [DOI] [PubMed] [Google Scholar]
- Dreyfus CF. Neurotransmitters and neurotrophins collaborate to influence brain development. Perspect Dev Neurobiol. 1998;5:389–399. [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, Johnson C, Fenster L, Barr DB. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102:228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Flaskos J, McLean WG, Hargreaves AJ. The toxicity of organophosphate compounds towards cultured PC12 cells. Toxicol Lett. 1994;70:71–76. doi: 10.1016/0378-4274(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Fujita K, Lazarovici P, Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Akaike N, Onodera H, Kogure K. Expression of 5-HT3 receptors in PC12 cells treated with NGF and 8-Br-cAMP. J Neurophysiol. 1992;67:812–819. doi: 10.1152/jn.1992.67.4.812. [DOI] [PubMed] [Google Scholar]
- Gupta RC. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol Mech Meth. 2004;14:103–143. doi: 10.1080/15376520490429175. [DOI] [PubMed] [Google Scholar]
- Homma K, Kitamura Y, Ogawa H, Oka K. Serotonin induces the increase in intracellular Ca2+ that enhances neurite outgrowth in PC12 cells via activation of 5-HT3 receptors and voltage-gated calcium channels. J Neurosci Res. 2006;84:316–325. doi: 10.1002/jnr.20894. [DOI] [PubMed] [Google Scholar]
- Icenogle LM, Christopher C, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Slotkin TA, Levin ED. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol Teratol. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Jacobsen N, Alfheim I, Jonsen J. Nickel and strontium distribution in some mouse tissues. Passage through placenta and mammary glands. Res Comm Chem Pathol Pharmacol. 1978;20:571–584. [PubMed] [Google Scholar]
- Jaga K, Dharmani C. The interrelation between organophosphate toxicity and the epidemiology of depression and suicide. Rev Environ Health. 2007;22:57–73. doi: 10.1515/reveh.2007.22.1.57. [DOI] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos affects phenotypic outcomes in a model of mammalian neurodevelopment: critical stages targeting differentiation in PC12 cells. Environ Health Perspect. 2006;114:667–672. doi: 10.1289/ehp.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson RR, Seidler FJ, Slotkin TA. Nonenzymatic functions of acetylcholinesterase splice variants in the developmental neurotoxicity of organophosphates: chlorpyrifos, chlorpyrifos oxon and diazinon. Environ Health Perspect. 2007;115:65–70. doi: 10.1289/ehp.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett DA, Navoa RV, Beckles RA, McLemore GL. Cognitive function and cholinergic neurochemistry in weanling rats exposed to chlorpyrifos. Toxicol Appl Pharmacol. 2001;174:89–98. doi: 10.1006/taap.2001.9198. [DOI] [PubMed] [Google Scholar]
- King SC, Tiller AA, Chang AS, Lam DM. Differential regulation of the imipramine-sensitive serotonin transporter by cAMP in human JAr choriocarcinoma cells, rat PC12 pheochromocytoma cells, and C33-14-B1 transgenic mouse fibroblast cells. Biochem Biophys Res Comm. 1992;183:487–491. doi: 10.1016/0006-291x(92)90508-i. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radical Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cd in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. Pesticides and polychlorinated biphenyls (PCBs): an analysis of the evidence that they impair children's neurobehavioral development. Mol Genet Metab. 2001;73:11–17. doi: 10.1006/mgme.2001.3177. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Godbold JH, Wolff MS. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107(suppl 3):431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM. Roles for neurotransmitters in development: possible interaction with drugs during the fetal and neonatal periods. In: Marois M, editor. Prevention of Physical and Mental Congenital Defects. Alan R. Liss; New York: 1985. pp. 375–380. [PubMed] [Google Scholar]
- Lee E, Williams Z, Goodman CB, Oriaku ET, Harris C, Thomas M, Soliman KFA. Effects of NMDA receptor inhibition by phencyclidine on the neuronal differentiation of PC12 cells. Neurotoxicology. 2006;27:558–566. doi: 10.1016/j.neuro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Alavanja MCR, Hoppin JA, Rusiecki JA, Kamel F, Blair A, Sandler DP. Mortality among pesticide applicators exposed to chlorpyrifos in the agricultural health study. Environ Health Perspect. 2007;115:528–534. doi: 10.1289/ehp.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Li WW, Casida JE. Organophosphorus neuropathy target esterase inhibitors selectively block outgrowth of neurite-like and cell processes in cultured cells. Toxicol Lett. 1998;98:139–146. doi: 10.1016/s0378-4274(98)00116-7. [DOI] [PubMed] [Google Scholar]
- Li X, DePetrillo PB. Corticosterone increases serotonin type-3 receptor mRNA in rat pheochromocytoma-12 cells. Neurosci Lett. 2002;331:143–145. doi: 10.1016/s0304-3940(02)00870-4. [DOI] [PubMed] [Google Scholar]
- Liu JP, Brannen KC, Grayson DR, Morrow AL, Devaud LL, Lauder JM. Prenatal exposure to the pesticide dieldrin or the GABA(A) receptor antagonist bicuculline differentially alters expression of GABA(A) receptor subunit mRNAs in fetal rat brainstem. Dev Neurosci. 1998;20:83–92. doi: 10.1159/000017302. [DOI] [PubMed] [Google Scholar]
- London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med. 2005;47:308–321. doi: 10.1002/ajim.20147. [DOI] [PubMed] [Google Scholar]
- May M. Disturbing behavior: neurotoxic effects in children. Environ Health Perspect. 2000;108:A262–A267. doi: 10.1289/ehp.108-a262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Canadas F, Cardona D, Sunol C, Campa L, Sanchez-Amate MC, Flores P, Sanchez-Santed F. Long-term monoamine changes in the striatum and nucleus accumbens after acute chlorpyrifos exposure. Toxicol Lett. 2008;176:162–167. doi: 10.1016/j.toxlet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Nagata K, Huang CS, Song JH, Narahashi T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res. 1997;769:211–218. doi: 10.1016/s0006-8993(97)00707-5. [DOI] [PubMed] [Google Scholar]
- Ostrea EM, Morales V, Ngoumgna E, Prescilla R, Tan E, Hernandez E, Ramirez GB, Cifra HL, Manlapaz ML. Prevalence of fetal exposure to environmental toxins as determined by meconium analysis. Neurotoxicology. 2002;23:329–339. doi: 10.1016/s0161-813x(02)00077-3. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, Diaz D, Dietrich J, Reyes A, Kinney PL. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Physicians for Social Responsibility. Pesticides and Children. Physicians for Social Responsibility; Washington DC: 1995. [Google Scholar]
- Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: What is the vulnerable period? Environ Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect. 2003a;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Violin JD, Slotkin TA. Nicotine is a developmental neurotoxicant and neuroprotectant: stage-selective inhibition of DNA synthesis coincident with shielding from effects of chlorpyrifos. Dev Brain Res. 2003b;147:183–190. doi: 10.1016/s0165-3806(03)00222-0. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera R, Andrews H, Hoepner L, Barr D, Whitehead D, Tang D, Whyatt RM. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaney SH, Smith DR. Manganese oxidation state mediates toxicity in PC12 cells. Toxicol Appl Pharmacol. 2005;205:271–281. doi: 10.1016/j.taap.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, Calamandrei G. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicol Appl Pharmacol. 2003;191:189–201. doi: 10.1016/s0041-008x(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamendrei G. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol Sci. 2006;93:105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008a;116:340–348. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Levin ED, Seidler FJ. Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environ Health Perspect. 2008b doi: 10.1289/ehp.11451. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006a;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Seidler FJ. Ameliorating the developmental neurotoxicity of chlorpyrifos: a mechanisms-based approach in PC12 cells. Environ Health Perspect. 2007a;115:1306–1313. doi: 10.1289/ehp.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Ryde IT, Tate CA, Seidler FJ. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine and divalent nickel. Environ Health Perspect. 2007b;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low-dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull. 2008c;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007a;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res Bull. 2007b;73:301–309. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod Toxicol. 2007c;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ Health Perspect. 2007c;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Targeting of neurotrophic factors, their receptors, and signaling pathways in the developmental neurotoxicity of organophosphates in vivo and in vitro. Brain Res Bull. 2008d;76:424–438. doi: 10.1016/j.brainresbull.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Ryde IT, Yanai J. Developmental neurotoxic effects of chlorpyrifos on acetylcholine and serotonin pathways in an avian model. Neurotoxicol Teratol. 2008e doi: 10.1016/j.ntt.2008.02.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006b;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Teng KK, Greene LA. Cultured PC12 cells: a model for neuronal function and differentiation. In: Celis JE, editor. Cell Biology: A Laboratory Handbook. Academic Press; San Diego: 1994. pp. 218–224. [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol. 2008;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuler SM, Hazen AA, Bowen JM. Release and metabolism of dopamine in a clonal line of pheochromocytoma (PC12) cells exposed to fenthion. Fund Appl Toxicol. 1989;13:484–492. doi: 10.1016/0272-0590(89)90284-4. [DOI] [PubMed] [Google Scholar]
- U.S. National Library of Medicine (2006). Superfund Chemicals in TOXMAP.
- Uzoukwu M, Sleight SD. Dieldrin toxicosis: fetotoxicosis, tissue concentrations, and microscopic and ultrastructural changes in guinea pigs. Am J Vet Res. 1972;33:579–583. [PubMed] [Google Scholar]
- Weiss B, Amler S, Amler RW. Pesticides. Pediatrics. 2004;113:1030–1036. [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Role of serotonin and other neurotransmitter receptors in brain development: basis for developmental pharmacology. Pharmacol Rev. 1991;43:553–561. [PubMed] [Google Scholar]
- Yanai J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann NY Acad Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, Schwartz DA, Krishnan KRR, Caron MG. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]




