Abstract
In the neocortex, neurons with similar response properties are often clustered together in column-like structures, giving rise to what has become known as functional architecture—the mapping of various stimulus feature dimensions onto the cortical sheet. At least partially, we owe this finding to the availability of several functional brain imaging techniques, both post-mortem and in-vivo, which have become available over the last two generations, revolutionizing neuroscience by yielding information about the spatial organization of active neurons in the brain. Here, we focus on how our understanding of such functional architecture is linked to the development of those functional imaging methodologies, especially to those that image neuronal activity indirectly, through metabolic or haemodynamic signals, rather than directly through measurement of electrical activity. Some of those approaches allow exploring functional architecture at higher spatial resolution than others. In particular, optical imaging of intrinsic signals reaches the striking detail of ∼50 μm, and, together with other methodologies, it has allowed characterizing the metabolic and haemodynamic responses induced by sensory-evoked neuronal activity. Here, we review those findings about the spatio-temporal characteristics of neurovascular coupling and discuss their implications for functional brain imaging, including position emission tomography, and non-invasive neuroimaging techniques, such as funtional magnetic resonance imaging, applicable also to the human brain.
INTRODUCTION
Experimental investigation of the metabolic counterpart of brain activity, and in particular its effects on blood flow, goes back at least as far as the late 19th century. Yet, in spite of some results obtained in humans (Broca, 1879; Mosso, 1881; 1894; Berger, 1901) and despite pioneering experiments on dogs, cats, and rabbits, and their sagacious interpretation (Roy and Sherrington, 1890), the limited availability of research tools hampered rigorous investigation, and many conclusions have therefore remained controversial. In addition, the eminent and influential English physiologist Leonard Hill, Hunterian Professor of the Royal College of Surgeons, had been convinced by his own work that brain function and brain circulation are not related (Hill, 1896). Thereafter, interest in the interactions between the brain and its blood circulation declined until the late 1920s, when the Bostonian neurosurgeon John Fulton reported that a patient with an arterio-venous malformation demonstrated audible changes in cerebral blood flow (CBF) when performing demanding visual tasks (Fulton, 1928). Proof of the existence of a neurovascular coupling, however, had to wait until the availability of radioactive blood-flow tracer-based methods made it possible to show directly that neuronal activation causes the brain vasculature to respond (Kety, 1950; Landau et al., 1955; Lassen, 1959; Lassen and Ingvar, 1961; Ingvar and Risberg, 1967; Sokoloff, 1969). The subsequent invention of positron emission tomography (PET) allowed the responses to be recorded on a local level even in humans (Ter-Pogossian et al., 1975; Hoffmann et al., 1976; Fox and Raichle, 1984; for historical overview, see Raichle, 1998; Table 1).
Table 1.
Overview of technologies and the findings they revealed about brain haemodynamic∕metabolic responses induced by neuronal activity.
| Technique | Finding | Authors |
|---|---|---|
| Intracranial pressure monitoring through aperture in cranial vault | Brain volume increases during mental activity | Mosso, 1881 |
| Intracranial pressure recordings in animals with simultaneous systemic blood pressure monitoring. Electrical peripheral stimulation and pharmacological manipulations. | Asphyxia and some putatively endogenous chemical agents raise intracranial pressure. Conclusion: “chemical products of cerebral metabolism…can cause variations of the calibre of the cerebral vessel.”Speculation: “the brain possesses an intrinsic mechanism by which its vascular supply can be varied locally in correspondence with local variations of functional activity.” | Roy and Sherrington, 1890 |
| Recordings of noise from arterio-venous malformation in cortex | Cerebral blood flow (CBF) increases in (occipital) cortex during visual stimulation | Fulton, 1928 |
| Cerebral arterio-venous difference of inhaled nitrous oxide (Kety-Schmidt method) | CBF and cerebral metabolic rate of oxygen utilization (CMRO2) increase during anxiety | Kety, 1950 |
| Regional clearing of radioisotopes (85KR, 133XE) injected into the blood circulation | CBF increase in speech-related areas during word-repetition tasks | Lassen and Ingvar, 1961,Ingvar and Risberg, 1967 |
| 2-(14C) deoxyglucose | The relationship between physiological function and energy metabolism in the central nervous system | Sokoloff et al., 1977 |
| Positron emission tomography (15O water as tracer) | Upon sensory stimulation, CBF in the somatosensory cortex grows much more than oxygen consumption | Fox and Raichle, 1986 |
| Optical imaging of intrinsic signals | Sensory-evoked changes in cerebral blood-volume (CBV) and -oxygenation allow functional brain mapping at a resolution of ∼50 μm (sub-columnar) | Grinvald et al., 1986;Frostig et al., 1990 |
| MRI using an intravenously injected contrast agent or employing the paramagnetic properties of deoxyhaemoglobin | fMRI allows exploitation of the activity-evoked haemodynamic responses to map functional architecture non-invasively in humans, at high spatial resolution, during complex cognitive tasks. | Belliveau et al., 1991;Ogawa et al., 1990 |
| Optical imaging spectroscopy | Early activity-evoked deoxygenation | Malonek and Grinvald, 1996,Mayhew et al., 1999 |
| Microscopy adapted for biological samples (in particular electron microscopy and two-photon fluorescence) | Reveals details of micro-vascular anatomy and allows study of CBF (velocity and density separately) in single micro-vessels. Vascular “machinery” underlying haemodynamic responses (astrocytes, pericytes) | Kleinfeld et al., 1998;Harrison et al., 2002;Chaigneau et al., 2003;Mulligan and MacVicar, 2004;Peppiatt et al., 2006 |
Over the last 20 years, interest in neurovascular coupling has burgeoned. Yet, as our knowledge grows, the picture of the interaction between brain function and the intricate underlying physiology has become increasingly complex. Indeed, it has become clear that neurovascular coupling relies on multiple mechanisms and signaling pathways (for recent reviews, see Haydon and Carmignoto, 2006; Lok et al., 2007). Moreover, recent data indicate that under certain circumstances, such as pre-ictal periods, vascular events can precede neuronal ones (Adelson et al., 1999; Salek-Haddadi et al., 2002; Schwartz, 2007; Zhao et al., 2007). Whereas the exact mechanisms underlying such phenomena remain unknown, these findings point to a tight yet complex entanglement of neuronal activity with physiology∕metabolism, and suggest a bilateral rather than unilateral interaction, which might have direct and indirect clinical relevance (in rats, seizures appear to have been predicted by monitoring the heart beat: Kerem and Geva, 2005) (for recent reviews, see Girouard and Iadecola, 2006; Suh et al., 2006).
Spatio-temporal characterization of neurovascular coupling and understanding of its underlying cellular mechanisms are of fundamental importance in the context of modern haemodynamics-based functional brain-imaging techniques. These techniques, as opposed to electro-encephalography, magneto-encephalography, and single- and multi-unit electrical recordings, make it possible to map the activity of large neuronal populations at high and very high spatial resolutions, and have thus become increasingly popular within the neuroscience community. Currently available techniques are PET (Fox and Raichle, 1984, 1986; Mintun et al., 1989), optical imaging of intrinsic signals (Grinvald et al., 1986; Frostig et al. 1990), and functional magnetic resonance imaging (fMRI) (Ogawa et al., 1990, 1992; Bandettini et al., 1992; Kwong et al., 1992). The challenge in using these techniques is to properly understand the neuronal correlates related to the measured functional brain imaging signals, as well as to increase the spatial resolution of the technologies involved (for recent reviews, see Ugurbil et al., 2003a, 2003b; Logothetis and Pfeuffer, 2004;Lauritzen, 2005; Nair, 2005).
The medical, physiological, and imaging aspects of neurovascular research are closely interconnected (see, e.g., Shulman et al., 2004). Here, we decided to focus on the functional brain-imaging perspective, an area in which some pressing questions remain largely unanswered:
To what extent do the various haemodynamic and neuronal processes co-localize in space and time? This issue is obviously related to the challenge of increasing the resolution of the functional imaging signals and their signal-to-noise ratio (SNR), in particular of fMRI. Haemodynamics-based functional signals are in most cases inadequate for studying dynamic aspects of neuronal events. This is because of the large difference between the time constants of haemodynamic responses, ranging from hundreds of milliseconds (onset of response) to tens of seconds (return to rest), and neuronal responses (in the range of milliseconds to tens of milliseconds). Yet, Ogawa and co-workers, using blood-oxygenation level dependent (BOLD) fMRI, succeeded in certain specific cases to probe neuronal activity on a time scale of tens of milliseconds by carefully designing the sensory stimulation paradigm (Ogawa et al., 2000). On the other hand, the haemodynamic responses’ temporal aspects do become relevant within the context of the signals’ spatial resolution, in the sense that the “static” (compared to the dynamics of neuronal events) functional image obtained via haemodynamics depends on the time at which the image was captured. Neuronal activation indeed triggers several different haemodynamic responses that induce haemodynamic activity patterns, each of which becomes dominant at a different time and co-localizes to a different extent with neuronal activity (Zhao et al., 2007 for a systematic study of the spatial resolution of different phases of the haemodynamic responses in rat barrel cortex, see Chen-Bee et al., 2007).
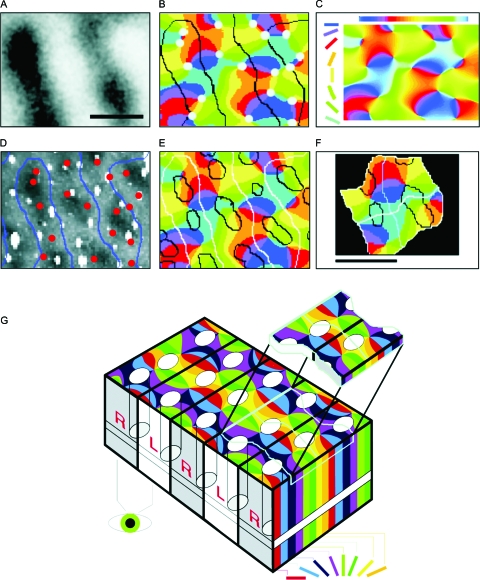
What spatial resolution can we hope to attain using haemodynamic signals? The ability to resolve neuronal dimensions is clearly beyond haemodynamics, as even when the magnification and SNR allow imaging of a single capillary (Kleinfeld et al., 1998; Chaigneau et al., 2003), a single vessel “senses” not one neuron, but many [capillary length ∼150–500 μm (Hudetz, 1997); median capillary length and distance ∼110 and 25 μm, respectively (Pawlik et al., 1981), whereas interneuronal distance is ∼30 μm and neuronal density is ∼40,000∕mm3 (Abeles, 1991; Braitenberg and Schüz, 1998)]. A less detailed yet highly informative spatial scale is that of “cortical columns;” i.e., sets of spatially clustered neurons that share similar functional properties and traverse the cortical sheet from the pia mater down to the white matter (Mountcastle, 1957; Hubel and Wiesel, 1962). In the view of many authors, these cortical columns, which have fuzzy borders, are probably the fundamental information-processing modules in the cortex. In the primate early visual cortex, those clusters have a diameter of ∼0.5 mm (Fig. 1).
Figure 1. High-resolution functional optical imaging reveals the relationship between pinwheels (detection of orientated edges, shape processing), ocular dominance columns (stereo vision, depth processing), and cytochrome oxidase blobs (color vision) in the primary visual cortex of the macaque monkey.
(A) Optical map of ocular dominance from a portion of striate cortex, ∼1.5 mm×1 mm. Dark bands represent columns dominated by input from the right eye. Scale bars: 500 μm. (B) Borders of the ocular dominance columns (black contours taken from A) were overlaid onto the discrete pinwheel map (the color in the map codes for the orientation preference of each cortical location, see color of oriented bars in C). Pinwheel centers are marked with circles: ∼70% are centered on ocular dominance columns. (C) Interpolation from the eight different orientations effectively used as visual stimulus yields a map of orientation preference, which is mostly continuous except at the pinwheel centers (B). (D) Cytochrome oxidase (CO)-rich blobs are marked (dark) on the histological photograph that corresponds exactly to the cortical area that was optically imaged. (E) Overlay of maps in B and D shows the relationships between blobs (D), iso-orientation domains (B, C), and ocular dominance columns (A, B). (F) A combined view of the various maps reveals the existence of recurrent units (“hypercolumns”): here, two such fundamental modules are magnified from (E). (G) On the macroscopic scale, the sequence of hypercolumns shown in (F) can be schematically represented in the “revised ice cube model:” black lines mark the borders between columns of neurons that receive signals from different eyes. White ovals represent groups of neurons responsible for color perception (blobs). The pinwheels are formed by neurons involved in the perception of shape, with each color marking a column of neurons that respond selectively to a particular orientation in space. Note that both the blobs and the pinwheel centers lie at the center of the R or L columns. The iso-orientation lines tend to cross borders of ocular dominance columns (black lines) at right angles. The top “slice” above the “ice cube” model depicts two adjacent fundamental modules (400 μm×800 μm). Each module contains a complete set of about 60,000 neurons, processing the three features of orientation, depth, and color. (Modified from Bartfeld and Grinvald, 1992.)
Which neuronal events cause which haemodynamic events? This critical issue raises many questions concerning the correct interpretation of the BOLD signal in terms of spiking activity versus subthreshold activation, or how to distinguish between excitation and inhibition (Arthurs and Boniface, 2002; Kim, 2003; Lauritzen and Gold, 2003; Logothetis, 2003; Logothetis and Wandell, 2004; Lauritzen, 2005). Answering such questions is a prerequisite for recovery of the various aspects of the underlying neuronal activity from the measured haemodynamic responses, and in particular for teasing apart input, output, and local processing within a given cortical location.
NEURONAL ACTIVITY EVOKES METABOLIC AND HAEMODYNAMIC RESPONSES
With the autoradiography studies conducted using the benchmark 2-[14C]-deoxyglucose (2DG) method (Sokoloff et al., 1977), it finally became clear that neuronal activity triggers metabolic responses. Moreover, those metabolic responses (here, glucose consumption) and neuronal activity were tightly co-localized (Sokoloff, 1981), as demonstrated by the high resolution of functional maps obtained in cat and monkey using the 2DG method (Kennedy et al., 1976; Tootell et al., 1981, 1982, 1988), as well as by staining selectively for cytochrome oxidase (Tootell et al., 1983, 1988), an enzyme that participates in the mitochondrial respiratory chain and thus accumulates in loci of increased metabolic activity. Spatial resolution of those methods easily attains the sub-millimeter scale, allowing the abovementioned cortical columns to be clearly visualized. A shortcoming of all these techniques, however, is that they are one-shot procedures, in that they do not yield signals that one can record concomitantly with the sensory stimulus, rather, the animal must be sacrificed and data can be obtained only from one single stimulus condition, so that dynamic studies with the 2DG and∕or cytochrome oxidase techniques are impossible. It is also impossible to compare activation patterns elicited on the same patch of cortex by different stimuli unless several activity-dependent radioactive markers are used. This latter shortcoming can be partially overcome if the PET version (Reivich et al., 1979) of the 2DG method is used. This, however, has a much lower spatial resolution; in addition, a relatively long time is needed for radio-isotope decay (tens of minutes at best, depending on the isotope), limiting the number of possible runs per subject and per session, and hence the numbers of stimuli that can be imaged.
These shortcomings do not apply to functional imaging based on haemodynamic responses. In that case, the time needed to record the response to a given stimulus, i.e., the minimal delay between one stimulus and the next, is set by the time scale of the haemodynamic responses (at worst, some tens of seconds). Thus, numerous repetitions of many different stimuli can be recovered even over comparatively long periods, a prerequisite for any plasticity study (Frostig et al., 1990; Prakash et al., 1996; Dinse et al., 1997; Crair et al., 1997; Sengpiel et al., 1998; for a recent review, see Roe, 2007).
PHYSIOLOGICAL IMPLICATIONS
The availability of techniques for the recording of blood flow (Landau et al., 1955; Kety et al., 1950) and of glucose utilization (Sokoloff et al., 1977) in animals and humans (Reivich et al., 1979; Raichle et al., 1983) quantitatively (Frackowiak et al., 1980; Mintun et al., 1984), and their validation for oxygen consumption (Mintun et al., 1984; Altman et al., 1991) by PET, had important consequences not only for functional brain imaging but also for the understanding of brain metabolism. Some 20 years ago, Fox and Raichle “shocked the Neuroscience world” (Research News, Science , 1997) with the results of their PET scans (Fox et al., 1986, 1988) when their data indicated a substantial mismatch between a large, activity-dependent regional increase in glucose consumption and only a small increase in regionally utilized oxygen (30–50% versus ∼5%: Fox et al., 1986, 1988). Their results raised the question of whether, in the living brain, neuronal activation causes a significant increase in oxidative metabolism or whether the increased energy demands are met by other mechanisms, e.g., anaerobic glycolysis of glucose into lactate (Raichle 1987, 1998; Fujita et al., 1999), despite the availability of sufficient oxygen to support oxidative phosphorylation. Further support for the latter scenario came from a peak in lactate concentration observed by magnetic resonance spectroscopy in correspondence to neuronal activation (Prichard et al., 1991; Sappey-Marinier et al., 1992).
Another plausible explanation for lactate production compatible with neuronal oxidative metabolism is provided by the astrocyte-to-neuron lactate shuttle hypothesis (ANLSH) (Pellerin and Magistretti, 1994; Pellerin et al., 1998), which postulates that glutamate released at activated synapses triggers astrocytic glycolysis, providing lactate for oxidative metabolism of neurons. Strong support for this theory came from a 13C-NMR study of the fate of 13C-glucose injected into the rat brain (Sibson et al., 1998). The authors concluded that the stoichiometric ratio of: (i) glutamate-glutamine astrocytic glutamate cycling to (ii) glucose-lactate metabolism is 1:1, meaning that glutamate cycling in astrocytes is the principal driving force behind brain glucose metabolism. Despite the elegance of the ANLSH and the persuasiveness of arguments in its favor (see, e.g., Hu and Wilson, 1997), the controversy regarding these issues is not yet resolved (Chih and Roberts Jr, 2003; Pellerin and Magistretti, 1994; for a review, see Schurr, 2006). Altogether, it seems likely that the energy-production mechanisms are multiple, eventually structured according to specific energy needs (depending eventually on the type of stimulus, Shulman et al., 2001) and∕or organized sequentially in time (Kasischke et al., 2004).
HIGH RESOLUTION FUNCTIONAL MAPS OBTAINEDIN VIVO BY OPTICAL IMAGING
Whereas activity-evoked changes in cerebral blood flow (CBF) and metabolism were successfully studied using radioactive methods, Jöbsis and co-workers showed that the corresponding changes in cerebral blood volume (CBV) can be adequately investigated by optical means. Thanks to the light-absorption properties of haemoglobin, this can be done simply by analyzing, at specific wavelengths, the intensity changes of light back-reflected from the exposed cortex (Jöbsis, 1977). Earlier pioneering work by Chance (Chance et al., 1962) and Jöbsis (Jöbsis et al., 1977) had shown that neuronal activity is accompanied not only by CBV and CBF responses, but also by oximetric signals that can be detected optically by monitoring the absorption and∕or fluorescence of intrinsic chromophores. The importance of those intrinsic optical signals for functional brain mapping was established only two decades later, when Grinvald and co-workers (Grinvald et al., 1986), familiar with Roy and Sherrington’s work as well as with that of Britten Chance, showed that the small light absorption changes evoked by neuronal activity allow exploring cortical functional architecture in vivo at columnar (sub-millimeter) resolution (for review, see Grinvald et al., 1988, 2000, 2005; Lieke et al., 1989).
Soon afterwards, the same group (Frostig et al., 1990; Ts’o et al., 1990) confirmed those results using high-resolution CCD-based imaging. They also investigated the spatio-temporal characteristics of the sensory-evoked intrinsic optical signals in vivo, as well as their physiological origin. To do this, they exploited the differential spectral properties of optical signals resulting from changes in the concentration of oxy- and deoxyhaemoglobin (HbO2 and Hbr, respectively), as well as in the oxidation state of cytochromes (Chance et al., 1962; Jöbsis, 1977; Jöbsis et al., 1977; LaManna et al., 1985, 1987), and studied the spatio-temporal properties of the sensory-evoked optical responses recorded upon illumination of the exposed primary visual cortex of anaesthetized monkeys at several wavelengths ranging from 480 to 940 nm. They found that those activity-evoked changes in intensity of back-reflected light could be mostly accounted for by haemodynamic processes, whereas there was little or no contribution of other intrinsic chromophores such as cytochromes (Frostig et al., 1990). The same conclusion was reached by later optical imaging spectroscopy studies using more sophisticated analyses (with the exception of some hints as to the presence of cytochrome-related signals reported in Mayhew, 1999, but no longer in subsequent publications by the same group, e.g., Mayhew et al., 2000). Care should, however be taken when using different imaging geometries and∕or longer wavelengths: indeed, changes in the oxidation state of cytochromes have been reported to contribute non-negligibly to the optical signals when imaging through the intact human skull (Kohl et al., 1998; Heekeren et al., 1999; Wobst et al., 2001).
This multi-wavelength imaging study (Frostig et al., 1990) also showed that the intrinsic optical signal is multi-component, the different components reflecting distinct haemodynamic processes. Those are: (i) changes in blood-volume (CBV, equivalent to changes in the overall haemoglobin concentration [Hb] in the cortical tissue); and (ii) changes in the oxygenation state of Hb. The authors also suggested the existence of a (iii) third component, resulting from light scattering changes. Those can be separated into fast and slow ones (Rector et al., 2001), and although these signals were detected in vitro already some 60 years ago (Hill and Keynes, 1949; later: Lipton, 1973; Cohen, 1973; Grinvald et al., 1982; MacVicar and Hochman, 1991), the detectability of the fast component in vivo is still controversial (Gratton et al., 1995; Steinbrink et al., 2000; Syre et al., 2003), as is the magnitude of the contribution of the slow one to intrinsic optical signals (Mc Loughlin and Blasdel, 1998; Kohl et al., 2000; Tanner et al., 2005, 2006).
The same study (Frostig et al., 1990) also showed that the optical maps obtained upon illumination at wavelengths that are strongly oximetric (i.e., sensitive to the oxygenation state of Hb), in particular in the orange∕red part of the spectrum where the absorption of Hbr dominates, reflect the underlying functional architecture more faithfully than those obtained at isosbestic wavelengths (i.e., where Hbr and HbO2 have equal absorptivity), and are sensitive mainly to changes in CBV, particularly using green wavelengths. The authors thus concluded: “oxygen delivery from the capillaries is better localized to the site of spiking neurons than the blood volume changes.”
The results obtained with optical imaging based on intrinsic signals reported since 1986 (Grinvald et al., 1986; Frostig et al., 1990; Ts’o et al., 1990) thus demonstrated for the first time that functional maps at very high resolution (Fig. 1) can be obtained in vivo by monitoring changes in intrinsic chromophores without any extrinsic contrast agents, i.e., by monitoring the amounts of HbO2 and∕or Hbr and, in particular, their relative concentration. Incidentally, this conclusion suggested the possibility of using the activity-dependent oximetric changes also as an intrinsic signal for magnetic resonance imaging (Ogawa et al., 1990, exploiting the different magnetic properties of Hbr and HbO2 (para- and diamagnetic, respectively). This prospect indeed led to the development of BOLD-fMRI (Ogawa et al., 1992; Kwong et al., 1992), offering a far better spatial resolution than that achievable with PET, yet without the use of an extrinsic radioactive contrast agent (Tank et al., 1992).
NEUROVASCULAR COUPLING INVESTIGATED BY OPTICAL IMAGING
Although CBV changes can be imaged at each single isosbestic wavelength and although oximetric changes can under certain circumstances be approximated by imaging in the orange-red part of the spectrum (600–630 nm), a rigorous separation of the various components of the haemodynamic response in the optical signals requires a many-wavelength, spectroscopic approach. Frostig’s study (Frostig et al., 1990) was limited to a few discrete wavelengths. It was extended by Malonek and Grinvald (1996), who used imaging spectroscopy to better disentangle the different activity-evoked haemodynamic responses, following earlier pioneering work by LaManna et al. (1985). First, sensory-evoked action spectra, i.e., the reflection spectrum recorded during stimulation and normalized to that recorded during rest, were obtained from the visual cortex of the anaesthetized cat (in the cited study by Malonek and Grinvald at a sampling rate of 2 Hz). Those action spectra were then fitted, for each time point, to a linear combination of the absorption spectra of HbO2 and Hbr according to a somewhat simplified spectroscopic model, and a third term was added to account for possible light-scattering changes. One year later, additional experiments were performed to obtain CBF recordings simultaneously with the spectroscopic measurements (Malonek et al., 1997).
The time courses obtained from these spectroscopic analyses suggested that, after stimulus onset, [Hbr] increases transiently. This increase in [Hbr] has been later referred to as the “initial dip” by the BOLD-fMRI research community (in fMRI, the [Hbr] increase shows up as a downward deflection of the signal, owing to the inverse dependency of the BOLD signal on [Hbr] changes). Malonek and Grinvald found, moreover, that this increase in [Hbr] was highly co-localized with areas of neuronal activity. On the other hand, the somewhat delayed hyperoxygenation resulting from the increased blood flow was seen in optical imaging spectroscopy as an increase in CBV and [HbO2] together with a decrease in [Hbr]. With respect to the initial dip, both delayed processes were found to be (i) much more prolonged; and (ii) less localized to neuronal active sites, in particular activating large surface vessels.
The authors interpreted the initial increase in [Hbr] as the result of an increased oxygen demand by the activated neurons (or surrounding glia) before the vasculature can react to provide more highly oxygenated blood, as indeed it does a few seconds later by increasing the blood volume and flow. Being a direct result of locally increased oxidative metabolism rather than of vascular activity, the initial dip is thus expected to tightly co-localize with sites of neuronal activation. On the other hand, the more global character of the later [HbO2] increase and [Hbr] decrease was interpreted as a consequence of a particularly generous design of the vascular response, which would overcompensate the metabolic needs by massive and largely non-local CBV and CBF responses (like “watering the entire garden for the sake of one thirsty flower”). The results obtained by Malonek and Grinvald were soon afterwards reproduced in the rat (Nemoto et al., 1999; but see also Nemoto et al., 1997) and in the awake macaque (Shtoyerman et al., 2000).
The findings of Malonek and Grinvald had several implications. First, they introduced the concept that a distinction has to be made between two kinds of activity-evoked haemodynamic processes: (i) those that are passive from the point of view of the vasculature; and (ii) those that result from active intervention of certain blood vessels in a specific neighborhood of the loci of the neuronal activation. In both kinds of processes, the vascular substrate spatially “filters” the neuronal-activity patterns. In the case of the active responses, the filtering is rather complex, given the different responses of the various vessel types and the macroscopic design of the cortical vessels with the largest active responses (Nemoto et al., 2004; Vanzetta et al., 2005; Berwick et al., 2005; Hillmann et al., 2007), as well as the largely unknown nature of the kernel convolving the neuronal patterns. On the other hand, the early deoxygenation, which results from increased oxygen consumption and is thus passive from the point of view of the vasculature, happens mostly at the capillary level, as those vessels have the strongest gas exchange with the surrounding tissue. The passive deoxygenation is thus supported by the vascular network having the highest density—that of the capillaries—and consequently co-localizes maximally with the neuronal (or glial) oxygen sinks. Nevertheless, this passive phenomenon also loses its spatial specificity with time, because the blood, although deoxygenated at the local level, spreads out quite rapidly as it flows away toward the draining veins. Clearly, then, to optimize the spatial resolution and SNR obtainable in functional imaging, the spatio-temporal dynamics of all those processes must be properly taken into account. Detailed characterization of their dynamic behavior is thus of paramount importance.
THE “INITIAL DIP”; OXIDATIVE METABOLISM DEBATE
Because BOLD fMRI uses Hbr as an intrinsic tracer, the optical imaging spectroscopy results of Malonek and Grinvald pointed to the possibility of exploiting the initial dip for the purpose of increasing the spatial resolution of non-invasive functional imaging in the human brain. Some reports, mainly from studies performed at high magnetic fields, confirmed the initial dip (Ernst and Hennig, 1994; Menon et al., 1995; Hu et al., 1997; Logothetis et al., 1999; Yacoub and Hu, 1999; Kim et al., 2000). Those confirmations, however, remained somewhat isolated because the initial dip, whose amplitude grows more rapidly with the field strength than the positive BOLD response (Yacoub and Hu, 1999), was missed in most of the low-field studies (Fransson et al., 1998; Mandeville et al., 1999a; Marota 1999, but see Yacoub and Hu, 1999) and even in some high-field experiments (Silva et al., 2000); in any case, when investigated with BOLD fMRI, the initial dip appeared to be somewhat elusive (Logothetis, 2000 versus Kim, 2000). In addition, doubts were raised with respect to the accuracy of the [Hbr] and the oxyhaemoglobin concentration ([HbO2]) time courses calculated by Malonek and Grinvald in their 1996 paper, since those had been obtained with a classical Beer-Lambert spectroscopic model, which approximates the optical path as constant rather than taking its wavelength dependency into account (Cope et al., 1991; Arridge et al., 1992). Subsequently, optical imaging spectroscopy results were more rigorously modeled and analyzed, mainly by two groups: one in Sheffield directed by John Mayhew (Mayhew et al., 1999) and the other in Berlin directed by Ulrich Dirnagl (Kohl et al., 2000) [Details in Supplemental Material 1. (The supplemental material contains some text and one figure about the “modified” Beer-Lambert spectroscopic model, and some text about the “sustained” negative BOLD signal.)]. It was justifiably claimed that the assumption of wavelength-independent optical path length in cortical tissue could be an oversimplification that might distort results and their interpretation.
More rigorous spectroscopic models were therefore proposed (Gratton et al., 1997; Matcher et al., 1997;Mayhew et al., 1999; Kohl et al., 2000) and their theoretical and∕or experimental application to imaging spectroscopy data obtained in vivo was elaborated by several groups(Mayhew et al., 1999, 2000; Jones et al., 2001; Lindaueret al., 2001; Sheth et al., 2004a). Concerning the existence of the initial dip, however, the conclusions were conflicting, with negative results being reported by the Berlin group (Kohl et al., 2000; Lindauer et al., 2001), affirmative results by the Sheffield and other groups (Mayhew et al., 1999, 2000; Jones et al., 2001; Sheth et al., 2004a), and inconclusive findings by others (Sheth et al., 2005). All in all, the published optical imaging spectroscopic studies have shown considerable differences, with regard not only to the existence of the initial dip, but also to other aspects of the calculated time courses. It remains a question, for example, whether or not [HbO2] transiently decreases during the early [Hbr] increase; the answer influences the interpretation of the calculated time courses (Buxton, 2001) in terms of the underlying physiological processes (such as oxygenation, CBV, and capillary recruitment). In some cases, those discrepancies may be due to slight differences in the spectroscopic models that evolve from earlier to later publications (for instance: Mayhew et al., 2000; Jones et al., 2001 as opposed to Mayhew et al., 1999. In this case, changes in cytochromes were found in the early study but not in the later one: there, changes in [Hbr] and [HbO2] sufficed to explain the data, suggesting that the spectroscopic model used in the later publications was more adequate than the one used before.)
It sometimes happens, however, that the same research group successively publishes contrasting results, even in the absence of any apparent changes in spectroscopic models. Thus, conclusions might differ concerning the presence not only of an early [HbO2] decrease, but also of the initial dip itself, which was found to exist, for example, in some studies (Devor et al., 2003, 2005) but not in others (Dunn et al., 2005). Those authors attributed the difference, at least in part, to a better SNR in the earlier studies, in which a slightly different stimulation paradigm had been used. Nevertheless, the error bars on the early [Hbr] trace (Fig. 6 in Dunn et al., 2005) are quite narrow, and the initial dip should thus probably have been detectable despite the noise. It can indeed be argued that a small early upward deflection of the [Hbr] can be seen in that figure, raising the question of whether it would have been significant on a parametric test (mono-versus biphasic function). The same argument applies in other studies (Kohl et al., 2000; Lindauer et al., 2001). In yet another publication by the group cited earlier, the elusive initial dip reappeared (Fig. 1 in Devor et al., 2007). This time, however, as opposed to their earlier findings (Devor et al., 2003, 2005) and despite the absence of any evident changes in preparation, spectroscopic approach or stimulation paradigm, no simultaneous decrease in [HbO2] was reported. Those conflicting conclusions highlight the need to rule out the various pitfalls inherent in the experimental procedures, the spectroscopic models, and the stimulation paradigm.
Also worth mentioning is the fact that too little importance is often accorded to species specificities, both in the development of spectroscopic models and in the interpretation, comparison, and generalization of their outcome. It is now well known that species differences can drastically affect the kinetics of several haemodynamic responses (e.g,. a biphasic relative to a monophasic Hbr timecourse in rats compared to that in mice; Prakash et al., 2007). The same is true for the animal’s physiological state (which must be rigorously assessed by multiple parameters, including cortical temperature, blood pressure, end-tidal CO2 level, blood gases, and depth and type of anaesthesia). Finally, differences have been reported to exist between “normal” sensory-evoked and pathologic activity (e.g., epilepsy: Bahar et al., 2006).
MORE DIRECT MEASUREMENTS OF CEREBRAL OXIDATIVE METABOLISM
An obvious difficulty in spectral studies is that the effects of CBF, CBV, and the cerebral metabolic rate of oxygen utilization (CMRO2) contribute in an interrelated manner to changes in [HbO2] and [Hbr], and all of these parameters vary, at least to a certain extent, on different temporal scales. As a consequence, the interpretation of the quantities measured optically remains somewhat ambiguous (and so do those measured by BOLD fMRI, although there the ambiguity can be reduced by calibrating the CBF-CMRO2 relationship using hypercapnia: Davis et al., 1998; Cohen et al., 2004). To interpret them quantitatively—and in some cases also qualitatively—in terms of oxygen metabolism or changes in blood flow or blood volume, a number of assumptions are needed concerning both the physical model (depth of signal provenance, single versus multi-compartments, etc.) and the choices of model parameters, such as baseline oxygen saturation and baseline haemoglobin concentration (Kohl et al., 2000; Jones et al., 2001; Lindauer et al., 2001; Dunn et al., 2005). Because the correct values of these parameters are not always known, the stability of the conclusions must be tested against those assumptions (e.g., Kohl et al., 2000; Berwick et al., 2005). Finally, the scattering and absorption properties of the cortical tissue also appear to differ from one area and∕or species to another [e.g., rat whisker barrel versus monkey striate cortex (Johnston and Vanzetta, unpublished observations, 2000)] and should thus be taken into account.
In an attempt to resolve the controversy regarding the existence of the initial dip and its interpretation in terms of early oxidative metabolism, while bypassing the numerous difficulties inherent in imaging spectroscopy in the living brain, we measured the phosphorescence decay times of an exogenous oxygen probe (an elegant method to measure oxygen tension, developed by Wilson et al., 1987). We injected the phosphorescence probe into the blood circulation of anaesthetized cats (Vanzetta and Grinvald, 1999, 2001). An initial hypo-oxygenation was observed in the cortical vasculature about 0.3 s after stimulus onset, and was followed 2–3 s later by a substantial hyper-oxygenation. However, using the same recording method in a study of rat whisker barrel cortex, another group (Lindauer et al., 2001) found—consistent with their spectroscopic data—no evidence for the initial dip, and attributed its occasional appearance (e.g., their Fig. 6) to chance. Possible causes of this discrepancy are discussed elsewhere (Vanzetta and Grinvald, 2001). In brief, important factors might include species differences (rats have shorter mean capillary length than cats or monkeys, implying a smaller initial dip), as well as technical pitfalls inherent in the fitting of multi-exponential decay curves. To prevent such pitfalls, in our original study we also tested our conclusions using multi-component analyses, obtaining results consistent with an early deoxygenation (briefly reported in the annotations of Vanzetta and Grinvald, 1999). Moreover, using the same phosphorescence-based method, another study (in rat somatosensory cortex, Ances, 2001b) concluded that CMRO2 increases following activation.
Despite additional evidence accumulating from BOLD-fMRI (Yacoub 2001; 1999; Kim et al., 2000;Logothetis et al., 1999), some sought an even more definitive argument favoring the existence of the initial dip and its interpretation in terms of a transient decoupling between a rapid rise in CMRO2 and a somewhat delayed increase in CBF. This was recently provided by the results of direct tissue-oxygen electrode recordings in the rat and cat brain (Ances et al., 2001; Thompson et al., 2003; for review, see Ances, 2004). Those measurements, performed in the parenchyma (cortex and lateral geniculate nucleus) rather than within the local blood microcirculation, clearly confirmed not only that neuronal activation evoked by a sensory stimulus indeed induces a rapid fall in tissue-oxygen tension, but also that this deoxygenation is better localized to spiking activity than the hyperoxygenation phase that succeeds it (Thompson et al., 2004, 2005), exactly as originally suggested by Malonek and Grinvald’s imaging spectroscopy study.
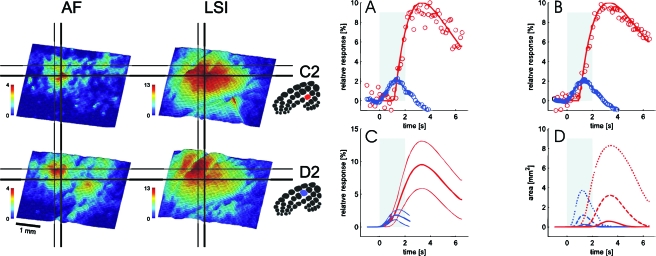
Finally and conclusively, following pioneering work by Chance and co-workers (Barlow et al., 1979) a sensory-evoked increase in oxidative metabolism was directly imaged by means of flavoprotein autofluorescence (Shibuki et al., 2003; Murakami et al., 2004; Reinert et al., 2004;Tohmi et al., 2006), and was found to precede the onset of the active vascular response by at least 0.5 s, as shown in Fig. 2 (Weber et al., 2004). Moreover, those flavoprotein autofluorescence responses were recently shown to accurately reflect the functional architecture in cat primary visual cortex (Husson et al., 2007). The high quality of the orientation pinwheel maps obtained by this method was at least as good as that of the maps obtained by intrinsic imaging.
Figure 2. Comparison of the spatio-temporal characteristics of sensory-evoked changes in oxidative metabolism and blood flow.
Left Panel: Activity maps obtained in rat whisker barrel cortex upon stimulation of two distinct whiskers (C2, top; D2, bottom, see insets for the corresponding somatotopy) from flavoprotein autofluorescence (AF) (left) and laser speckle imaging (LSI) (right). The color code depicts the percentage of signal increase from baseline. The horizontal and vertical lines facilitate the comparison across sub-panels, showing that the spatial pattern of the blood flow response was considerably less focal than that of the changes in oxidative metabolism, although their peaks of the activation roughly co-localized. Right Panel: Time course of AF and LSI responses. (A) AF (blue) and LSI (red) depict time courses from a single trial (2 s stimulation of vibrissa C2, average over area of >50% peak activation). Data points are represented by markers. Solid lines are fitted gamma curves. The shaded rectangle shows the stimulation. (B) Signal-averaged time course (20 trials). Note the excellent agreement between the single trial (A) and the average data (B). (C) Grand average (50% of the peak activation of C2 and D2 stimulations) fitted gamma curves (thick lines) ±1 standard deviation (thin lines) of the AF (blue) and LSI (red) responses. (D) Mean time course of the size of the activated area for AF (blue) and LSI (red). Shown are the areas with >25% (solid), >50% (dashed), and >75% (dotted) of the peak response. Note that the active area determined from AF is considerably smaller than that obtained from LSI. In addition, onset of the latter signal is delayed relative to the former, supporting the concept that the oxidative metabolism response is faster and more localized to neuronal activation than the blood flow response (with permission from: Weber et al., 2004).
In light of the support derived from numerous independent approaches, including multiple wavelength imaging, imaging spectroscopy, and intravascular oxygen measurements with phosphorescence probes, extra-vascular oxygen electrodes, and intracellular mitochondrial energy markers (the flavoproteins), one wonders whether it is still justified to consider the initial dip and its interpretation as controversial.
IMPLICATIONS FOR MODELING CEREBRAL HAEMODYNAMICS AND OXYGEN METABOLISM
The initial dip has important consequences for modeling of cerebral haemodynamics and oxygen metabolism. Once a sensory-evoked increase in oxidative metabolism has been shown to exist, we can draw important conclusions about its size from the fact that oxygenation at the level of the parenchyma and the local microcirculation initially drops. What those data suggest is that the initial need for oxygen is so great that it exceeds the oxygen supply provided by the blood flow, in agreement with the hypothesis that, at least initially, energy is produced mostly via oxygen-expensive aerobic rather than oxygen-inexpensive anaerobic metabolism. Whereas this conclusion is just opposite to that drawn by Fox and Raichle (1986) from their PET data, a posteriori, this contradiction might not come as a surprise. Indeed, PET has a much lower time resolution (tens of seconds) than that of imaging spectroscopy (tenths of seconds). As a consequence, the initial dip, which is short (1–3 s), was virtually impossible to see in the study by Fox and Raichle, due to the masking effect of the subsequent increase in oxygenation∕blood-flow, which is 5-10 times larger (depending on the duration of the stimulus) and prolonged (Vanzetta and Grinvald, 1999; Kim et al., 2000; Jones et al., 2001; Ances et al., 2001a). Consistently, also other methodologies such as oxygen-dependent phosphorescence quenching and BOLD-fMRI, proven to be able to detect the initial dip at high temporal resolution (e.g., Vanzetta and Grinvald, 1999; Yacoub and Hu, 1999, respectively, 100 and 300 ms), fail to do so when time resolution is too low (e.g., Ances et al., 2001b; Kennerley et al., 2005, respectively, 1.7 and 2 s).
Even if the cortex deals with its energy needs aerobically, there remains the question as to why the activity-induced increase in blood flow should be as large as it is, both in amplitude and in spatial extent. A rationale for this overcompensation of tissue-oxygen needs (and in particular for the spatial aspect, or “vascular overspill”) was originally suggested in terms of protecting every part of the brain against even the slightest risk of anoxia (Malonek and Grinvald, 1996). A more quantitative explanation is based on the “oxygen-limitation model” and its modifications (Gjedde et al., 1991, 1999; Buxton and Frank, 1997; Hyder et al., 1998). This model assumes that oxygen metabolism and CBF are constantly coupled, although with a much larger fractional change in CBF. In such a case, the large activity-induced increase in CBF would reflect a necessity, resulting from a mechanistic limitation on the ability to increase cerebral oxygen delivery through CBF. In other words, the CBF response is only apparently disproportionate: in reality it is coupled to the rise in CMRO2 and its size is right for the purpose. Supporting evidence comes from experiments in human primary visual cortex (Hoge et al., 1999), showing a simple graded relationship between CBF and oxygen metabolism during visual stimulation.
The central point of the oxygen-limitation model is that oxygen transfer from capillaries to the surrounding tissue depends non-linearly on blood flow. Thus, in the case of capillary oxygenation close to the arterial one (high flow regime), even small increases in tissue-oxygen delivery occur only when there are very large increases in blood flow. This concept can be grasped intuitively if we take into account that the faster the red blood cells flow, the less time they spend next to the active neurons or glia and the less time they have to lose their oxygen. From the modeling perspective, in the oxygen-limitation model the large increase in CBF is needed to support even a small increase in oxygen consumption because the oxygen extraction fraction (E) must drop with increased flow (this means that the CBF increase needed to satisfy a given oxygen demand grows with the “baseline” flow rate). This necessity derives from two basic assumptions in the original model (Buxton and Frank, 1997). The first is that tissue PO2 is near zero and the diffusion distance from capillary to mitochondria is fixed. The diffusion gradient for oxygen is then determined mainly by the capillary PO2, and in order to raise it, E must be reduced. The second assumption is that oxygen delivery to the tissue can be increased only by an increase in perfusion. At steady state, the product of E and CBF must then match this increased oxygen flux out of the capillary following the oxygen gradient; CBF must therefore increase more than oxygen metabolism in order to overcome the decrease in E.
Whereas Buxton and Frank’s oxygen delivery model could adequately explain the large mismatch between CMRO2 and CBF (1:10–1:5) found by Fox and Raichle, it was not able to explain other data. In humans, for example, CMRO2∕CBF ratios as small as ∼1:2 were found during sensory stimulation (Seitz and Roland, 1992) and only ∼1:1 during cognitive stimulation (Roland et al., 1987). Other PET studies (Gjedde et al., 1990; Raichle et al., 1976;Roland et al., 1987) also showed that at rest, human cortical gray matter values of CBF, CMRO2, and CMRGlc (cerebral metabolic rate for glucose) are regionally coupled. A CMRO2∕CBF ratio that varies with time upon continuous stimulation has also been reported (Mintun et al., 2002). To address the difficulties encountered by the Buxton and Frank model with those very different experimental data, Gjedde and co-workers (Gjedde, 1997) used a compartmental model to show that changes in the oxygen diffusivity parameter can modulate the CMRO2∕CBF ratio (Gjedde et al., 1999; Vafaee and Gjedde, 2000).
Using a somewhat different formalism, that weakened the strong supra-linear behavior of CBF at high levels of capillary oxygenation, Hyder and colleagues (Hyder et al., 1998) were able to account for the various published CMRO2∕CBF ratios. In their model, this was accomplished by allowing the effective vessel-to-tissue oxygen delivery to change, depending on local capillary PO2, hematocrit, and CBV, as might be achieved, for example, by functional capillary recruitment. As a consequence, “the oxygen diffusivity properties of the capillary bed, which are adjusted with respect to perfusion, play a crucial part in regulating cerebral oxygen delivery in vivo” (Hyder et al., 1998). As we show below, such a scenario for CBF∕CBV regulation at the capillary level has important implications for functional imaging.
The initial dip also introduced the important notion that the relationship between CBF and oxygen metabolism can change quite rapidly, or in other words, that CBF and CMRO2 are not always in equilibrium. Mayhew and co-workers showed that steady-state haemodynamic models of the Buxton and Frank or the Hyder type cannot handle these dynamics adequately (Mayhew et al., 2001). Rather, an increase in [Hbr] owing to increased oxygen consumption prior to compensation by the increased CBF suggests that CBF and CMRO2 become temporarily uncoupled. As pointed out by several groups, one way to account for such dynamics is to assume that oxygen can significantly accumulate in the parenchyma to form an oxygen reserve that can temporally cover local oxygen consumption increases before CBF begins to grow (Valabregue et al., 2000; Zheng et al., 2002; Aubert and Costalat, 2002; Valabregue et al., 2003). Importantly, relaxation of the zero tissue oxygen tension hypothesis originally set up by Buxton and Frank (1997) results not only from modeling considerations, but is justified also by direct experimental data (Ances et al., 2001a) showing that tissue PO2 increases upon prolonged stimulation (and also by the recent data of Offenhauser et al., 2005). Dynamic modeling by several groups (Hathout et al., 1999; Valabregue et al., 2000; Aubert and Costalat, 2002; Zheng et al., 2002; Valabregue et al., 2003) has yielded encouraging results. The models successfully reproduce a large variety of experimental results, including BOLD responses characterized by an initial dip as well as an overshoot of amplitude ratios compatible with the data (but dependent also on the model’s parameters). A dynamic version of the oxygen-limitation model (Zheng et al., 2002) was proposed by the Sheffield group, who then plugged the resulting “oxygen transfer to tissue” model into a more complete theoretical framework (Friston et al., 2000) that models haemodynamics-based functional imaging signals all the way from neuronal activity up to the measurable signals; i.e., the BOLD fMRI responses.
To achieve the ultimate goal of reconstructing neuronal activity from the haemodynamic responses measured, for example, via the fMRI signals (Buxton et al., 2004), it appears that current haemodynamic models have yet to be made more realistic physiologically (Hayashi et al., 2003; Fantini, 2002). For instance, usually only one vascular compartment is modeled, neglecting the fact that arteries, arterioles, capillaries, venules, and veins all have different responses. To take those into account, a three-compartment model was recently proposed (Zheng et al., 2005). However, as the model gets more complex, the number of parameters to be experimentally determined naturally grows, eventually requiring multiple, complementary recording strategies (Kennerley et al., 2005). The neuronal part of the model will also need to be formalized more accurately, e.g., by formalizing the neuronal∕glial compartmentalized energy metabolism (Aubert et al., 2007) and its changes linked to neuronal signaling. Another important question concerns the way in which those changes are communicated to the local microvasculature; experimental data suggest that astrocytes are likely to play a key role in this connection (Zonta et al., 2003; Jakovcevic and Harder 2007). Supporting results were recently obtained by two-photon microscopy, a particularly valuable technique in this context owing to its high spatial resolution (Mulligan and MacVicar, 2004; Takano et al., 2006; Chuquet et al., 2007). Other glial cells (in isolated retina: Metea and Newman, 2006) and interneurons (in cortical slices: Cauli B et al., 2004) evidently also play an important role in neurovascular coupling.
Finally, in most haemodynamic modeling it is assumed that CBF depends directly on neuronal activity; changes in CBF then cause CBV changes, assumed to occur predominantly in venules and veins. The Balloon (Buxton et al., 1998) or Windkessel (Mandeville et al., 1999b) Models or their modifications (Kong et al., 2004) are then used to formalize the CBV-CBF relation, which is needed to calculate the BOLD fMRI signal expected from a given neuronal response. The predicted timing relationships, i.e., CBF leading CBV changes, were found by Sheth and co-workers (Sheth et al., 2005) but not by others, whose data rather showed CBV leading CBF (Malonek et al., 1997); the data of the Sheffield group (Jones et al., 2001) show that CBV leads at response onset, whereas CBF leads later on. Results from our laboratory obtained in the awake macaque (Deneux et al., 2006) also suggest that CBV leads CBF changes by at least 500 ms. Exchanging the roles of CBV and CBF in the Balloon Model equations thus improved the fit of the model predictions to the experimental data, but without attaining satisfactory agreement, probably also because the largest CBV changes occur in arterioles rather than in venules or veins (Nemoto et al., 2004; Vanzetta et al., 2005; Hillmann et al., 2007). In any case, it becomes increasingly apparent that CBV and CBF are linked in a more complex way than by a simple power-law CBV=CBFα (“Grubb’s law,” Grubb et al., 1974), and that they depend also on state of activation, vessel type, and size (Hutchinson et al., 2006).
ACTIVITY-EVOKED CHANGES IN BLOOD-VOLUME AND FLOW
Whereas the oxygen-tension drop in the parenchyma detected with oxygen microelectrodes (Thompson et al., 2003; Ances et al., 2001a) can be explained in terms of an increased CMRO2, it is less simple to explain the initial dip at the level of the local microvasculature. Indeed, a possible contribution to an increase in [Hbr] comes not only from augmented oxygen extraction, but also from an increase in CBV (Buxton, 2001), especially if one assumes that it is mostly the venous part of the local vessels that inflates (Buxton et al., 1998; Hathout et al., 1999). To take an extreme case, the overall local oxygen tension (average over compartments) can in principle decrease even without any increase in oxygen consumption, simply due to a large enough venous ballooning, provided that the increase in CBF remains small. A strong argument in favor of the increased oxygen extraction scenario would be the detection of a decrease in [HbO2] simultaneously with the [Hbr] increase during the initial dip. Indeed, if oxygen consumption grows and CBV (and, implicitly assumed, CBF) remains unchanged, [Hbr] must increase at the expense of [HbO2]. However, no such decrease in [HbO2] was seen in the original spectroscopy experiments in our lab (Malonek and Grinvald, 1996; Malonek et al., 1997). Even when the initial dip was later detected by means of more sophisticated spectroscopic approaches, the authors did not see a corresponding decrease in [HbO2] or failed to provide conclusive evidence for it (Mayhew et al., 1999 versus Mayhew et al., 2000; Devor et al., 2003 versus Devor et al., 2007; Sheth et al., 2004a, 2004b).
We addressed this issue in our phosphorescence quenching experiments (Vanzetta and Grinvald, 1999, 2001) by measuring CBV simultaneously with the microvascular oxygen tension. The observed delay of ∼300 ms between the onset of the initial dip and of changes (an increase) in CBV strongly indicated that the detected drop in PO2 in the local microvasculature was indeed a result of increased oxygen extraction, although we could not rule out the special case of pure blood redistribution (dilatations in some vascular compartments and constrictions in others, with no net overall volume increase). This latter hypothesis was ruled out, however, in a complementary study (Vanzetta et al., 2005) in which, using a new fluorescent contrast agent to image CBV changes in the various microvascular compartments, we found no vasoconstriction, and the temporal order of the dilatation was arterioles, arteries, capillaries, venules, veins. Moreover, the ordering of both the amplitudes and timings were inconsistent with the prevalently venous response suggested by the Balloon Model.
The question of how to interpret the initial dip at the microvasculature level also gained some clarity from two recent elegant experiments, one performed with optical imaging (Fukuda et al., 2006b) and the other with fMRI (Nagaoka et al., 2006). In both, the active vascular responses—and thus the changes in CBV—were pharmacologically blocked; yet, not only was the initial dip clearly present, but it was actually enhanced, proving that it cannot be explained by CBV effects alone.
Why, then, was no [HbO2] decrease detected? Originally, functional recruitment of capillaries was proposed as an explanation (Malonek et al., 1997). However, intravital microscopy (Hudetz, 1997) suggests only a minor role for this somewhat controversial concept (Lindauer et al., 2001;Villringer and Dirnagl, 1995; Kuschinsky and Paulson, 1992). Nevertheless, functional recruitment of red blood cells to the capillary bed has been observed in scanning laser-Doppler flowmetry experiments in the cerebellar cortex of rats upon stimulation of climbing and parallel fibers (Akgoren and Lauritzen, 1999), and functional capillary recruitment has been deduced indirectly from comparing the ratio between CBF and cerebral metabolic rate for glucose (CMRGlu) upon stimulation and at rest in human parietal cortex (Kuwabara et al., 1992). Moreover, accumulating experimental evidence from microscopic investigations shows that capillaries are indeed endowed with the “machinery” needed to control their diameter (Rodriguez-Baeza et al., 1998; Harrison et al., 2002) and that this machinery responds to stimulation (Peppiatt et al., 2006, in retina).
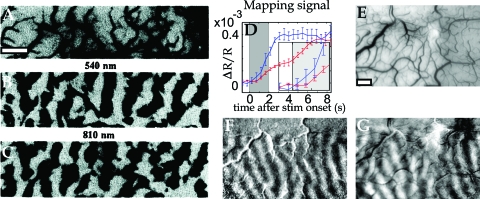
From a more macroscopic perspective, it is worth noting that Frostig and colleagues (1990) obtained high quality CBV-based functional maps by illuminating the cortex at isosbestic wavelengths. To get rid of the artifacts from large blood vessels, they used a “differential” approach, i.e., they computed their maps by comparing two “orthogonal” visual stimuli (i.e., known to activate complementary patches of cortex but to elicit an almost identical pattern of macro-vessels). The high resolution of their “blood volume-based” functional map (Fig. 3) showed, almost 20 years ago, that CBV is indeed regulated also at the capillary level; if this were not the case, the displayed high-resolution differential maps of ocular dominance would have been impossible to obtain. The same results were reproduced later in monkey and cat visual cortex (Vanzetta et al., 2004, 2005; Vanzetta and Grinvald, 2001).
Figure 3. Capillary character of blood volume (CBV) responses, shown by the morphology of high-resolution differential maps obtained by optical imaging.
(A) Image of the cortical vasculature in the primary visual cortex of an anaesthetized macaque. (B, C) Functional maps of ocular dominance columns obtained at 540 and 810 nm, two near-isosbestic wavelengths, at which the contribution of CBV changes dominates the signals. (D) Time course of the mapping signal, showing that the appearance of oximetric maps (obtained at 605 nm, blue trace) slightly precedes that of the CBV maps (obtained at 570 nm, red trace). The larger 570 nm signal was downscaled in the figure by a factor of 4 for display purposes. Inset: Zoom into the first second of the responses. (E) Image of the cortical vasculature in the primary visual cortex of an awake macaque. (F, G) Functional maps of ocular dominance columns obtained at a strongly oximetric wavelength (605 nm) and at an isosbestic wavelength (570 nm) chosen to image CBV signals. Note that (i) the patterns of the blood-volume maps are validated by the oximetric maps, known to reflect the underlying functional architecture; and (ii) the high spatial resolution of the CBV maps strongly suggests that the functional patches can only be of capillary origin. Scale bars: 1 mm. (A–C) Modified from Frostig et al., 1990; (D–G) modified from Vanzetta et al., 2004.
Moreover, our principal component analysis of optical data obtained in the awake macaque shows that the functional architecture correlates better with the CBV response of the capillaries than with the CBV changes in the other vascular compartments (Vanzetta et al., 2004). However, oximetric functional maps tend to appear some 300 ms before the CBV ones (Vanzetta et al., 2004, and unpublished observations, 1998), in agreement with the hypothesis that local oxygen extraction precedes the active vascular response. Also, the macrovascular component of CBV-based maps is much larger than in oximetric ones, at least upon optical imaging and at least in cat and monkey primary visual cortex (Vanzetta et al., 2004; Vanzetta and Grinvald, 2001).
All in all, however, it would appear that, with respect to the initial dip, the expectation raised by conclusions drawn from optical imaging (Malonek and Grinvald, 1996; Grinvald et al., 2000; Vanzetta et al., 2004) have been only partially fulfilled in BOLD fMRI. In fact, the studies showing single-condition maps using the initial dip in BOLD-fMRI remain isolated and∕or controversial (Kim et al., 2000; Kim 2000; Logothetis, 2000; Duong et al., 2001), whereas CBF- and CBV-based signals, shown to be less adequate for high-resolution functional mapping in optical imaging, have yielded spectacular single-condition maps in fMRI (respectively: Duong et al., 2001; Zhao et al., 2005). Interestingly, with the help of statistical methods it was shown that, at least in rodent barrel cortex, also when using optical imaging the spatial resolution of CBV-based optical signals can be as good as that of oximetric ones, and that the amount of the spread and the precision of the map depend less on etiology (CBV, CBF, or oximetry) and more on timing: the earlier, the better (Sheth et al., 2004). Recent spatio-temporal point-spread-function measurements of the positive BOLD fMRI signal at 7 T in humans (Shmuel et al., 2007) would appear to be in agreement with those conclusions.
Why are the signals of preference for obtaining high-resolution functional maps not the same with optical imaging and fMRI? To answer this question, it must be kept in mind that, despite their similarity, fMRI and optical imaging do not measure exactly the same thing, and consequently also the BOLD signal and Hbr-weighted optical maps differ—in particular with respect to their time course (Moon et al., 2007). A possible cause for these differences is that the BOLD-fMRI signal also depends explicitly on vascular volume, whereas—at least in principle—optical imaging spectroscopy yields functional maps that depend only on local changes in [Hbr]. Moreover, photons are cheap, and here we refer less to the price of the high-field magnets needed to enhance the signals coming from small vessels than to their cost in terms of SNR (artifacts related to field inhomogeneities grow with field strength). Geometrical considerations are also important; usually, the optical imaging signal is contaminated by contributions from the macrovasculature, which lies preferentially on the cortical surface. If these vessels are removed, however, good functional maps can also be obtained from CBV measurements (for removal by differential imaging see Frostig et al., 1990; for removal by post-processing, see Vanzetta et al., 2004). On the other hand, thin MRI slices can be placed within the gray matter, avoiding the large vessels known to run preferentially parallel to the cortical sheet on its inner or outer surface. Recent results indeed show that if the fMRI sequence is adequately chosen, the resulting functional images can even reach sub-laminar resolution (Zhao et al., 2006; Goense and Logothetis, 2006). Finally, since the blood flows through the active area (∼0.5–1.8 mm∕s in capillaries; Hudetz, 1997), only the first part of the initial dip appears to be useful for columnar mapping by BOLD fMRI (∼2 s from stimulus onset, Duong et al., 2000), thus severely limiting the attainable SNR (interestingly, this was found to be true also for CBV mapping: Sheth et al., 2005; CBV and CBF spread retrogradely towards arteries and oximetry anterogradely towards veins). Altogether, it appears that the higher SNR attainable with optical imaging than with fMRI is a major reason for the differences encountered in the two methodologies with respect to the spatial resolution of functional maps obtained with the various haemodynamic responses.
The temporal impulse response functions of BOLD-based, CBV-based, and contrast-enhanced CVB-based fMRI signals have been recently measured (Silva et al., 2007) and found to be around 1.5 s, which thus shows that neurovascular control mechanisms relevant for haemodynamics-based fMRI have a temporal resolution better than 1.5 s. Yet, the contrast-enhanced CBV fMRI signal was found to be slightly faster and narrower (∼0.5 s difference). This improved time resolution is particularly interesting when taken together with the fact that the use of contrast agents makes it possible to drastically enhance the SNR of the CBV-weighted fMRI signal, allowing high quality single-condition maps to be obtained at columnar resolution (Moon et al., 2007; Fukuda et al., 2006a; Zhao et al., 2005). Contrast agent-enhanced fMRI is currently limited to animal studies, underscoring the importance of further exploration of alternatives to the classical BOLD signal for achieving high resolution with fMRI in humans (Kim and Ogawa, 2002). Examples are Hahn Spin Echo BOLD fMRI (Yacoub et al., 2003, 2005; Harshbarger and Song, 2006), perfusion-based fMRI (Pfeuffer et al., 2002), and other, non-haemodynamics-based fMRI signals (Darquie et al., 2001; Le Bihan et al., 2006).
INTERPRETING HAEMODYNAMIC RESPONSES IN TERMS OF NEURONAL ACTIVITY
The precise neuronal origin of the haemodynamic responses measured by optical imaging, laser Doppler flowmetry, or fMRI is still relatively unknown, in spite of its importance. Knowing, for example, whether a measured BOLD response reflects neuronal spiking, sub-threshold membrane potential changes, or both, is crucial for correctly interpreting the measurement in terms of input, output, and local processing activity in a given cortical volume. It is beyond the scope of this review to cover these important issues. Some of those subjects have been recently reviewed by others (Arthurs and Boniface, 2002; Attwell and Iadecola, 2002; Kim, 2003; Lauritzen and Gold, 2003; Logothetis, 2003; Logothetis and Wandell, 2004; Lauritzen, 2005). (To illustrate the complexity of the neuronal-to-haemodynamic transformation, in Supplemental Material 2 we briefly discuss the principal findings concerning the “sustained” negative BOLD signal, as well as their implications.)
All in all, the high complexity of the interactions between neuronal activity and brain microcirculatory responses set a formidable challenge to accurately translating the haemodynamic responses (measured, e.g., by fMRI) into neuronal ones. A universal neuronal-to-haemodynamic transfer function, valid for all tasks, brain areas, and structures might not even exist, especially when different volume scales are to be considered (Kim et al., 2004). It is to be hoped that these issues will be elucidated by further research, eventually taking into account the interaction between ongoing neuronal activity and the brain’s haemodynamics (Fox and Raichle, 2007).
CONCLUSIONS
This review covers what seem to us to be important aspects of the coupling between neuronal activity and haemodynamics from the neuroimaging perspective. In comparison to the knowledge available before modern neuroimaging techniques became more or less standard tools of neuroscience, impressive progress has been made, from the strictly neuroscientific perspective as well as from the physiological and biophysical modeling points of view. Some key issues, however, remain to be elucidated, mostly concerning the precise neuronal origin of the haemodynamic signals. For example, how are haemodynamic signals affected by inhibitory activity, excitatory one, their proportion and interplay? Indeed, since the spiking of inhibitory neuronal populations is likely to pose the same metabolic challenge as that of excitatory ones, haemodynamic activity can only be imaged in bulk tissue, where the action of inhibitory neurons can be inferred by their effect in decreasing the activity of the target neuronal populations. Moreover, to what extent are the various haemodynamic responses triggered by spiking and to what extent by synaptic activity? Do the different haemodynamic processes reflect different aspects of the local network activity (input, output, local processing)? Answers to these questions will help us to find reliable ways of translating the recorded haemodynamic signals into the neuronal responses needed by neuroscientists to investigate how the brain functions. Currently available optical approaches include two-photon microscopy (Denk et al., 1990; Svoboda et al., 1997; Kleinfeld et al., 1998; Ohki et al., 2005), optical coherence tomography (Huang et al., 1991; Maheswari, 2003; Culver et al., 2003), laser-speckle (Briers, 2001; Dunn et al., 2001; Durduran et al., 2004) and red blood cell-tracking based flowmetry (Grinvald et al., 2004; Vanzetta et al., 2006) on wild-type and genetically modified animals, as well as intra-operative optical imaging (Hagelund et al., 1993; Shoham and Grinvald, 2001; Zhao et al., 2007) and near-infrared imaging through the intact skull in healthy humans (Gratton et al., 2006, 1997, 1995; Franceschini and Boas, 2004), eventually aiming for millisecond resolution in the human brain. These and future technologies will undoubtedly prove useful in tackling the ambitious goal of understanding the workings of the brain.
References
- Abeles, M (1991). Corticonics: neural circuits of the cerebral cortex, 1st ed., Cambridge University Press, Cambridge. [Google Scholar]
- Adelson, P D, Nemoto, E, Scheuer, M, Painter, M, Morgan, J, and Yonas, H (1999). “Noninvasive continuous monitoring of cerebral oxygenation periictally using near infrared spectroscopy: a preliminary report.” Epilepsia 10.1111/j.1528-1157.1999.tb02030.x 40, 1484–1489. [DOI] [PubMed] [Google Scholar]
- Akgoren, N, and Lauritzen, M (1999). “Functional recruitment of red blood cells to rat brain microcirculation accompanying increased neuronal activity in cerebellar cortex.” NeuroReport 10, 3257–3263. [DOI] [PubMed] [Google Scholar]
- Altman, D I, Lich, L L, and Powers, W J (1991). “Brief inhalation method to measure cerebral oxygen extraction fraction with PET: accuracy determination under pathologic conditions.” J. Nucl. Med. 32, 1738–1741. [PubMed] [Google Scholar]
- Ances, B M (2004). “Coupling of changes in cerebral blood flow with neural activity: what must initially dip must come back up.” J. Cereb. Blood Flow Metab. 10.1097/01.WCB.0000103920.96801.12 24, 1–6. [DOI] [PubMed] [Google Scholar]
- Ances, B M, Buerk, D G, Greenberg, J H, and Detre, J A (2001a). “Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats.” Neurosci. Lett. 306, 106–110. [DOI] [PubMed] [Google Scholar]
- Ances, B M, Wilson, D F, Greenberg, J H, and Detre, J A (2001b). “Dynamic changes in cerebral blood flow, O2 tension, and calculated cerebral metabolic rate of O2 during functional activation using oxygen phosphorescence quenching.” J. Cereb. Blood Flow Metab. 10.1097/00004647-200105000-00005 21, 511–516. [DOI] [PubMed] [Google Scholar]
- Arridge, S R, Cope, M, and Delpy, D T (1992). “The theoretical basis for the determination of optical pathlengths in tissue: temporal and frequency analysis.” Phys. Med. Biol. 10.1088/0031-9155/37/7/005 37, 1531–1560. [DOI] [PubMed] [Google Scholar]
- Arthurs, O J, and Boniface, S (2002). “How well do we understand the neural origins of the fMRI BOLD signal?” Trends Neurosci. 10.1016/S0166-2236(00)01995-0 25, 27–31. [DOI] [PubMed] [Google Scholar]
- Attwell, D, and Iadecola, C (2002). “The neural basis of functional brain imaging signals.” Trends Neurosci. 10.1016/S0166-2236(02)02264-6 25, 621–625. [DOI] [PubMed] [Google Scholar]
- Aubert, A, and Costalat, R (2002). “A model of the coupling between brain electrical activity, metabolism and hemodynamics: application to the interpretation of functional neuroimaging.” Neuroimage 17, 1162–1181. [DOI] [PubMed] [Google Scholar]
- Aubert, A, Pellerin, L, Magistretti, P J, and Costalat, R (2007). “A coherent neurobiological framework for functional neuroimaging provided by a model integrating compartmentalized energy metabolism.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0605864104 104, 4188–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar, S, Suh, M, Zhao, M, and Schwartz, T H (2006). “Multiwavelength intrinsic optical signal imaging of acute focal seizures in rat neocortex. The ‘epileptic dip.’” NeuroReport 10.1097/01.wnr.0000209010.78599.f5 17, 499–503. [DOI] [PubMed] [Google Scholar]
- Bandettini, P A, Wong, E C, Hinks, R S, Tikofsky, R S, and Hyde, J S (1992). “Time course EPI of human brain function during task activation.” Magn. Reson. Med. 10.1002/mrm.1910250220 25, 390–397. [DOI] [PubMed] [Google Scholar]
- Barlow, C H, Harden, W R, III, Harken, A H, Simson, M B, Haselgrove, C, Chance, B, O’Connor, M, and Austin, G (1979). “Fluorescence mapping of mitochrondrial redox changes in heart and brain.” Crit. Care Med. 7, 402–406. [DOI] [PubMed] [Google Scholar]
- Bartfeld, E, and Grinvald, A (1992). “Relationships between orientation-preference pinwheels, cytochrome oxidase blobs, and ocular-dominance columns in primate striate cortex.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.89.24.11905 89, 11905–11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau, J W, Kennedy, D N, Jr, McKinstry, R C, Buchbinder, B R, Weisskoff, R M, Cohen, M S, Vevea, J M, Brady, T J, and Rosen, B R (1991). “Functional mapping of the human visual cortex by magnetic resonance imaging.” Science 10.1126/science.1948051 254, 716–719. [DOI] [PubMed] [Google Scholar]
- Berger, H (1901). Zur Lehre von der Blutzirkulation in der Schädelhöhle des Menschen. Verlag von Gustav, Fischer, Jena, Germany. [Google Scholar]
- Berwick, J, Johnston, D, Jones, M, Martindale, J, Redgrave, P, McLoughlin, N, Schiessl, I, and Mayhew, J E (2005). “Neurovascular coupling investigated with two-dimensional optical imaging spectroscopy in rat whisker barrel cortex.” Eur. J. Neurosci. 10.1111/j.1460-9568.2005.04347.x 22, 1655–1666. [DOI] [PubMed] [Google Scholar]
- Braitenberg, V, and Schüz, A (1998). Cortex: statistics and geometry of neuronal connectivity, 2nd ed., Springer-Verlag, Berlin. [Google Scholar]
- Briers, J D (2001). “Laser doppler, speckle and related techniques for blood perfusion mapping and imaging.” Physiol. Meas 22, R35–R66. [DOI] [PubMed] [Google Scholar]
- Broca, P (1879). “Sur la temperatures morbides locales.” Bull. Acad. Natl. Med. 2S, 1331–1347. [Google Scholar]
- Buxton, R B (2001). “The elusive initial dip.” Neuroimage 13, 953–958. [DOI] [PubMed] [Google Scholar]
- Buxton, R B, and Frank, L R (1997). “A model of the coupling between cerebral blood flow and oxygen metabolism during neural stimulation.” J. Cereb. Blood Flow Metab. 10.1097/00004647-199701000-00009 17, 64–72. [DOI] [PubMed] [Google Scholar]
- Buxton, R B, Uludag, K, Dubowitz, D J, and Liu, T T (2004). “Modeling the haemodynamic response to brain activation.” Neuroimage 23, S220–S233. [DOI] [PubMed] [Google Scholar]
- Buxton, R B, Wong, E C, and Frank, L R (1998). “Dynamics of blood flow and oxygenation changes during brain activation: the balloon model.” Magn. Reson. Med. 10.1002/mrm.1910390602 39, 855–864. [DOI] [PubMed] [Google Scholar]
- Cauli, B, Tong, X K, Rancillac, A, Serluca, N, Lambolez, B, Rossier, J, and Hamel, E (2004). “Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways.” J. Neurosci. 10.1523/JNEUROSCI.3065-04.2004 24, 8940–8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau, E, Oheim, M, Audinat, E, and Charpak, S (2003). “Two-photon imaging of capillary blood flow in olfactory bulb glomeruli.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.2133652100 100, 13081–13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance, B, Cohen, P, Jöbsis, F, and Schoener, B (1962). “Intracellular oxidation-reduction states in vivo.” Science 10.1126/science.137.3529.499 137, 499–508. [DOI] [PubMed] [Google Scholar]
- Chen-Bee, C H, Agoncillo, T, Xiong, Y, and Frostig, R D (2007). “The triphasic intrinsic signal: implications for functional imaging.” J. Neurosci. 10.1523/JNEUROSCI.0326-07.2007 27, 4572–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih, C-P, and Roberts, E L, Jr (2003). “Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis.” J. Cereb. Blood Flow Metab. 10.1097/01.WCB.0000081369.51727.6F 23, 1263–1281. [DOI] [PubMed] [Google Scholar]
- Chuquet, J, Hollender, L, and Nimchinsky, E A (2007). “High-resolution in vivo imaging of the neurovascular unit during spreading depression.” J. Neurosci. 10.1523/JNEUROSCI.0721-07.2007 27, 4036–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, E R, Rostrup, E, Sidaros, K, Lund, T E, Paulson, O B, Ugurbil, K, and Kim, S G (2004). “Hypercapnic normalization of BOLD fMRI: comparison across field strengths and pulse sequences.” Neuroimage 23, 613–624. [DOI] [PubMed] [Google Scholar]
- Cohen, L B (1973). “Changes in neuron structure during action potential propagation and synaptic transmission.” Physiol. Rev. 53, 373–418. [DOI] [PubMed] [Google Scholar]
- Cope, M, van der, Zee P, Essenpreis, M, Arridge, S R, and Delpy, D T (1991). “Data analysis methods for near infrared spectroscopy of tissue: problems in determining the relative cytochrome aa3 concentration.” Proc. SPIE 10.1117/12.44196 1431, 251–262. [DOI] [Google Scholar]
- Crair, M C, Ruthazer, E S, Gillespie, D C, and Stryker, M P (1997). “Relationship between the ocular dominance and orientation maps in visual cortex of monocularly deprived cats.” Neuron 10.1016/S0896-6273(00)80941-1 19, 307–318. [DOI] [PubMed] [Google Scholar]
- Culver, J P, Siegel, A M, Stott, J J, and Boas, D A (2003). “Volumetric diffuse optical tomography of brain activity.” Opt. Lett. 10.1364/OL.28.002061 28, 2061–2063. [DOI] [PubMed] [Google Scholar]
- Darquie, A, Poline, J B, Poupon, C, Saint-Jalmes, H, and Le Bihan, D (2001). “Transient decrease in water diffusion observed in human occipital cortex during visual stimulation.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.151125698 98, 9391–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, T L, Kwong, K K, Weisskoff, R M, and Rosen, B R (1998). “Calibrated functional MRI: mapping the dynamics of oxidative metabolism.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.95.4.1834 95, 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneux, T, Vanzetta, I, Frédéric Chavane, F, Olivier Faugeras, O, and Masson, G S (2006). “Linearity and non-linearity of sensory-evoked neuronal and hemodynamic responses in awake monkey V1.” 12th Annual Meeting of the Organization for Human Brain Mapping in Florence, Italy.
- Denk, W, Strickler, J H, and Webb, W W (1990). “Two-photon laser scanning fluorescence microscopy.” Science 10.1126/science.2321027 248, 73–76. [DOI] [PubMed] [Google Scholar]
- Devor, A, Dunn, A K, Andermann, M L, Ulbert, I, Boas, D A, and Dale, A M (2003). “Coupling of total haemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex.” Neuron 10.1016/S0896-6273(03)00403-3 39, 353–359. [DOI] [PubMed] [Google Scholar]
- Devor, A, Tian, P, Nishimura, N, Teng, I C, Hillman, E M, Narayanan, S N, Ulbert, I, Boas, D A, Kleinfeld, D, and Dale, A M (2007). “Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal.” J. Neurosci. 10.1523/JNEUROSCI.0134-07.2007 27, 4452–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor, A, Ulbert, I, Dunn, A K, Narayanan, S N, Jones, S R, Andermann, M L, Boas, D A, and Dale, A M (2005). “Coupling of the cortical haemodynamic response to cortical and thalamic neuronal activity.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0407789102 102, 3822–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinse, H R, Godde, B, Hilger, T, Haupt, S S, Spengler, F, and Zepka, R (1997). “Short-term functional plasticity of cortical and thalamic sensory representations and its implication for information processing.” Adv. Neurol. 73, 159–178. [PubMed] [Google Scholar]
- Dunn, A K, Bolay, H, Moskowitz, M A, and Boas, D A (2001). “Dynamic imaging of cerebral blood flow using laser speckle.” J. Cereb. Blood Flow Metab. 10.1097/00004647-200103000-00002 21, 195–201. [DOI] [PubMed] [Google Scholar]
- Dunn, A K, Devor, A, Dale, A M, Boas, D A (2005). “Spatial extent of oxygen metabolism and haemodynamic changes during functional activation of the rat somatosensory cortex.” Neuroimage 27, 279–290. [DOI] [PubMed] [Google Scholar]
- Duong, T Q, Kim, D-S, Ugurbil, K, and Kim, S-G (2000). “Spatiotemporal dynamics of the BOLD fMRI signals: toward mapping submillimeter cortical columns using the early negative response.” Magn. Reson. Med. 44, 231–242. [DOI] [PubMed] [Google Scholar]
- Duong, T Q, Kim, D-S, Ugurbil, K, and Kim, S-G (2001). “Localized cerebral blood flow response at submillimeter columnar resolution.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.191101098 98, 10904–10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durduran, T, Burnett, M G, Yu, G, Zhou, C, Furuya, D, Yodh, A G, Detre, J A, and Greenberg, J H (2004). “Spatiotemporal quantification of cerebral blood flow during functional activation in rat somatosensory cortex using laser-speckle flowmetry.” J. Cereb. Blood Flow Metab. 10.1097/00004647-200405000-00005 24, 518–525. [DOI] [PubMed] [Google Scholar]
- See EPAPS Document No. E-HJFOA5-2-003802 for supplemental material. This document can be reached through a direct link in the online article’s HTML reference section or via the EPAPS home page (http://www.aip.org/pubservs/epaps.html).
- Ernst, T, and Hennig, J (1994). “Observation of a fast response in functional MR.” Magn. Reson. Med. 10.1002/mrm.1910320122 32, 146–149. [DOI] [PubMed] [Google Scholar]
- Fantini, S (2002). “A haemodynamic model for the physiological interpretation of in vivo measurements of the concentration and oxygen saturation of haemoglobin.” Phys. Med. Biol. 10.1088/0031-9155/47/18/402 47, N249–N257. [DOI] [PubMed] [Google Scholar]
- Fox, P T, and Raichle, M E (1984). “Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography.” J. Neurophysiol. 51, 1109–1120. [DOI] [PubMed] [Google Scholar]
- Fox, P T, and Raichle, M E (1986). “Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.83.4.1140 83, 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, P T, and Raichle, M E (2007). “Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging.” Nat. Rev. Neurosci. 10.1038/nrn2201 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Fox, P T, Raichle, M E, Mintun, M A, and Dence, C (1988). “Nonoxidative glucose consumption during focal physiologic neural activity.” Science 10.1126/science.3260686 241, 462–464. [DOI] [PubMed] [Google Scholar]
- Frackowiak, R S, Lenzi, G L, Jones, T, and Heather, J D (1980). “Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values.” J. Comput. Assist. Tomogr. 10.1097/00004728-198012000-00001 4, 727–736. [DOI] [PubMed] [Google Scholar]
- Franceschini, M A, and Boas, D A (2004). “Noninvasive measurement of neuronal activity with near-infrared optical imaging.” Neuroimage 21, 372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson, P, Kruger, G, Merboldt, K D, and Frahm, J (1998). “Temporal characteristics of oxygenation-sensitive MRI responses to visual activation in humans.” Magn. Reson. Med. 10.1002/mrm.1910390608 39, 912–919. [DOI] [PubMed] [Google Scholar]
- Friston, K J, Mechelli, A, Turner, R, and Price, C J (2000). “Nonlinear responses in fMRI: the balloon model, volterra kernels, and other haemodynamics.” Neuroimage 12, 466–477. [DOI] [PubMed] [Google Scholar]
- Frostig, R D, Lieke, E E, Ts’o, D Y, and Grinvald, A (1990). “Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.87.16.6082 87, 6082–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, H, Kuwabara, H, Reutens, D C, and Gjedde, A (1999). “Oxygen consumption of cerebral cortex fails to increase during continued vibrotactile stimulation.” J. Cereb. Blood Flow Metab. 10.1097/00004647-199903000-00004 19, 266–271. [DOI] [PubMed] [Google Scholar]
- Fukuda, M, Moon, C H, Wang, P, Moon, C H, and Kim, S G (2006a). “Mapping iso-orientation columns by contrast agent-enhanced functional magnetic resonance imaging: reproducibility, specificity, and evaluation by optical imaging of intrinsic signal.” J. Neurosci. 10.1523/JNEUROSCI.3098-06.2006 26, 11821–11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, M, Wang, P, Moon, C H, Tanifuji, M, and Kim, S G (2006b). “Spatial specificity of the enhanced dip inherently induced by prolonged oxygen consumption in cat visual cortex: implication for columnar resolution functional MRI.” Neuroimage 30, 70–87. [DOI] [PubMed] [Google Scholar]
- Fulton, J F (1928). “Observations upon the vascularity of the human occipital lobe during visual activity.” Brain 51, 310–320. [PMC free article] [PubMed] [Google Scholar]
- Girouard, H, and Iadecola, C (2006). “Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease.” J. Appl. Physiol. 10.1152/japplphysiol.00966.2005 100, 328–335. [DOI] [PubMed] [Google Scholar]
- Gjedde, A (1991). “Is oxygen diffusion limiting for blood-brain transfer of oxygen?” In: Brain work and mental activity, Alfred Benzon Symp. 31, Lassen, N A, Ingvar, D H, Raichle, M E, and Friberg, L, eds., Munksgaard, Copenhagen, Denmark, pp. 177–184. [Google Scholar]
- Gjedde, A (1997). “The relation between brain function and cerebral blood flow and metabolism.” In: Cerebrovascular disease (Batjer, H H, ed.), Lippincott-Raven, Philadelphia, PA, pp. 23–40. [Google Scholar]
- Gjedde, A, Kuwabara, H, and Hakim, A M (1990). “Reduction of functional capillary density in human brain after stroke.” J. Cereb. Blood Flow Metab. 10, 317–326. [DOI] [PubMed] [Google Scholar]
- Gjedde, A, Poulsen, P H, and Ostergaard, L (1999). “On the oxygenation of haemoglobin in the human brain.” Adv. Exp. Med. Biol. 471, 67–81. [DOI] [PubMed] [Google Scholar]
- Goense, J BM, and Logothetis, N K (2006). “Laminar specificity in monkey V1 using high-resolution SE-fMRI.” Magn. Reson. Imaging 10.1016/j.mri.2005.12.032 24, 381–392. [DOI] [PubMed] [Google Scholar]
- Gratton, G, Brumback, C R, Gordon, B A, Pearson, M A, Low, K A, and Fabiani, M (2006). “Effects of measurement method, wavelength, and source-detector distance on the fast optical signal.” Neuroimage 32, 1576–1590. [DOI] [PubMed] [Google Scholar]
- Gratton, G, Corballis, P M, Cho, E, Fabiani, M, and Hood, D C (1995). “Shades of gray matter: noninvasive optical images of human brain responses during visual stimulation.” Psychophysiology 10.1111/j.1469-8986.1995.tb02102.x 32, 505–509. [DOI] [PubMed] [Google Scholar]
- Gratton, G, Fabiani, M, Corballis, P M, and Gratton, E (1997). “Noninvasive detection of fast signals from the cortex using frequency-domain optical methods.” Ann. N.Y. Acad. Sci. 10.1111/j.1749-6632.1997.tb46202.x 820, 286–298. [DOI] [PubMed] [Google Scholar]
- Grinvald, A, Bonhoeffer, T, Vanzetta, I, Pollack, A, Aloni, E, Ofri, R, and Nelson, D (2004). “High-resolution functional optical imaging: from the neocortex to the eye,” Ophthalmol. Clin. North America 17, 53–67. [DOI] [PubMed] [Google Scholar]
- Grinvald, A, Frostig, R D, Lieke, E, and Hildesheim, R (1988). “Optical imaging of neuronal activity,” Physiol. Rev. 68, 1285–1366. [DOI] [PubMed] [Google Scholar]
- Grinvald, A, Lieke, E, Frostig, R D, Gilbert, C D, and Wiesel, T N (1986). “Functional architecture of cortex revealed by optical imaging of intrinsic signals,” Nature (London) 10.1038/324361a0 324, 361–364. [DOI] [PubMed] [Google Scholar]
- Grinvald, A, Manker, A, and Segal, M (1982). “Visualization of the spread of electrical activity in rat hippocampal slices by voltage-sensitive optical probes,” J. Physiol. (London) 333, 269–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald, A, Sharon, D, Slovin, H, and Vanzetta, I (2005). “Intrinsic signal imaging in the neocortex: implications for haemodynamic based functional imaging.” In: Imaging in neuroscience and development: a laboratory manual. Cold Spring Harbor Laboratory Press, New York, Yuste, R, and Konnerth, A, eds., Sec. 7, Chap. 89, pp. 655–671. [Google Scholar]
- Grinvald, A, Shmuel, A, Vanzetta, I, Shtoyerman, E, Shoham, D, and Arieli, A (2000). “Intrinsic signal imaging in the neocortex.” In: Imaging neurons: a laboratory manual. Yuste, R, Lanni, F, and Konnerth, A, eds., Cold Spring Harbor Laboratory Press, New York, Sec. 6, Chap. 45. [Google Scholar]
- Grubb, R L, Raichle, M E, Eichling, J O, and Ter-Pergossian, M M (1974). “The effects of changes in PACO2 on cerebral blood volume, blood flow and vascular mean transit time,” Stroke 5, 630–639. [DOI] [PubMed] [Google Scholar]
- Haglund, M M, Ojemann, G A, and Blasdel, G G (1993). “Optical imaging of bipolar cortical stimulation,” J. Neurosurg. 78, 785–793. [DOI] [PubMed] [Google Scholar]
- Harrison, R V, Harel, N, Panesar, J, and Mount, R J (2002). “Blood capillary distribution correlates with haemodynamic-based functional imaging in cerebral cortex,” Cereb. Cortex 12, 225–233. [DOI] [PubMed] [Google Scholar]
- Harshbarger, T B, and Song, A W (2006). “Endogenous functional CBV contrast revealed by diffusion weighting,” NMR Biomed. 10.1002/nbm.1067 19, 1020–1027. [DOI] [PubMed] [Google Scholar]
- Hathout, G M, Varjavand, B, and Gopi, R K (1999). “The early response in fMRI: a modelling approach,” Magn. Reson. Med. 41, 550–554. [DOI] [PubMed] [Google Scholar]
- Hayashi, T, et al. (2003). “A theoretical model of oxygen delivery and metabolism for physiologic interpretation of quantitative cerebral blood flow and metabolic rate of oxygen,” J. Cereb. Blood Flow Metab. 10.1097/01.WCB.0000090506.76664.00 23, 1314–1323. [DOI] [PubMed] [Google Scholar]
- Haydon, P G, and Carmignoto, G (2006). “Astrocyte control of synaptic transmission and neurovascular coupling,” Physiol. Rev. 86, 1009–1031. [DOI] [PubMed] [Google Scholar]
- Heekeren, H R, Kohl, M, Obrig, H, Wenzel, R, von Pannwitz, W, Matcher, S J, Dirnagl, U, Cooper, C E, and Villringer, A (1999). “Noninvasive assessment of changes in cytochrome-c oxidase oxidation in human subjects during visual stimulation.” J. Cereb. Blood Flow Metab. 10.1097/00004647-199906000-00002 19, 592–603. [DOI] [PubMed] [Google Scholar]
- Hill, D K, and Keynes, R D (1949). “Opacity changes in stimulated nerve.” J. Physiol. (London) 108, 278–281. [PubMed] [Google Scholar]
- Hill, L (1896). The physiology and pathology of the cerebral circulation: an experimental research.Churchill, London, UK. [Google Scholar]
- Hillman, E M, Devor, A, Bouchard, M B, Dunn, A K, Krauss, G W, Skoch, J, Bacskai, B J, Dale, A M, and Boas, D A (2007). “Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation.” Neuroimage 35, 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, E J, Phelps, M E, Mullani, N A, Higgins, C S, and Ter-Pogossian, M M (1976). “Design and performance characteristics of a whole-body positron transaxial tomograph.” J. Nucl. Med. 17, 493–502. [PubMed] [Google Scholar]
- Hoge, R D, Atkinson, J, Gill, B, Crelier, G R, Marrett, S, and Pike, G B (1999). “Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.96.16.9403 96, 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X, Le, T H, and Ugurbil, K (1997). “Evaluation of the early response in fMRI in individual subjects using short stimulus duration.” Magn. Reson. Med. 10.1002/mrm.1910370612 37, 877–884. [DOI] [PubMed] [Google Scholar]
- Hu, Y B, and Wilson, G S (1997). “A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor.” J. Neurochem. 69, 1484–1490. [DOI] [PubMed] [Google Scholar]
- Huang, D, et al. (1991). “Optical coherence tomography.” Science 10.1126/science.1957169 254, 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel, D H, and Wiesel, T N (1962). “Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex.” J. Physiol. (London) 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz, A G (1997). “Blood flow in the cerebral capillary network: a review emphasizing observations with intravital microscopy.” Microcirculation 4, 233–252. [DOI] [PubMed] [Google Scholar]
- Husson, T R, Mallik, A K, Zhang, J X, and Issa, N P (2007). “Functional imaging of primary visual cortex using flavoprotein autofluorescence.” J. Neurosci. 10.1523/JNEUROSCI.2156-07.2007 27, 8665–8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson, E B, Stefanovic, B, Koretsky, A P, and Silva, A C (2006). “Spatial flow-volume dissociation of the cerebral microcirculatory response to mild hypercapnia.” Neuroimage 32, 520–530. [DOI] [PubMed] [Google Scholar]
- Hyder, F, Shulman, R G, and Rothman, D L (1998). “A model for the regulation of cerebral oxygen delivery.” J. Appl. Physiol. 85, 554–564. [DOI] [PubMed] [Google Scholar]
- Ingvar, D H, and Risberg, J (1967). “Increase of regional cerebral blood flow during mental effort in normals and in patients with focal brain disorders.” Exp. Brain Res. 3, 195–211. [DOI] [PubMed] [Google Scholar]
- Jakovcevic, D, and Harder, D R (2007). “Role of astrocytes in matching blood flow to neuronal activity.” Curr. Top Dev. Biol. 79, 75–97. [DOI] [PubMed] [Google Scholar]
- Jöbsis, F F (1977). “Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters.” Science 10.1126/science.929199 198, 1264–1267. [DOI] [PubMed] [Google Scholar]
- Jöbsis, F F, Keizer, J H, LaManna, J C, and Rosenthal, M J (2001). “Reflectance spectrophotometry of cytochrome aa3 in vivo.” J. Appl. Physiol.: Respir., Environ. Exercise Physiol. 43, 858–872. [DOI] [PubMed] [Google Scholar]
- Jones, M, Berwick, J, Johnston, D, and Mayhew, J (2001). “Concurrent optical imaging spectroscopy and laser-doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex.” Neuroimage 13, 1002–1015. [DOI] [PubMed] [Google Scholar]
- Kasischke, K A, Vishwasrao, H D, Fisher, P J, Zipfel, W R, and Webb, W W (2004). “Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis.” Science 10.1126/science.1096485 305, 99–103. [DOI] [PubMed] [Google Scholar]
- Kennedy, C, DesRosiers, M H, Sakurada, O, Shinohara, M, Reivich, M, Jehle, J, and Sokoloff, L (1976). “Metabolic mapping of the primary visual system of the monkey by means of the autoradiographic [14C]-deoxyglucose technique.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.73.11.4230 73, 4230–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley, A J, Berwick, J, Martindale, J, Johnston, D, Papadakis, N, and Mayhew, J E (2005). “Concurrent fMRI and optical measures for the investigation of the haemodynamic response function.” Magn. Reson. Med. 10.1002/mrm.20511 54, 354–365. [DOI] [PubMed] [Google Scholar]
- Kerem, D H, and Geva, A B (2005). “Forecasting epilepsy from the heart rate signal.” Med. Biol. Eng. Comput. 10.1007/BF02345960 43, 230–239. [DOI] [PubMed] [Google Scholar]
- Kety, S S (1950). “Circulation and metabolism of the human brain in health and disease.” Am. J. Med. 10.1016/0002-9343(50)90363-9 8, 205–217. [DOI] [PubMed] [Google Scholar]
- Kim, D S (2000). “Can current fMRI techniques reveal the micro-architecture of cortex.” Nat. Neurosci. 10.1038/74771 3, 414. [DOI] [PubMed] [Google Scholar]
- Kim, D S, Duong, T Q, and Kim, S G (2000). “High-resolution mapping of isoorientation columns by fMRI.” Nat. Neurosci. 10.1038/72109 3, 164–169. [DOI] [PubMed] [Google Scholar]
- Kim, D S, Ronen, I, Olman, C, Kim, S G, Ugurbil, K, and Toth, L J (2004). “Spatial relationship between neuronal activity and BOLD functional MRI.” Neuroimage 21, 876–885. [DOI] [PubMed] [Google Scholar]
- Kim, S G (2003). “Progress in understanding functional imaging signals.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0730809100 100, 3550–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S G, and Ogawa, S (2002). “Insights into new techniques for high resolution functional MRI.” Curr. Opin. Neurobiol. 10.1016/S0959-4388(02)00355-0 12, 607–615. [DOI] [PubMed] [Google Scholar]
- Kleinfeld, D, Mitra, P P, Helmchen, F, and Denk, W (1998). “Fluctuations and stimulus induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.95.26.15741 95, 15741–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl, M, Lindauer, U, Royl, G, Kühl, M, Gold, L, Villringer, A, and Dirnagl, U (2000). “Physical model for the spectroscopic analysis of cortical intrinsic optical signals.” Phys. Med. Biol. 10.1088/0031-9155/45/12/317 45, 3749–3764. [DOI] [PubMed] [Google Scholar]
- Kohl, M, Nolte, C, Heekeren, H R, Horst, S, Scholz, U, Obrig, H, and Villringer, A (1998). “Changes in cytochrome oxidation in the occipital cortex during visual stimulation: improvement in sensitivity by the determination of the wavelength dependence of the differential pathlength factor.” Phys. Med. Biol. 10.1088/0031-9155/43/6/028 43, 1771–1782. [DOI] [PubMed] [Google Scholar]
- Kong, Y, Zheng, Y, Johnston, D, Martindale, J, Jones, M, Billings, S, and Mayhew, J (2004). “A model of the dynamic relationship between blood flow and volume changes during brain activation.” J. Cereb. Blood Flow Metab. 10.1097/00004647-200412000-00007 24, 1382–1392. [DOI] [PubMed] [Google Scholar]
- Kuschinsky, W, and Paulson, O B (1992). “Capillary circulation in the brain.” Cerebrovasc Brain Metab. Rev. 4, 261–286. [PubMed] [Google Scholar]
- Kuwabara, H, Ohta, S, Brust, P, Meyer, E, and Gjedde, A (1992). “Density of perfused capillaries in living human brain during functional activation.” Prog. Brain Res. 91, 209–215. [DOI] [PubMed] [Google Scholar]
- Kwong, K K, et al. (1992). “Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.89.12.5675 89, 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaManna, J C, Pikarsky, S M, Sick, T J, and Rosenthal, M (1985). “A rapid scanning spectrophotometer designed for biological tissues in vitro or in vivo.” Anal. Biochem. 10.1016/0003-2697(85)90145-9 144, 483–493. [DOI] [PubMed] [Google Scholar]
- LaManna, J C, Sick, T J, Pikarsky, S M, and Rosenthal, M (1987). “Detection of an oxidizable fraction of cytochrome oxidase in intact rat brain.” Am. J. Physiol.: Cell Physiol. 253, C477–C483. [DOI] [PubMed] [Google Scholar]
- Landau, W M, Freygang, W H, Rowland, L P, Sokoloff, L, and Kety, S S (1955). “The local circulation of the living brain: values in the unanesthetized and anesthetized cat.” Trans. Am. Neurol. Assoc. 80, 125–129. [PubMed] [Google Scholar]
- Lassen, N A (1959). “Cerebral blood flow and oxygen consumption in man.” Physiol. Rev. 39, 183–238. [DOI] [PubMed] [Google Scholar]
- Lassen, N A, and Ingvar, D H (1961). “The blood flow of the cerebral cortex determined by radioactive krypton.” Experientia 10.1007/BF02157946 17, 42–43. [DOI] [PubMed] [Google Scholar]
- Lauritzen, M (2005). “Reading vascular changes in brain imaging: is dendritic calcium the key?” Nat. Rev. Neurosci. 10.1038/nrn1589 6, 77–85. [DOI] [PubMed] [Google Scholar]
- Lauritzen, M, and Gold, L (2003). “Brain function and neurophysiological correlates of signals used in functional neuroimaging.” J. Neurosci. 23, 3972–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan, D, Urayama, S, Aso, T, Hanakawa, T, and Fukuyama, H (2006). “Direct and fast detection of neuronal activation in the human brain with diffusion MRI.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0600644103 103, 8263–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieke, E E, Frostig, R D, Arieli, A, Ts’o, D Y, Hildesheim, R, and Grinvald, A (1989). “Optical imaging of cortical activity: real-time imaging using extrinsic dye-signals and high resolution imaging based on slow intrinsic-signals.” Annu. Rev. Physiol. 10.1146/annurev.ph.51.030189.002551 51, 543–559. [DOI] [PubMed] [Google Scholar]
- Lindauer, U, Royl, G, Leithner, C, Kühl, M, Gold, L, Gethmann, J, Kohl-Bareis, M, Villringer, A, and Dirnagl, U (2001). “No evidence for early decrease in blood oxygenation in rat whisker cortex in response to functional activation.” Neuroimage 13, 988–1001. [DOI] [PubMed] [Google Scholar]
- Lipton, P (1973). “Effects of membrane depolarization on light scattering by cerebral cortical slices.” J. Physiol. (London) 231, 365–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis, N K (2000). “Can current fMRI techniques reveal the micro-architecture of cortex.” Nat. Neurosci. 10.1038/74768 3, 413. [DOI] [PubMed] [Google Scholar]
- Logothetis, N K (2003). “The underpinnings of the BOLD functional magnetic resonance imaging signal.” J. Neurosci. 23, 3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis, N K, Guggenberger, H, Peled, S, and Pauls, J (1999). “Functional imaging of the monkey brain.” Nat. Neurosci. 10.1038/9210 2, 555–562. [DOI] [PubMed] [Google Scholar]
- Logothetis, N K, and Pfeuffer, J (2004). “On the nature of the BOLD fMRI contrast mechanism.” Magn. Reson. Imaging 10.1016/j.mri.2004.10.018 22, 1517–1531. [DOI] [PubMed] [Google Scholar]
- Logothetis, N K, and Wandell, B A (2004). “Interpreting the BOLD signal.” Annu. Rev. Physiol. 10.1146/annurev.physiol.66.082602.092845 66, 735–769. [DOI] [PubMed] [Google Scholar]
- Lok, J, Gupta, P, Guo, S, Kim, W J, Whalen, M J, van Leyen, K, and Lo, E H (2007). “Cell-cell signaling in the neurovascular unit.” Neurochem. Res. 32, 2032–2045. [DOI] [PubMed] [Google Scholar]
- MacVicar, B A, and Hochman, D (1991). “Imaging of synaptically evoked intrinsic optical signals in hippocampal slices.” J. Neurosci. 11, 1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswari, R U, Takaoka, H, Kadono, H, Homma, R, and Tanifuji, M (2003). “Novel functional imaging technique from brain surface with optical coherence tomography enabling visualization of depth resolved functional structure in vivo.” J. Neurosci. Methods 10.1016/S0165-0270(02)00370-9 124, 83–92. [DOI] [PubMed] [Google Scholar]
- Malonek, D, Dirnagl, U, Lindauer, U, Yamada, K, Kanno, I, and Grinvald, A (1997). “Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.94.26.14826 94, 14826–14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek, D, and Grinvald, A (1996). “Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping.” Science 10.1126/science.272.5261.551 272, 551–554. [DOI] [PubMed] [Google Scholar]
- Mandeville, J B, Marota, J J, Ayata, C, Moskowitz, M A, Weisskoff, R M, and Rosen, B R (1999a). “MRI measurement of the temporal evolution of relative CMRO2 during rat forepaw stimulation.” Magn. Reson. Med. 42, 944–951. [DOI] [PubMed] [Google Scholar]
- Mandeville, J B, Marota, J J, Ayata, C, Zaharchuk, G, Moskowitz, M A, Rosen, B R, and Weisskoff, R M (1999b). “Evidence of a cerebrovascular postarteriole Windkessel with delayed compliance.” J. Cereb. Blood Flow Metab. 10.1097/00004647-199906000-00012 19, 679–689. [DOI] [PubMed] [Google Scholar]
- Marota, J J, Ayata, C, Moskowitz, M A, Weisskoff, R M, Rosen, B R, and Mandeville, J B (1999). “Investigation of the early response to rat forepaw stimulation.” Magn. Reson. Med. 41, 247–252. [DOI] [PubMed] [Google Scholar]
- Matcher, S J, Cope, M, and Delpy, D T (1997). “In vivo measurements of the wavelength dependence of tissue-scattering coefficients between 760–900 nm measured with time resolved spectroscopy.” Appl. Opt. 36, 386–396. [DOI] [PubMed] [Google Scholar]
- Mayhew, J, Johnston, D, Berwick, J, Jones, M, Coffey, P, and Zheng, Y (2000). “Spectroscopic analysis of neural activity in brain: increased oxygen consumption following activation of barrel cortex.” Neuroimage 12, 664–675. [DOI] [PubMed] [Google Scholar]
- Mayhew, J, Johnston, D, Martindale, J, Jones, M, Berwick, J, and Zheng, Y (2001). “Increased oxygen consumption following activation of brain: theoretical footnotes using spectroscopic data from barrel cortex.” Neuroimage 13, 973–985. [DOI] [PubMed] [Google Scholar]
- Mayhew, J, Zheng, Y, Hou, Y, Vuksanovic, B, Berwick, J, Askew, S, and Coffey, P (1999). “Spectroscopic analysis of changes in remitted illumination: the response to increased neural activity in brain.” Neuroimage 10, 304–326. [DOI] [PubMed] [Google Scholar]
- Mc Loughlin, N P, and Blasdel, G G (1998). “Wavelength-dependent differences between optically determined functional maps from macaque striate cortex.” Neuroimage 7, 326–336. [DOI] [PubMed] [Google Scholar]
- Menon, R S, Ogawa, S, Hu, X, Strupp, J P, Anderson, P, and Ugurbil, K (1995). “BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals.” Magn. Reson. Med. 10.1002/mrm.1910330323 33, 453–459. [DOI] [PubMed] [Google Scholar]
- Metea, M R, and Newman, E A (2006). “Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling.” J. Neurosci. 10.1523/JNEUROSCI.4048-05.2006 26, 2862–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun, M A, Fox, P T, and Raichle, M E (1989). “A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography.” J. Cereb. Blood Flow Metab. 9, 96–103. [DOI] [PubMed] [Google Scholar]
- Mintun, M A, Raichle, M E, Martin, W R, and Herscovitch, P (1984). “Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography.” J. Nucl. Med. 25, 177–187. [PubMed] [Google Scholar]
- Mintun, M A, Vlassenko, A G, Shulman, G L, and Snyder, A Z (2002). “Time related increase of oxygen utilization in continuously activated human visual cortex.” Neuroimage 162, 531–537. [DOI] [PubMed] [Google Scholar]
- Moon, C H, Fukuda, M, Park, S H, and Kim, S G (2007). “Neural interpretation of blood oxygenation level-dependent fMRI maps at submillimeter columnar resolution.” J. Neurosci. 10.1523/JNEUROSCI.0445-07.2007 27, 6892–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosso, A (1881). Über den Kreislauf des Blutes im Menschlichen Gehirn. Von Veit, Leipzig, Germany. [Google Scholar]
- Mosso, A (1894). La Temperatura del Cervello, Treves, Milan, Italy.
- Mountcastle, V B (1957). “Modality and topographic properties of single neurons of cat’s somatic sensory cortex.” J. Neurophysiol. 20, 408–434. [DOI] [PubMed] [Google Scholar]
- Mulligan, S J, and MacVicar, B A (2004). “Calcium transients in astrocyte endfeet cause cerebrovascular constrictions.” Nature (London) 10.1038/nature02827 431, 195–199. [DOI] [PubMed] [Google Scholar]
- Murakami, H, Kamatani, D, Hishida, R, Takao, T, Kudoh, M, Kawaguchi T, Tanaka, R, and Shibuki, K (2004). “Short-term plasticity visualized with flavoprotein autofluorescence in the somatosensory cortex of anaesthetized rats.” Eur. J. Neurosci. 10.1111/j.1460-9568.2004.03237.x 19, 1352–1360. [DOI] [PubMed] [Google Scholar]
- Nagaoka, T, Zhao, F, Wang, P, Harel, N, Kennan, R P, Ogawa, S, and Kim, S G (2006). “Increases in oxygen consumption without cerebral blood volume change during visual stimulation under hypotension condition.” J. Cereb. Blood Flow Metab. 10.1038/sj.jcbfm.9600251 26, 1043–1051. [DOI] [PubMed] [Google Scholar]
- Nair, D G (2005). “About being BOLD.” Brain Res. Dev. Brain Res. 50, 229–243. [DOI] [PubMed] [Google Scholar]
- Nemoto, M, Nomura, Y, Sato, C, Tamura, M, Houkin, K, Koyanagi, I, and Abe, H (1999). “Analysis of optical signals evoked by peripheral nerve stimulation in rat somatosensory cortex: dynamic changes in haemoglobin concentration and oxygenation.” J. Cereb. Blood Flow Metab. 10.1097/00004647-199903000-00002 19, 246–259. [DOI] [PubMed] [Google Scholar]
- Nemoto, M, Nomura, Y, Tamura, M, Sato, C, Houkin, K, and Abe, H (1997). “Optical imaging and measuring of local haemoglobin concentration and oxygenation changes during somatosensory stimulation in rat cerebral cortex.” Adv. Exp. Med. Biol. 428, 521–531. [DOI] [PubMed] [Google Scholar]
- Nemoto, M, Sheth, S, Guiou, M, Pouratian, N, Chen, J W, and Toga, A W (2004). “Functional signal- and paradigm-dependent linear relationships between synaptic activity and haemodynamic responses in rat somatosensory cortex.” J. Neurosci. 10.1523/JNEUROSCI.4870-03.2004 24, 3850–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenhauser, N, Thomsen, K, Caesar, K, and Lauritzen, M (2005). “Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow.” J. Physiol. (London) 565, 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, S, Lee, T M, Kay, A R, and Tank, D W (1990). “Brain magnetic resonance imaging with contrast dependent on blood oxygenation.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.87.24.9868 87, 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, S, Lee, T M, Stepnoski, R, Chen, W, Zhu, X H, and Ugurbil, K (2000). “An approach to probe some neural systems interaction by functional MRI at neural time scale down to milliseconds.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.97.20.11026 97, 11026–11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, S, Tank, D W, Menon, R, Ellermann, J M, Kim, S-G, Merkle, H, and Ugurbil, K (1992). “Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.89.13.5951 89, 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki, K, Chung, S, Ch’ng, Y H, Kara, P, and Reid, R C (2005). “Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex.” Nature (London) 10.1038/nature03274 433, 597–603. [DOI] [PubMed] [Google Scholar]
- Pawlik, G, Rackl, A, and Bing, R J (1981). “Quantitative capillary topography and blood flow in the cerebral cortex of cats: an in vivo microscopic study.” Brain Res. 208, 35–58. [DOI] [PubMed] [Google Scholar]
- Pellerin, L, and Magistretti, P J (1994). “Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.91.22.10625 91, 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin, L, Pellegri, G, Bittar, P G, Charnay, Y, Bouras, C, Martin, J, Stella, N, and Magistretti, P J (1998). “Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle.” Dev. Neurosci. (Basel, Switz.) 20, 291–299. [DOI] [PubMed] [Google Scholar]
- Peppiatt, C M, Howarth, C, Mobbs, P, and Attwell, D (2006). “Bidirectional control of CNS capillary diameter by pericytes.” Nature (London) 10.1038/nature05193 443, 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer, J, Adriany, G, Shmuel, A, Yacoub, E, Van De Moortele, P F, Hu, X, and Ugurbil, K (2002). “Perfusion-based high-resolution functional imaging in the human brain at 7 Tesla.” Magn. Reson. Med. 10.1002/mrm.10154 47, 903–911. [DOI] [PubMed] [Google Scholar]
- Prakash, N, Biag, J D, Sheth, S A, Mitsuyama, S, Theriot, J, Ramachandra, C, and Toga, A W (2007). “Temporal profiles and 2-dimensional oxy-, deoxy-, and total-haemoglobin somatosensory maps in rat versus mouse cortex.” Neuroimage , online publication 21 May 2007. 10.1016/j. neuroimage.2007.04.063. [DOI] [PMC free article] [PubMed]
- Prakash, N, Cohen-Cory, S, and Frostig, R D (1996). “RAPID and opposite effects of BDNF and NGF on the functional organization of the adult cortex in vivo.” Nature (London) 10.1038/381702a0 381, 702–706. [DOI] [PubMed] [Google Scholar]
- Prichard, J, Rothman, D, Novotny, E, Petroff, O, Kuwabara, T, Avison, M, Howseman, A, Hanstock, C, and Shulman, R (1991). “Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation.” Proc. Natl. Acad. Sci. U.S.A. 88, 5829–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M E (1987). “Circulatory and metabolic correlates of brain function in normal humans.” In: The nervous system V, handbook of physiology, Plum, F, ed., Am. Physiol. Soc., Bethesda, MD, pp. 643–674. [Google Scholar]
- Raichle, M E (1998). “Behind the scenes of functional brain imaging: a historical and physiological perspective.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.95.3.765 95, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M E, Grubb, R L, Gado, M H, Eichling, J O, and Ter-Pogossian, M M (1976). “Correlation between regional cerebral blood flow and oxidative metabolism.” Arch. Neurol. 33, 523–526. [DOI] [PubMed] [Google Scholar]
- Raichle, M E, Martin, W R, Herscovitch, P, Mintun, M A, and Markham, J (1983). “Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation.” J. Nucl. Med. 24, 790–798. [PubMed] [Google Scholar]
- Rector, D M, Rogers, R F, Schwaber, J S, Harper, R M, and George, J S (2001). “Scattered-light imaging in vivo tracks fast and slow processes of neurophysiological activation.” Neuroimage 14, 977–994. [DOI] [PubMed] [Google Scholar]
- Reinert, K C, Dunbar, R L, Gao, W, Chen, G, and Ebner, T J (2004). “Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo.” Neurophysiology 92, 199–211. [DOI] [PubMed] [Google Scholar]
- Reivich, M, Kuhl, D, Wolf, A, Greenberg, J, Phelps, M, Ido, T, Casella, V, Fowler, J, Hoffman, E, Alavi, A, Som, P, and Sokoloff, L (1979). “The [18F] fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man.” Circ. Res. 44, 127–137. [DOI] [PubMed] [Google Scholar]
- Research News (1997). “What makes brain neurons run?” Science 10.1126/science.276.5310.196 276, 196–198. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Baeza, A, Reina-De La Torre, F, Ortega-Sanchez, M, and Sahuquillo-Barris, J (1998). “Perivascular structures in corrosion casts of the human central nervous system: a confocal laser and scanning electron microscope study.” Anat. Rec. 252, 176–184. [DOI] [PubMed] [Google Scholar]
- Roe, A W (2007). “Long-term optical imaging of intrinsic signals in anesthetized and awake monkeys.” Appl. Opt. 10.1364/AO.46.001872 46, 1872–1880. [DOI] [PubMed] [Google Scholar]
- Roland, P E, Eriksson, L, Atone-Elander, S, and Widen, L (1987). “Does mental activity change the oxidative metabolism of the brain?” J. Neurosci. 7, 2373–2389. [PMC free article] [PubMed] [Google Scholar]
- Roy, C, and Sherrington, C (1890). “On the regulation of the blood supply of the brain.” J. Physiol. (London) 11, 85–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek-Haddadi, A, Merschhemke, M, Lemieux, L, and Fish, D R (2002). “Simultaneous EEG Correlated Ictal fMRI.” Neuroimage 16, 32–40. [DOI] [PubMed] [Google Scholar]
- Sappey-Marinier, D, Calabrese, G, Fein, G, Hugg, J W, Biggins, C, and Weimer, M W (1992). “Effect of photic stimulation on human visual cortex lactate and phosphates usinh 1H and 31P magnetic resonance spectroscopy.” J. Cereb. Blood Flow Metab. 12, 584–592. [DOI] [PubMed] [Google Scholar]
- Schurr, A (2006). “Lactate: the ultimate cerebral oxidative energy substrate?” J. Cereb. Blood Flow Metab. 10.1038/sj.jcbfm.9600174 26, 142–152. [DOI] [PubMed] [Google Scholar]
- Schwartz, T H (2007). “Neurovascular coupling and epilepsy: haemodynamic markers for localizing and predicting seizure onset.” Epilepsy Curr. 7, 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz, R J, and Roland, P E (1992). “Vibratory stimulation increases and decreases the regional cerebral blood flow and oxidative metabolism: a positron emission tomography (PET) study.” Acta Neurol. Scand. 86, 60–67. [DOI] [PubMed] [Google Scholar]
- Sengpiel, F, Godecke, I, Stawinski, P, Hubener, M, Lowel, S, and Bonhoeffer, T (1998). “Intrinsic and environmental factors in the development of functional maps in cat visual cortex.” Neuropharmacology 10.1016/S0028-3908(98)00034-3 37, 607–621. [DOI] [PubMed] [Google Scholar]
- Sheth, S A, Nemoto, M, Guiou, M, Walker, M, Pouratian, N, Hageman, N, and Toga, A W (2004a). “Columnar specificity of microvascular oxygenation and volume responses: implications for functional brain mapping.” J. Neurosci. 10.1523/JNEUROSCI.4526-03.2004 24, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth, S A, Nemoto, M, Guiou, M, Walker, M, Pouratian, N, and Toga, A W (2004b). “Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and haemodynamic responses.” Neuron 10.1016/S0896-6273(04)00221-1 42, 347–355. [DOI] [PubMed] [Google Scholar]
- Sheth, S A, Nemoto, M, Guiou, M W, Walker, M A, and Toga, A W (2005). “Spatiotemporal evolution of functional haemodynamic changes and their relationship to neuronal activity.” J. Cereb. Blood Flow Metab. 10.1038/sj.jcbfm.9600091 25, 830–841. [DOI] [PubMed] [Google Scholar]
- Shibuki, K, Hishida, R, Murakami, H, Kudoh, M, Kawaguchi, T, Watanabe, M, Watanabe, S, Kouuchi, T, and Tanaka, R (2003). “Dynamic imaging of somatosensorycortical activity in the rat visualized by flavoprotein autofluorescence.” J. Physiol. (London) 10.1113/jphysiol.2003.040709 549, 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel, A, Yacoub, E, Chaimow, D, Logothetis, N K, and Ugurbil, K (2007). “Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla.” Neuroimage 35, 539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham, D, and Grinvald, A (2001). “The cortical representation of the hand in macaque and human area S-I: high resolution optical imaging.” J. Neurosci. 21, 6820–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtoyerman, E, Arieli, A, Slovin, H, Vanzetta, I, and Grinvald, A (2000). “Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys.” J. Neurosci. 20, 8111–8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman, R G, Hyder, F, and Rothman, D L (2001). “Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging.” Trends Neurosci. 98, 6417–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman, R G, Rothman, D L, Behar, K L, and Hyder, F (2004). “Energetic basis of brain activity: implications for neuroimaging.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.27.10.489 27, 489–495. [DOI] [PubMed] [Google Scholar]
- Sibson, N R, Dhankhar, A, Mason, G F, Rothman, D L, Behar, K L, and Shulman, R G (1998). “Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.95.1.316 95, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, A C, Koretsky, A P, and Duyn, J H (2007). “Functional MRI impulse response for BOLD and CBV contrast in rat somatosensory cortex.” Magn. Reson. Med. 10.1002/mrm.21246 57, 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, A C, Lee, S P, Iadecola, C, and Kim, S G (2000). “Early temporal characteristics of cerebral blood flow and deoxyhaemoglobin changes during somatosensory stimulation.” J. Cereb. Blood Flow Metab. 10.1097/00004647-200001000-00025 20, 201–206. [DOI] [PubMed] [Google Scholar]
- Sokoloff, L (1969). “Cerebral circulation and behavior in man: strategy and findings.” In: Psychochemical research in man, Mandell, A J, and Mandell, M P, eds., Academic, New York: 237–252. [Google Scholar]
- Sokoloff, L (1981). “Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose.” J. Cereb. Blood Flow Metab. 1, 7–36. [DOI] [PubMed] [Google Scholar]
- Sokoloff, L, Reivich, M, Kennedy, C, Des Roisers, M H, Patlak, C S, Pettigrew, K D, Sakurada, O, and Shinohara, M (1977). “The 14C deoxyglucose method for the measurement of local cerebral glucose utilization, theory, procedure and normal values in the conscious and anesthetized albino rat.” J. Neurochem. 10.1111/j.1471-4159.1977.tb10649.x 28, 897–916. [DOI] [PubMed] [Google Scholar]
- Steinbrink, J, Kohl, M, Obrig, H, Curio, G, Syre, F, Thomas, F, Wabnitz, H, Rinneberg, H, and Villringer, A (2000). “Somatosensory evoked fast optical intensity changes detected non-invasively in the adult human head.” Neurosci. Lett. 10.1016/S0304-3940(00)01395-1 291, 105–108. [DOI] [PubMed] [Google Scholar]
- Suh, M, Ma, H, Zhao, M, Sharif, S, and Schwartz, T H (2006). “Neurovascular coupling and oximetry during epileptic events.” Mol. Neurobiol. 33, 181–197. [DOI] [PubMed] [Google Scholar]
- Svoboda, K, Denk, W, Kleinfeld, D, and Tank, D W (1997). “In vivo dendritic calcium dynamics in neocortical pyramidal neurons.” Nature (London) 10.1038/385161a0 385, 161–165. [DOI] [PubMed] [Google Scholar]
- Syre, F, Obrig, H, Steinbrink, J, Kohl, M, Wenzel, R, and Villringer, A (2003). “Are VEP correlated fast optical signals detectable in the human adult by non-invasive nearinfrared spectroscopy (NIRS)?” Adv. Exp. Med. Biol. 530, 421–431. [DOI] [PubMed] [Google Scholar]
- Takano, T, Tian, G F, Peng, W, Lou, N, Libionka, W, Han, X, and Nedergaard, M (2006). “Astrocyte-mediated control of cerebral blood flow.” Nat. Neurosci. 10.1038/nn1623 9, 260–267. [DOI] [PubMed] [Google Scholar]
- Tank, D W, Ogawa, S, and Ugurbil, K (1992). “Mapping the brain with MRI.” Ecologist 2, 525–528. [DOI] [PubMed] [Google Scholar]
- Tanner, K, Beitel, E, D’Amico, E, Mantulin, W W, and Gratton, E (2006). “Effects of vasodilation on intrinsic optical signals in the mammalian brain: a phantom study.” J. Biomed. Opt. 10.1117/1.2398920 11, 064020. [DOI] [PubMed] [Google Scholar]
- Tanner, K, D’Amico, E, Kaczmarowski, A, Kukreti, S, Malpeli, J, Mantulin, W W, and Gratton, E (2005). “Spectrally resolved neurophotonics: a case report of haemodynamics and vascular components in the mammalian brain.” J. Biomed. Opt. 10.1117/1.2137291 10, 064009. [DOI] [PubMed] [Google Scholar]
- Ter-Pogossian, M M, Phelps, M E, Hoffman, E J, and Mullani, N A (1975). “A positron-emission transaxial tomograph for nuclear imaging (PETT).” Radiology 114, 89–98. [DOI] [PubMed] [Google Scholar]
- Thompson, J K, Peterson, M R, and Freeman, R D (2003). “Single-neuron activity and tissue oxygenation in the cerebral cortex.” Science 10.1126/science.1079220 299, 1070–1072. [DOI] [PubMed] [Google Scholar]
- Thompson, J K, Peterson, M R, and Freeman, R D (2004). “High-resolution neurometabolic coupling revealed by focal activation of visual neurons.” Nat. Neurosci. 10.1038/nn1308 7, 919–920. [DOI] [PubMed] [Google Scholar]
- Thompson, J K, Peterson, M R, and Freeman, R D (2005). “Separate spatial scales determine neural activity-dependent changes in tissue oxygen within central visual pathways.” J. Neurosci. 10.1523/JNEUROSCI.2127-05.2005 25, 9046–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmi, M, Kitaura, H, Komagata, S, Kudoh, M, and Shibuki, K (2006). “Enduring critical period plasticity visualized by transcranial flavoprotein imaging in mouse primary visual cortex.” J. Neurosci. 10.1523/JNEUROSCI.1643-06.2006 26, 11775–11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell, R B, Hamilton, S L, Silverman, M S, and Switkes, E (1988). “Functional anatomy of macaque striate cortex. I. Ocular dominance, binocular interactions, and baseline conditions.” J. Neurosci. 8, 1500–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell, R B, Silverman, M S, and De Valois, R L (1981). “Spatial frequency columns in primary visual cortex.” Science 10.1126/science.7292014 214, 813–815. [DOI] [PubMed] [Google Scholar]
- Tootell, R B, Silverman, M S, Switkes, E, and De Valois, R L (1982). “Deoxyglucose analysis of retinotopic organization in primate striate cortex.” Science 10.1126/science.7134981 218, 902–904. [DOI] [PubMed] [Google Scholar]
- Tootell, R B, Silverman, M S, De Valois, R L, and Jacobs, G H (1983). “Functional organization of the second cortical visual area in primates.” Science 10.1126/science.6301017 220, 737–739. [DOI] [PubMed] [Google Scholar]
- Ts’o, D Y, Frostig, R D, Lieke, E E, and Grinvald, A (1990). “Functional organization of primate visual cortex revealed by high resolution optical imaging.” Science 10.1126/science.2165630 249, 417–420. [DOI] [PubMed] [Google Scholar]
- Ugurbil, K, et al. (2003a). “Ultrahigh field magnetic resonance imaging and spectroscopy.” Magn. Reson. Imaging 10.1016/j.mri.2003.08.027 21, 1263–1281. [DOI] [PubMed] [Google Scholar]
- Ugurbil, K, Toth, L, and Kim, D S (2003b). “How accurate is magnetic resonance imaging of brain function?” Trends Neurosci. 10.1016/S0166-2236(02)00039-5 26, 108–114. [DOI] [PubMed] [Google Scholar]
- Vafaee, M S, and Gjedde, A (2000). “Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex.” J. Cereb. Blood Flow Metab. 10.1097/00004647-200004000-00012 20, 747–754. [DOI] [PubMed] [Google Scholar]
- Valabregue, R, Aubert, A, Burger, J, Bittoun, J, and Costalat, R (2003). “Relation between cerebral blood flow and metabolism explained by a model of oxygen exchange.” J. Cereb. Blood Flow Metab. 10.1097/01.WCB.0000055178.31872.38 23, 536–545. [DOI] [PubMed] [Google Scholar]
- Valabregue, R, Costalat, R, Burger, J, and Bittoun, J (2000). “Relation between cerebral blood flow and metabolism revisited by a model of oxygen exchange.” In: 8th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Denver, CO. [DOI] [PubMed]
- Vanzetta, I, Deneux, T, Masson, G S, and Faugeras, O (2006). “Cerebral blood flow recorded at high sensitivity in two dimensions using high resolution optical imaging.” In: Biomedical imaging: nano to macro, 3rd IEEE International Symposium, IEEE, New York, 1264–1267.
- Vanzetta, I, and Grinvald, A (1999). “Increased cortical oxidative metabolism due to sensory stimulation: implications for functional brain imaging.” Science 10.1126/science.286.5444.1555 286, 1555–1558. [DOI] [PubMed] [Google Scholar]
- Vanzetta, I, and Grinvald, A (2001). “Evidence and lack of evidence for the initial dip in the anesthetized rat: implications for human functional brain imaging.” Neuroimage 13, 959–967. [DOI] [PubMed] [Google Scholar]
- Vanzetta, I, Hildesheim, R, and Grinvald, A (2005). “Compartment-resolved imaging of activity-dependent dynamics of cortical blood volume and oximetry.” J. Neurosci. 10.1523/JNEUROSCI.3032-04.2005 25, 2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzetta, I, Slovin, H, Omer, D B, and Grinvald, A (2004). “Columnar resolution of blood volume and oximetry functional maps in the behaving monkey: implications for fMRI.” Neuron 10.1016/j.neuron.2004.04.004 42, 843–854. [DOI] [PubMed] [Google Scholar]
- Weber, B, Burger, C, Wyss, M T, von Schulthess, G K, Scheffold, F, and Buck, A (2004). “Optical imaging of the spatiotemporal dynamics of cerebral blood flow and oxidative metabolism in the rat barrel cortex.” Eur. J. Neurosci. 10.1111/j.1460-9568.2004.03735.x 20, 2664–2670. [DOI] [PubMed] [Google Scholar]
- Villringer, A, and Dirnagl, U (1995). “Coupling of brain activity and cerebral blood-flow—basis of functional neuroimaging.” Cerebrovasc Brain Metab. Rev. 7, 240–276. [PubMed] [Google Scholar]
- Wilson, D F, Vanderkooi, J M, Green, T J, Maniara, G, DeFeo, S P, and Bloomgarden, D C (1987). “A versatile and sensitive method for measuring oxygen.” Adv. Exp. Med. Biol. 215, 71–77. [DOI] [PubMed] [Google Scholar]
- Wobst, P, Wenzel, R, Kohl, M, Obrig, H, and Villringer, A (2001). “Linear aspects of changes in deoxygenated haemoglobin concentration and cytochrome oxidase oxidation during brain activation.” Neuroimage 13, 520–530. [DOI] [PubMed] [Google Scholar]
- Yacoub, E, Duong, T Q, Van De Moortele, P F, Lindquist, M, Adriany, G, Kim, S G, Ugurbil, K, and Hu, X (2003). “Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields.” Magn. Reson. Med. 10.1002/mrm.10433 49, 655–664. [DOI] [PubMed] [Google Scholar]
- Yacoub, E, and Hu, X (1999). “Detection of the early negative response in fMRI at 1.5 Tesla.” Magn. Reson. Med. 41, 1088–1092. [DOI] [PubMed] [Google Scholar]
- Yacoub, E, Shmuel, A, Pfeuffer, J, Van De Moortele, P F, Adriany, G, Ugurbil, K, and Hu, X (2001). “Investigation of the initial dip in fMRI at 7 Tesla.” NMR Biomed. 10.1002/nbm.715 14, 408–412. [DOI] [PubMed] [Google Scholar]
- Yacoub, E, Van De Moortele, P F, Shmuel, A, and Ugurbil, K (2005). “Signal and noise characteristics of Hahn SE and GE BOLD fMRI at 7 T in humans.” Neuroimage 24, 738–750. [DOI] [PubMed] [Google Scholar]
- Zhao, F, Wang, P, Hendrich, K, and Kim, S-G (2005). “Spatial specificity of cerebral blood volume-weighted fMRI responses at columnar resolution.”Neuroimage 27, 416–424. [DOI] [PubMed] [Google Scholar]
- Zhao, F, Wang, P, Hendrich, K, Ugurbil, K, and Kim, S G (2006). “Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into haemodynamic regulation.” Neuroimage 30, 1149–1160. [DOI] [PubMed] [Google Scholar]
- Zhao, F Q, Jin, T, Wang, P, and Kim, S-G (2007). “Improved spatial localization of post-stimulus BOLD undershoot relative to positive BOLD.” Neuroimage 34, 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, M, Suh, M, Ma, H, Perry, C, Geneslaw, A, and Schwartz, T H (2007). “Focal increases in perfusion and decreases in haemoglobin oxygenation precede seizure onset in spontaneous human epilepsy.” Epilepsia 10.1111/j.1528-1167.2007.01229.x 48, 2059–2067. [DOI] [PubMed] [Google Scholar]
- Zheng, Y, Johnston, D, Berwick, J, Chen, D, Billings, S, and Mayhew, J (2005). “A three-compartment model of the haemodynamic response and oxygen delivery to brain.” Neuroimage 28, 925–939. [DOI] [PubMed] [Google Scholar]
- Zheng, Y, Martindale, J, Johnston, D, Jones, M, Berwick, J, and Mayhew, J (2002). “A model of the haemodynamic response and oxygen delivery to brain.” Neuroimage 16, 617–637. [DOI] [PubMed] [Google Scholar]
- Zonta, M, Angulo, M C, Gobbo, S, Rosengarten, B, Hossmann, K A, Pozzan, T, and Carmignoto, G (2003). “Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation.” Nat. Neurosci. 10.1038/nn980 6, 43–50. [DOI] [PubMed] [Google Scholar]