Abstract
PDZK1 is a four PDZ domain-containing scaffold protein that binds to scavenger receptor class B, type I (SR-BI), the high density lipoprotein receptor, by its first PDZ domain (PDZ1). PDZK1 knock-out mice exhibit a >95% decrease in hepatic SR-BI protein and consequently an ∼70% increase in plasma cholesterol in abnormally large high density lipoprotein particles. These defects are corrected by hepatic overexpression of full-length PDZK1 but not the PDZ1 domain alone, which partially restores SR-BI protein abundance but not cell surface expression or function. We have generated PDZK1 knock-out mice with hepatic expression of four PDZK1 transgenes encoding proteins with nested C-terminal truncations: pTEM, which lacks the three C-terminal residues (putative PDZ-binding motif), and PDZ1.2, PDZ1.2.3, or PDZ1.2.3.4, which contain only the first two, three, or four N-terminal PDZ domains, respectively, but not the remaining C-terminal sequences. Hepatic overexpression of pTEM restored normal hepatic SR-BI abundance, localization, and function. Hepatic overexpression of PDZ1.2 or PDZ1.2.3 partially restored SR-BI abundance (∼12 or ∼30% of wild type, respectively) but did not (PDZ1.2) or only slightly (PDZ1.2.3) restored hepatic SR-BI cell surface localization and function. Hepatic overexpression of PDZ1.2.3.4 completely restored SR-BI protein abundance, cell surface expression, and function (normalization of plasma cholesterol levels). Thus, all four PDZ domains in PDZK1, but not PDZ1-3 alone, are sufficient for its normal control of the abundance, localization, and therefore function of hepatic SR-BI, whereas the residues C-terminal to the PDZ4 domain, including the C-terminal putative PDZ-binding domain, are not required.
SR-BI3 plays a significant role in lipoprotein metabolism as the high density lipoprotein (HDL) receptor (1). Through a mechanism called selective uptake (2-4), SR-BI mediates the uptake of cholesteryl esters from HDL and other lipoproteins into cells (5-10) as well as the bidirectional movement of unesterified cholesterol between cells and lipoproteins (11, 12).
SR-BI is most highly expressed in the liver and steroidogenic tissues (5), which exhibit the highest levels of HDL selective cholesterol uptake (2, 4). Deficiency of SR-BI in SR-BI knock-out (KO) mice leads to elevated (∼2.2-fold) plasma cholesterol levels transported in abnormally large HDL particles with an unesterified cholesterol:total cholesterol ratio roughly double that of wild-type (WT) mice (13-16). This dyslipidemia is a consequence of the reduced uptake of cholesterol from plasma HDL by the liver and can be reversed by hepatic SR-BI transgene expression (17).
Hepatic SR-BI activity is controlled by the intracellular adaptor protein PDZK1 (18-20). PDZK1 is a four PDZ domain-containing scaffold protein (see Fig. 1A, top left) that binds to the C termini of numerous membrane-associated transporter proteins, including ion channels (e.g. cystic fibrosis transmembrane conductance regulator) and cell surface receptors (19, 21). Its most N-terminal PDZ domain, PDZ1, binds to the C terminus of SR-BI (18) but ostensibly not to SR-BII, a minor, alternatively spliced form of SR-BI whose 39-residue C-terminal sequence differs from that of SR-BI (20, 22-27). Deletion of the most C-terminal amino acid of SR-BI abolishes its interaction with the PDZ1 domain of PDZK1 (28). PDZK1 is most highly expressed in the kidney and, like SR-BI, is expressed in the liver and intestines but exhibits very low expression levels in the steroidogenic tissues in which SR-BI is abundantly expressed (5, 18, 19).
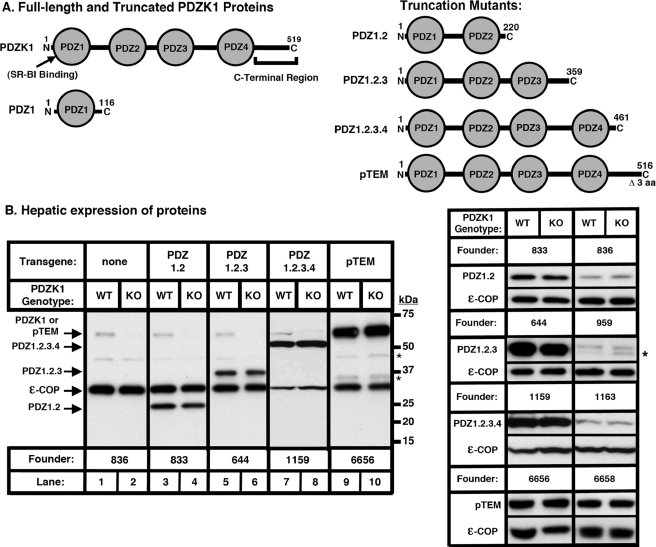
FIGURE 1.
Hepatic expression of PDZ1.2, PDZ1.2.3, PDZ1.2.3.4 and pTEM transgenes. A, left, schematic diagrams of the full-length PDZK1 protein (SR-BI binding site and the region designated “C-Terminal Region” are indicated) and PDZ1 (30). A, right, schematic diagrams of the proteins encoded by the PDZ1.2 (top), PDZ1.2.3 (second), PDZ1.2.3.4 (third), and pTEM (bottom) transgenes. B, left panels, immunoblot analysis of hepatic expression of PDZ1.2, PDZ1.2.3, PDZ1.2.3.4, and pTEM proteins in PDZ1.2, PDZ1.2.3, PDZ1.2.3.4, and pTEM transgenic mice. Hepatic protein levels are shown in nontransgenic WT and PDZK1 KO mice (lanes 1 and 2), WT [PDZ1.2-Tg] and PDKZI KO [PDZ1.2-Tg] mice (lanes 3 and 4), WT[PDZ1.2.3-Tg] and PDZK1 KO [PDZ1.2.3-Tg] mice (lanes 5 and 6), WT [PDZ1.2.3.4-Tg] and PDZK1 KO [PDZ1.2.3.4-Tg] mice (lanes 7 and 8), and WT [pTEM-Tg] and PDZK1 KO [pTEM-Tg] mice (lanes 9 and 10). The data for PDZ1.2.3.4-Tg were from an independent blot. B, right panels, immunoblot analysis of steady-state levels of hepatic expression of PDZ1.2, PDZ1.2.3, PDZ1.2.3.4, and pTEM proteins in corresponding transgenic WT and PDZK1 KO mice from two different founders for each transgene.ε-COP (∼34 kDa) was used as a loading control. * indicates background bands.
In vivo, PDZK1 regulates SR-BI expression in a tissue-specific, post-transcriptional manner (20). PDZK1 KO mice show a dramatic, >95% reduction in hepatic SR-BI protein levels and a partial decrease in SR-BI abundance in the small intestine but no change in steroidogenic tissues (20). Because of this dramatic decrease in hepatic SR-BI protein levels, PDZK1 KO mice exhibit abnormally high levels of plasma cholesterol (∼1.7-fold), which is transported primarily in abnormally large HDL particles (20). This effect on plasma lipoprotein metabolism in PDZK1 KO mice is similar to, although not as severe as, those changes found in SR-BI KO mice (14). Hepatic overexpression of a murine SR-BI transgene in SR-BI/PDZK1 double knock-out mice results in cell surface expression and normal function of SR-BI (29). Hepatic SR-BI is, therefore, able to function normally without PDZK1 provided it is localized on the cell surface at steady-state levels similar to those in wild-type hepatocytes (29). The mechanism by which PDZK1 regulates hepatic SR-BI abundance and/or localization in nontransgenic animals remains unclear.
Previously we showed that hepatic overexpression of full-length PDZK1 in PDZK1-deficient mice restored normal hepatic SR-BI protein levels and function but had little, if any, effect on hepatic SR-BI in WT mice (30). However, hepatic overexpression of a truncated mutant transgene of PDZK1, PDZ1-Tg, encoding primarily the PDZ1 domain but not the other PDZ domains (see Fig. 1A, left) of PDZK1, was not sufficient to restore normal SR-BI function in PDZK1 KO mice (30). In a dominant negative fashion, hepatic overexpression of PDZ1-Tg in WT mice (∼24-fold greater than PDZK1 levels in WT mice) resulted in plasma lipoproteins that resembled those in PDZK1 KO mice (hypercholesterolemia and large HDL) because of a 75% reduction in hepatic SR-BI protein levels and intracellular mislocalization away from the cell surface of the remaining SR-BI protein (30). These results suggested that the PDZ1 domain can control both the abundance and intracellular localization of hepatic SR-BI and that normal regulation of SR-BI by PDZK1 is likely to require that other portions of PDZK1 interact with other, as yet unidentified, cellular components.
The three PDZ domains C-terminal to PDZ1 (PDZ2, PDZ3, and PDZ4), and the 66-residue C-terminal tail of PDZK1 (see Fig. 1A, top left) are apparently not involved in directly binding to SR-BI (18, 19). The role(s) of these features of PDZK1 in the regulation of hepatic SR-BI is unknown. Other hepatic proteins (such as OATP1A1 (31)) have been shown to interact with the PDZ domains of PDZK1. Nakamura et al. (32) have shown that the C-terminal region of rat PDZK1 and its phosphorylation are required for optimal PDZK1 transgene-mediated induction of increased SR-BI protein levels in cultured cells. Additionally PDZK1 has a putative PDZ-interacting motif at its C terminus (the final three residues: Thr-Glu-Met, or TEM) (18, 19, 21). PDZK1 is known to form heterodimers with other PDZ proteins (NHERF1 and NHERF2) through interactions between the PDZ domains of PDZK1 and the C termini of the target proteins (33). It is conceivable that PDZK1 might form hetero- or homodimers or higher order oligomers via its TEM PDZ-binding motif.
Here we have analyzed the impact of the PDZ2, PDZ3, PDZ4, and C-terminal domains of PDZK1, including the putative C-terminal PDZ-binding motif, on the abundance, localization, and function of hepatic SR-BI in vivo. We compared the effects of hepatic expression in WT or PDZK1 KO mice of four transgenes (see Fig. 1A, right): pTEM-Tg, expressing a PDZK1 protein product where the three C-terminal residues (TEM) are deleted, and PDZ1.2-Tg, PDZ1.2.3-Tg, and PDZ1.2.3.4-Tg, encoding respectively only the N-terminal two, three, or four PDZ domains but not the remaining C-terminal portions of PDZK1. We found that hepatic overexpression of both PDZ1.2.3.4 (2.8-10.6-fold) and pTEM-Tg (24.6-44.3-fold) in PDZK1 KO mice restored normal hepatic SR-BI protein levels and function and had little effect on SR-BI function in WT mice. Hepatic overexpression of PDZ1.2-Tg (1.3-5.5-fold) did not affect SR-BI abundance or function in WT mice. In PDZK1 KO mice, the phenotypes of PDZ1.2-Tg overexpression were similar to those of PDZ1-Tg (30). SR-BI protein levels were partially restored (∼12% of WT), although the SR-BI protein was mislocalized intracellularly, resulting in no change in plasma cholesterol levels. Hepatic expression or overexpression (0.2-7.9-fold) of PDZ1.2.3-Tg in WT mice had no effect on SR-BI. However, in PDZK1 KO mice, it resulted in a small (∼15%) decrease in plasma cholesterol levels. This was accompanied by an increase in hepatic SR-BI protein levels (to 30% of WT) and a partial restoration of steady-state SR-BI cell surface localization and function. Thus, all four PDZ domains in PDZK1, but not PDZ1-3 alone, are sufficient for its normal control of the abundance, localization, and therefore function of hepatic SR-BI. The C-terminal 58 amino acids of PDZK1, including the putative phosphorylation site located on a serine within the last 15 amino acids of the protein (32) and the putative PDZ binding site TEM, are not required for normal SR-BI localization and function.
EXPERIMENTAL PROCEDURES
Generation of PDZ1.2, PDZ1.2.3, PDZ1.2.3.4, and pTEM Transgenic Mice—PDZ1.2, PDZ1.2.3, PDZ1.2.3.4, and pTEM transgenic mice were generated using the pLIV-LE6 plasmid, kindly provided by Dr. John M. Taylor (Gladstone Institute of Cardiovascular Disease, University of California, San Francisco, CA). The pLIV-LE6 plasmid contains the promoter, first exon, first intron, and part of the second exon of the human apoE gene, the polyadenylation sequence, and a part of the hepatic control region of the apoE/C-I gene locus (34).
To generate PDZ1.2-Tg, we subcloned a 700-bp DNA fragment encoding the N-terminal 220 amino acids of murine PDZK1 between the KpnI and XhoI sites of the pLIV-LE6 plasmid (PDZ1.2-Tg). The same approach was used to generate PDZ1.2.3-Tg (1.1-kb insert, N-terminal 359 amino acids), PDZ1.2.3.4-Tg (1.4-kb insert, N-terminal 461 residues), and pTEM-Tg (1.6-kb insert, N-terminal 516 amino acids resulting in the deletion of the C-terminal 3 residues) (see Fig. 1A, right). The sequences of the recombinant plasmids were confirmed by DNA sequencing. These constructs were linearized by SacII/SpeI digestion, and the resulting 5.7-kb (PDZ1.2-Tg), 6.1-kb (PDZ1.2.3-Tg), 6.4-kb (PDZ1.2.3.4-Tg), and 6.6-kb (pTEM-Tg) constructs were used to generate transgenic mice using standard procedures (35).
Founder animals for these transgenes in an FVB/N genetic background were identified by PCR performed on tail DNA using the following oligonucleotide primers: for the PDZ1.2-Tg and PDZ1.2.3-Tg, one primer corresponding to the PDZK1 cDNA sequence (CAATGGTGTCTTTGTCGACAAG) and one corresponding to the 3′-end of the human apoE gene sequence included in the cloning vector (AGCAGATGCGTGAAACTTGGTGA); for the PDZ1.2.3.4-Tg and pTEM-Tg, one primer corresponding to the PDZK1 cDNA sequence (GAGGCAGCTGGCTTGAAGAAC) and the same 3′-primer used for PDZ1.2-Tg. Founders expressing PDZ1.2-Tg, PDZ1.2.3-Tg, PDZ1.2.3.4-Tg, or pTEM-Tg were crossed with PDZK1 KO mice (129SvEv background). Heterozygous pups expressing the transgenes were crossed with WT and PDZK1 KO mice to obtain transgenic and control non-transgenic WT and PDZK1 KO mice, thus ensuring that the mixed genetic backgrounds of experimental and control mice in each founder line were matched. PDZK1 KO mice were genotyped as described previously (36).
Animals—All mice were on a 25:75 FVB/N:129SvEv genetic background and were maintained on a normal chow diet (36), and ∼6-15-week-old male mice were used for experiments. Three independent founder lines for each of the transgenes pTEM-Tg, PDZ1.2-Tg, and PDZ1.2.3-Tg and two independent founder lines for PDZ1.2.3.4-Tg were generated. Plasma cholesterol results presented here are pooled from two founders each expressing either pTEM-Tg, PDZ1.2-Tg, PDZ1.2.3-Tg, or PDZ1.2.3.4-Tg. All procedures on transgenic and non-transgenic mice were performed in accordance with the guidelines of the Beth Israel-Deaconess Medical Center and the Massachusetts Institute of Technology Committee on Animal Care.
Blood and Tissue Sampling, Processing, and Analysis—Plasma and liver samples were collected and processed, and total plasma cholesterol levels and individual size-fractionated fast performance liquid chromatography (FPLC) cholesterol profiles were obtained as described previously (30). Total plasma cholesterol data from founders encoding the same mutation were pooled (see “Results”).
For immunoblotting, protein samples (∼30 μg) from total liver lysates were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride or nitrocellulose membranes, incubated with either a rabbit polyclonal anti-SR-BI anti-peptide antibody (mSR-BI495-112) (1:1000) or a rabbit polyclonal anti-PDZK1 anti-peptide antibody (1:20,000) (30) followed by an anti-rabbit IgG conjugated to horseradish peroxidase (Invitrogen; 1:10,000), and visualized by ECL chemiluminescence (GE Healthcare, catalog number RPN2132). Immunoblotting using a polyclonal anti-ε-COP (1:5000) (37) antibody was used for loading controls. The relative amounts of proteins were determined by quantitation using an Eastman Kodak Co. Image Station 440 CF and Kodak 1D software. Multiple independent determinations of band intensities from serially diluted samples were additionally made to ensure that the intensities were both in the linear range of the detector and reproducible.
Immunoperoxidase Analysis—Livers were harvested, fixed, and frozen, and 5-μm sections were stained with the anti-mSR-BI495-112 antibody and biotinylated anti-rabbit IgG, visualized by immunoperoxidase staining, and counterstained with Harris modified hematoxylin as described previously (20).
Statistical Analysis—Data are shown as the means ± S.D. Statistically significant differences were determined by either pairwise comparisons of values using the unpaired t test with (if variances differed significantly) or without Welch's correction or by one-way analysis of variance followed by the Tukey-Kramer multiple comparison post-test when comparing three or more sets of data. Mean values for experimental groups are considered statistically significantly different for p < 0.05 for both types of tests.
RESULTS
Ikemoto et al. (18) discovered that the cytoplasmic adaptor protein PDZK1 binds to SR-BI via its PDZ1 domain. We recently established that, unlike the case with full-length PDZK1, hepatic overexpression of a truncated form of PDZK1, PDZ1 (containing only the short N terminus and the PDZ1 domain (Fig. 1A, left)), is insufficient to restore normal levels of hepatic SR-BI protein abundance, localization (to the sinusoidal surfaces), and function in PDZK1 KO mice (30). To begin to explore the roles of the other PDZ domains and the C-terminal domain, we generated four additional 3′-truncated PDZK1 cDNA expression constructs (PDZ1.2-Tg, PDZ1.2.3-Tg, PDZ1.2.3.4-Tg, and pTEM-Tg) that encode the C-terminally truncated proteins shown in Fig. 1A (right). These constructs were used for hepatic transgene overexpression in both wild-type (WT [PDZ1.2-Tg], WT [PDZ1.2.3-Tg], WT [PDZ1.2.3.4-Tg], and WT [pTEM-Tg]) and PDZK1 knock-out (PDZK1 KO [PDZ1.2-Tg], PDZK1 KO [PDZ1.2.3-Tg], PDZK1 KO [PDZ1.2.3.4-Tg], and PDZK1 KO [pTEM-Tg]) mice on a mixed FVB/129SvEv background.
Hepatic levels of PDZK1, PDZ1.2, PDZ1.2.3, PDZ1.2.3.4, and pTEM proteins in non-transgenic and transgenic mice from two founders each were determined by immunoblotting (representative results are shown in Fig. 1B). Estimates of transgenic protein levels relative to levels of endogenous PDZK1 in WT non-transgenic mice were made from multiple immunoblots. The steady-state levels of PDZ1.2 protein were 5.5 ± 1.6-fold (founder line 833) and 1.3 ± 0.3-fold (founder line 836) higher than those of endogenous PDZK1 in nontransgenic WT mice (Fig. 1B, lanes 3 and 4, and top right-hand panels). PDZ1.2.3 protein levels were 7.9 ± 3.4-fold higher (founder 644) than or 17 ± 3% (founder 959) of WT non-transgenic PDZK1 (Fig. 1B, lanes 5 and 6, and upper middle right-hand panels). PDZ1.2.3.4 protein levels were 10.6 ± 1.0-fold (founder 1159) and 2.8 ± 1.1-fold (founder 1163) higher than that of WT non-transgenic endogenous PDZK1 (Fig. 1B, lanes 7 and 8, and lower middle right-hand panels). pTEM protein was expressed 44.3 ± 10.5-fold (founder 6656) and 24.6 ± 6.2-fold (founder 6658) higher than endogenous PDZK1 levels in nontransgenic WT mice (Fig. 1B, lanes 9 and 10, and bottom right-hand panels). Thus, only in pTEM-Tg-expressing mice were there very high levels of protein overexpression in both founders.
Analysis by immunoblotting also showed that the levels of endogenous hepatic PDZK1 in WT [PDZ1.2-Tg] were slightly, but statistically significantly, lower than those in nontransgenic WT mice (69 ± 14% of WT levels of endogenous PDZK1, p = 0.0176) and that in WT [PDZ1.2.3-Tg] mice PDZK1 levels were similar to those in nontransgenic WT mice (98 ± 22% of WT levels of endogenous PDZK1, p = 0.7534). In addition, no apparent effect of PDZ1.2.3.4-Tg on endogenous PDZK1 was observed. Thus overexpression of PDZ1.2-Tg, but not other PDZK1 truncated mutants, was capable of affecting endogenous PDZK1 levels albeit slightly.
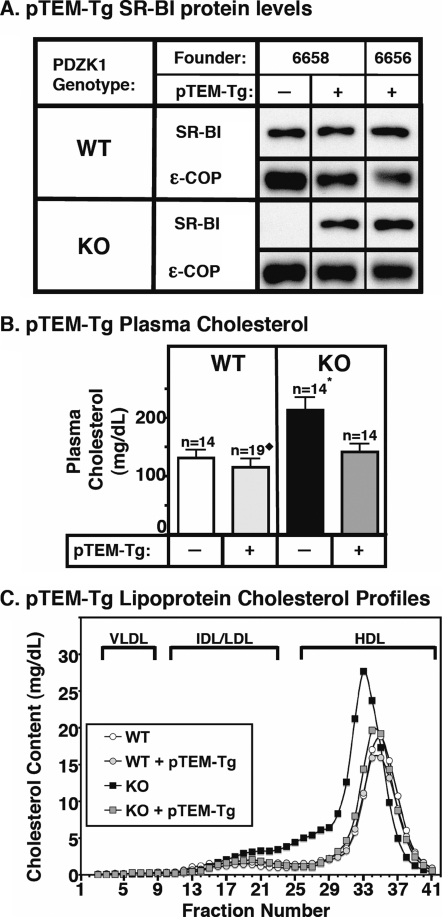
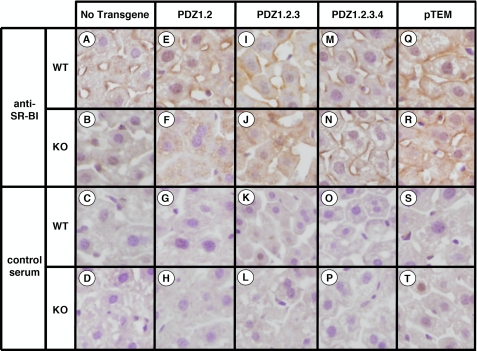
Effects of the pTEM-Tg on Hepatic SR-BI Expression and Lipoprotein Metabolism—In WT mice, hepatic overexpression of pTEM resulted in no statistically significant changes in steady-state levels of SR-BI in either founder (immunoblotting; Fig. 2A, top half of the panel; ε-COP used as loading control). As in non-transgenic WT mice, the majority of the steady-state SR-BI protein was localized on the cell surface in both founders. A representative immunohistochemistry image of a liver sample stained with anti-SR-BI polyclonal antiserum is shown in Fig. 3Q and should be compared with the immunostaining of liver samples from nontransgenic WT (Fig. 3A) and PDZK1 KO (Fig. 3B) mice. Controls for these and other samples stained with nonimmune serum are shown in Fig. 3, lower two rows, and indicate a very low nonspecific background immunostaining. In results pooled from two founder lines of WT [pTEM-Tg] mice, there was a small, but statistically significant, decrease in plasma cholesterol levels compared with non-transgenic controls when cholesterol values from both founders were combined (Fig. 2B, left panel). A similar, but not statistically significant, decrease was seen when the results for each of the founders were analyzed individually. The relevance of the small effect of pTEM in the pooled results is unclear because ∼21-fold hepatic overexpression of full-length PDZK1 in WT mice does not significantly alter plasma cholesterol levels (30). No changes in WT [pTEM-Tg] mice were observed in the size distribution of lipoproteins (FPLC lipoprotein cholesterol profiles) in which the bulk of the cholesterol was found in normal sized HDL particles (Fig. 2C, white and gray circles). These results suggest that the very high overexpression of the pTEM-Tg in WT mice may result in a slight increase in SR-BI function.
FIGURE 2.
Effects of expression of the pTEM transgene on hepatic SR-BI protein levels (A) and plasma lipoprotein cholesterol (B and C) in WT and PDZK1 KO mice. A, liver lysates (∼30 μg of protein) from mice with the indicated genotypes with (+) or without (-) the pTEM transgene were analyzed by immunoblotting, and SR-BI (∼82-kDa band) was visualized by chemiluminescence. ε-COP (∼34 kDa) was used as a loading control. Replicate experiments with multiple exposures and sample loadings were used to determine the relative levels of expression of SR-BI. WT and WT [pTEM-Tg] samples shown were all from a single gel but reordered for clarity of presentation. B, plasma was harvested from mice with the indicated genotypes and pTEM transgene. Total plasma cholesterol levels were determined in individual samples by enzymatic assay, and results from the indicated numbers of animals (n) were pooled by genotype. Independent WT and KO control animals for each founder line were generated to ensure that the mixed genetic backgrounds for experimental and control mice were matched. * indicates that the PDZK1 KO plasma cholesterol levels were statistically significantly different (p ≤ 0.001) from those plasma cholesterol levels of WT, WT [pTEM-Tg], and PDZK1 KO [pTEM-Tg] mice. ♦ indicates that the WT [pTEM-Tg] plasma cholesterol levels were statistically significantly different from those plasma cholesterol levels of WT (p ≤ 0.05), PDZK1 KO (p ≤ 0.001), and PDZK1 KO [pTEM-Tg] (p ≤ 0.001) mice. C, plasma samples (described in B) from individual animals were size-fractionated by FPLC, and the total cholesterol content of each fraction was determined by an enzymatic assay. The chromatograms are representative of multiple individually determined profiles. Approximate elution positions of native very low density lipoprotein (VLDL), intermediate density lipoprotein/low density lipoprotein (IDL/LDL), and HDL particles are indicated by brackets and were determined as described previously (14).
FIGURE 3.
Immunohistochemical analysis of hepatic SR-BI in WT and PDZK1 KO nontransgenic (A-D), PDZ1.2 transgenic (E-H), PDZ1.2.3 transgenic (I-L), PDZ1.2.3.4 transgenic (M-P), and pTEM transgenic (Q-T) mice. Livers from mice with the indicated genotypes and transgenes were fixed, frozen, and sectioned. The sections (5 μm) were then incubated with either polyclonal anti-SR-BI antiserum (top two rows) or control rabbit serum (bottom two rows) followed by a biotinylated anti-rabbit IgG secondary antibody and then stained using immunoperoxidase reagents and hematoxylin counterstaining. (Magnification, ×1200.) The samples from transgenic animals are from the following founder lines: 836 (E and G), 833 (F and H), 644 (I-L), 1163 (M-P), and 6656 (Q-T). Images are representative of the staining patterns observed in all founder lines for each transgene.
In PDZK1 KO mice, hepatic overexpression of pTEM corrected the previously reported abnormal phenotypes related to SR-BI and lipoprotein metabolism. In PDZK1 KO mice the pTEM-Tg restored the steady-state abundance of hepatic SR-BI protein from <5% of wild type (20) to typical wild-type levels (immunoblotting; Fig. 2A, bottom half of the panel); it was properly localized on the surfaces of the hepatocytes (immunohistochemistry; representative image in Fig. 3R). Consequently in PDZK1 KO mice, the pTEM-Tg restored plasma cholesterol levels from the ∼1.7-fold elevation seen in nontransgenic PDKZ1 KO mice to those seen in wild-type controls (Fig. 2B, right panel). The pTEM-Tg also restored the lipoprotein size distribution in PDZK1 KO [pTEM-Tg] from that with abnormally large HDL particles in the nontransgenic PDZK1 KO animals to that with normal sized HDL seen in the nontransgenic WT mice (Fig. 2C, black and gray squares). Note that the HDL peak in the PDZK1 KO sample is shifted to the left relative to that in WT plasma, indicating larger lipoprotein particles. Therefore, overexpression of pTEM leads to normal SR-BI protein levels in both WT and PDZK1 KO mice and fully complements the PDZK1 deficiency in PDZK1 KO mice with respect to hepatic SR-BI protein levels, cellular localization, and function in lipoprotein metabolism. This indicates that the C-terminal three amino acids (TEM), a putative PDZ-interacting motif, are not required for apparently normal PDZK1-mediated SR-BI protein expression, localization, and function.
Effects of PDZ1.2-Tg, PDZ1.2.3-Tg, and PDZ1.2.3.4-Tg on Lipoprotein Metabolism and Hepatic SR-BI Expression—In WT mice, we found that hepatic expression of PDZ1.2-Tg (1.3- and 5.5-fold higher than that of endogenous PDZK1 in WT), PDZ1.2.3 (0.2- and 7.9-fold), and PDZ1.2.3.4-Tg (2.8- and 10.6-fold) did not lead to statistically significant changes in plasma cholesterol levels (Figs. 4B, 5B, and 6B, left panels) or in the size distributions of lipoproteins (FPLC lipoprotein cholesterol profiles; Figs. 4C, 5C, and 6C, white and gray circles). This suggested that these transgenes might not alter SR-BI activity in WT mice. Indeed when we examined the effects of the PDZ1.2-Tg, PDZ1.2.3-Tg, and PDZ1.2.3.4-Tg on hepatic SR-BI protein abundance (immunoblotting analysis; Figs. 4A, 5A, and 6A, top half of the panels) and localization (immunohistochemistry; Fig. 3, E, I, and M) in WT mice, we observed no effects in the transgenic WT mice on SR-BI relative to nontransgenic WT mice. We cannot exclude the possibility that higher levels of hepatic expression of one or more of these transgenes might alter SR-BI abundance, localization, and/or function in WT mice. For example, 24-fold hepatic overexpression of PDZ1 in WT mice results in a dominant negative phenotype resembling that in nontransgenic PDZK1 KO mice (no cell surface expression or function of SR-BI (30)). Given the similarities of some of the phenotypes of PDZK1 KO [PDZ1.2-Tg] and PDZK1 KO [PDZ1.2.3-Tg] mice with PDZK1 KO [PDZ1-Tg] mice (see below and Fenske et al. (30)), it seems possible that higher expression levels of the PDZ1.2-Tg and PDZ1.2.3-Tg also might have resulted in dominant negative phenotypes in WT mice.
FIGURE 4.
Effects of expression of the PDZ1.2 transgene on hepatic SR-BI protein levels (A) and plasma lipoprotein cholesterol (B and C) in WT and PDZK1 KO mice. A, liver lysates (∼30 μg of protein) from mice with the indicated genotypes with (+) or without (-) the PDZ1.2 transgene were analyzed by immunoblotting, and SR-BI (∼82-kDa band) was visualized by chemiluminescence. ε-COP (∼34 kDa) was used as a loading control. Note the faint SR-BI band in the nontransgenic PDZK1 KO lane. Replicate experiments with multiple exposures and sample loadings were used to determine the relative levels of expression of SR-BI. All bands in adjacent panels were from a single gel. WT and WT [PDZ1.2-Tg] data were all from a single gel but reordered for clarity of presentation. B, plasma was harvested from mice with the indicated genotypes and PDZ1.2 transgene. Total plasma cholesterol levels were determined in individual samples by enzymatic assay, and results from the indicated numbers of animals (n) were pooled by genotype. Independent WT and KO control animals for each founder line were generated to ensure that the mixed genetic backgrounds for experimental and control mice were matched. † indicates that the nontransgenic WT plasma cholesterol levels were not statistically significantly different from those plasma cholesterol levels of WT [PDZ1.2-Tg]. ‡ indicates that PDZK1 KO plasma cholesterol levels were not statistically significantly different from those plasma cholesterol levels of PDZK1 KO [PDZ1.2-Tg] mice. WT and WT [PDZ1.2-Tg] plasma cholesterol levels were statistically significantly different (p ≤ 0.01) from those plasma cholesterol levels of PDZK1 KO and PDZK1 KO [PDZ1.2-Tg] mice. C, plasma samples (described in B) from individual animals were size-fractionated by FPLC, and the total cholesterol content of each fraction was determined by an enzymatic assay. The chromatograms are representative of multiple individually determined profiles. Approximate elution positions of native very low density lipoprotein (VLDL), intermediate density lipoprotein/low density lipoprotein (IDL/LDL), and HDL particles are indicated by brackets and were determined as described previously (14).
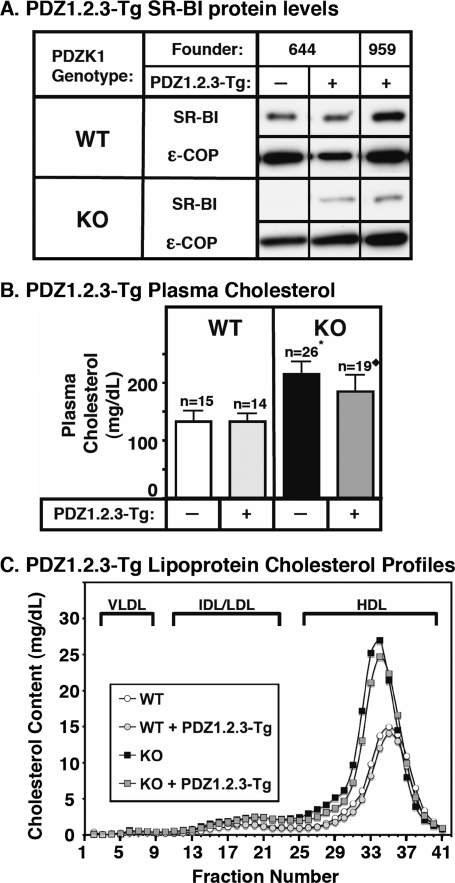
FIGURE 5.
Effects of expression of the PDZ1.2.3 transgene on hepatic SR-BI protein levels (A) and plasma lipoprotein cholesterol (B and C) in WT and PDZK1 KO mice. A, liver lysates (∼30 μg of protein) from mice with the indicated genotypes with (+) or without (-) the PDZ1.2.3 transgene were analyzed by immunoblotting, and SR-BI (∼82-kDa band) was visualized by chemiluminescence. ε-COP (∼34 kDa) was used as a loading control. Note the faint SR-BI band in the nontransgenic PDZK1 KO lane. Replicate experiments with multiple exposures and sample loadings were used to determine the relative levels of expression of SR-BI. All samples in adjacent panels shown were from a single gel. WT and WT [PDZ1.2.3-Tg] samples shown were all from a single gel but reordered for clarity of presentation. B, plasma was harvested from mice with the indicated genotypes and PDZ1.2.3 transgene. Total plasma cholesterol levels were determined in individual samples by enzymatic assay, and results from the indicated numbers of animals (n) were pooled by genotype. Independent WT and KO control animals for each founder line were generated to ensure that the mixed genetic backgrounds for experimental and control mice were matched. * indicates that the nontransgenic PDZK1 KO plasma cholesterol levels were statistically significantly different (p ≤ 0.001) from those plasma cholesterol levels of WT, WT [PDZ1.2.3-Tg], and PDZK1 KO [PDZ1.2.3-Tg] mice. ♦ indicates that PDZK1 KO [PDZ1.2.3-Tg] plasma cholesterol levels were statistically significantly different (p ≤ 0.001) from those plasma cholesterol levels of WT, WT [PDZ1.2.3-Tg], and PDZK1 KO mice. C, plasma samples (described in B) from individual animals were size-fractionated by FPLC, and the total cholesterol content of each fraction was determined by an enzymatic assay. The chromatograms are representative of multiple individually determined profiles. Approximate elution positions of native very low density lipoprotein (VLDL), intermediate density lipoprotein/low density lipoprotein (IDL/LDL), and HDL particles are indicated by brackets and were determined as described previously (14).
FIGURE 6.
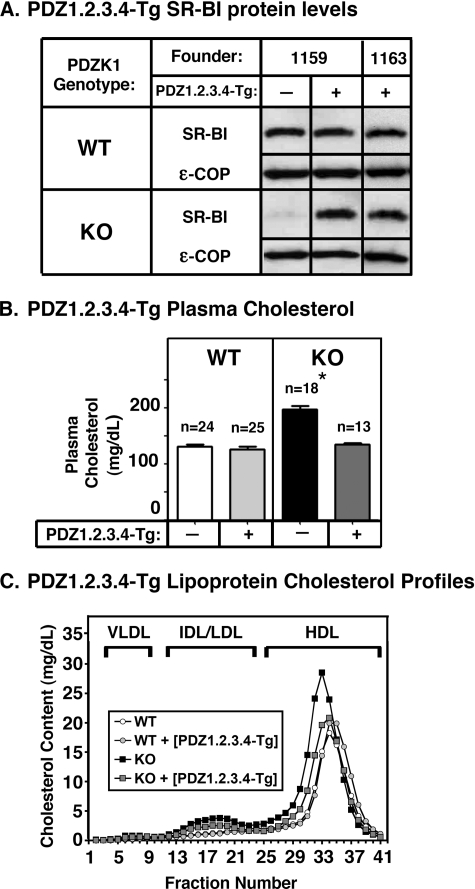
Effects of expression of the PDZ1.2.3.4 transgene on hepatic SR-BI protein levels (A) and plasma lipoprotein cholesterol (B and C) in WT and PDZK1 KO mice. A, liver lysates (∼30 μg of protein) from mice with the indicated genotypes with (+) or without (-) the PDZ1.2.3.4 transgene were analyzed by immunoblotting, and SR-BI (∼82-kDa band) was visualized by chemiluminescence. ε-COP (∼34 kDa) was used as a loading control. Note the faint SR-BI band in the nontransgenic PDZK1 KO lane. Replicate experiments with multiple exposures and sample loadings were used to determine the relative levels of expression of SR-BI. All samples in adjacent panels shown were from a single gel. WT and WT [PDZ1.2.3.4-Tg] samples shown were all from a single gel but reordered for clarity of presentation. B, plasma was harvested from mice with the indicated genotypes and PDZ1.2.3.4 transgene. Total plasma cholesterol levels were determined in individual samples by enzymatic assay, and results from the indicated numbers of animals (n) were pooled by genotype. Independent WT and KO control animals for each founder line were generated to ensure that the mixed genetic backgrounds for experimental and control mice were matched. * indicates that the nontransgenic PDZK1 KO plasma cholesterol levels were statistically significantly different (p ≤ 0.001) from those plasma cholesterol levels of WT, WT [PDZ1.2.3.4-Tg], and PDZK1 KO [PDZ1.2.3.4-Tg] mice. C, plasma samples (described in B) from individual animals were size-fractionated by FPLC, and the total cholesterol content of each fraction was determined by an enzymatic assay. The chromatograms are representative of multiple individually determined profiles. Approximate elution positions of native very low density lipoprotein (VLDL), intermediate density lipoprotein/low density lipoprotein (IDL/LDL), and HDL particles are indicated by brackets and were determined as described previously (14).
In PDZK1 KO mice, 5.5-fold hepatic overexpression of the truncated PDZ1.2 protein in founder line 833 and approximately endogenous levels (1.3-fold) of PDZ1.2 in founder line 836 had virtually no effect on the abnormally high plasma cholesterol levels (Fig. 4B, right panel) or the size distribution of lipoproteins (FPLC lipoprotein cholesterol profiles) in which the bulk of the cholesterol was found in abnormally large HDL particles (Fig. 4C, black and gray squares). These results are similar to those observed for hepatic overexpression of the PDZ1 protein, which was not sufficient to restore normal SR-BI function in PDZK1 KO mice (30).
However, in PDZK1 KO mice, ∼8-fold (founder line 644) and only 17% (founder line 959) expression of PDZ1.2.3-Tg led to a small, but significant (p ≤ 0.001) decrease in the abnormally high plasma cholesterol levels for the pooled data (Fig. 5B, right panel), although there was no discernible corresponding change in the size distribution of lipoproteins (FPLC lipoprotein cholesterol profiles) in which the bulk of the cholesterol was found in abnormally large HDL particles (Fig. 5C, black and gray squares). A virtually identical relative reduction in plasma cholesterol levels was observed in each founder line when considered independently. However, this reduction was not statistically significant in founder line 959 (17% expression), although it was significant in founder line 644 (p ≤ 0.01).
In PDZK1 KO mice, 10.6- and 2.8-fold overexpression of PDZ1.2.3.4-Tg (founder lines 1159 and 1163, respectively) led to normalization of plasma cholesterol levels (Fig. 6B, right panel) and near normalization of the size distribution of lipoproteins (FPLC lipoprotein cholesterol profiles; Fig. 6C, black and gray squares). Thus, whereas the N-terminal PDZ1 and PDZ2 domain-containing region of PDZK1 (PDZ1.2) was insufficient to restore SR-BI function in PDZK1 KO mice, SR-BI function was partially restored by expression of protein containing the first three PDZ domains (PDZ1.2.3) and essentially completely restored by protein containing all four PDZ domains (PDZ1.2.3.4) but not the C-terminal domain (residues 462-516) (Fig. 1A).
When we examined hepatic SR-BI protein abundance and localization in the transgenic mice, we found that although PDZ1.2-Tg had virtually no effect on the abnormally high plasma cholesterol levels in PDZK1 KO mice SR-BI protein levels in PDKZ1 KO [PDZ1.2-Tg] mice increased from <5% to 12 ± 5% of nontransgenic WT levels (immunoblotting; Fig. 4A, lower panels). However, the majority of the detectable steady-state hepatic SR-BI protein in PDZK1 KO [PDZ1.2-Tg] mice was not localized on the cell surface but was instead observed intracellularly (immunohistochemistry; representative image in Fig. 3F). This intracellular localization would prohibit SR-BI function by preventing interaction with plasma HDL particles at the cell surface. These results in PDZK1 KO [PDZ1.2-Tg] mice are similar to those observed previously in PDZK1 KO [PDZ1-Tg] mice (30), although the increase in intracellular SR-BI in PDZK1 KO [PDZ1.2-Tg] mice was only about half that in PDZK1 KO [PDZ1-Tg] mice (21 ± 6% of WT). This quantitative difference may be due, in part, to the higher level of transgene expression in PDZK1 KO [PDZ1-Tg] mice (24-versus 1.2-5.5-fold).
Compared with nontransgenic PDZK1 KO mice, PDZK1 KO [PDZ1.2.3-Tg] mice, which exhibited a small decrease in plasma cholesterol levels, exhibited a relatively large (>6-fold) increase in hepatic SR-BI abundance (30 ± 7 versus <5% of nontransgenic WT levels) (immunoblotting; Fig. 5A, lower panel). However, unlike hepatocytes in PDZK1 KO [PDZ1-Tg] (30) and PDZK1 KO [PDZ1.2-Tg] (this study) mice, at least some of the SR-BI protein in PDZK1 KO [PDZ1.2.3-Tg] hepatocytes was observed on the cell surface (immunohistochemistry; representative image in Fig. 3J). We were unable to quantitate precisely the relative amounts of intracellular and surface SR-BI, but visual inspection suggested that there was substantially less surface SR-BI on PDZK1 KO [PDZ1.2.3-Tg] hepatocytes than seen in nontransgenic WT mice. This cell surface localization of SR-BI on PDZK1 KO [PDZ1.2.3-Tg] hepatocytes could account for the small decrease in plasma cholesterol levels that was observed in these mice (Fig. 5B).
PDZK1 KO [PDZ1.2.3.4-Tg] mice, which exhibited plasma cholesterol levels not significantly different from WT mice, exhibited an essentially WT level of hepatic SR-BI protein (91 ± 4%; immunoblotting; Fig. 6A, lower panel). This was accompanied by restoration of wild-type-like SR-BI cell surface localization (immunohistochemistry; representative image in Fig. 3N; compare with Fig. 3A).
Thus, we conclude that the first three PDZ domains of PDZK1, expressed as a truncated PDZK1 protein, exhibit partial PDZK1 function in that PDZ1.2.3 was able not only to partially restore SR-BI abundance, as can PDZ1 (30) and PDZ1.2, but also to restore partial cell surface localization of SR-BI and consequently partial SR-BI function. Furthermore we found that all four PDZ domains of PDZK1 are sufficient to restore virtually normal SR-BI abundance and function. The C-terminal 58 amino acids of PDZK1, including the putative phosphorylation site located on a serine within the last 15 amino acids of the protein (32) and the putative PDZ binding site TEM, are not necessary for normal SR-BI localization and function.
DISCUSSION
The first PDZ domain, PDZ1, of the cytoplasmic adaptor protein PDZK1 (Fig. 1A, left) binds to the C terminus of SR-BI, and as a consequence, PDZK1 controls hepatic SR-BI protein expression and lipoprotein metabolism (18, 20, 28, 30). Overexpression in hepatocytes of PDZK1 KO mice of a transgene encoding the full-length PDZK1 completely restores the abundance, surface localization, and function of SR-BI to that seen in WT mice (30). Overexpression (24-fold) in hepatocytes of PDZK1 KO mice of a truncated PDZK1 transgene (PDZ1-Tg) encoding only the N terminus and PDZ1 domain (PDZ1 protein) is not sufficient to restore SR-BI cell surface localization or function. However, it does result in an increase in SR-BI abundance (from <5 to 24% of WT levels, although the SR-BI is mislocalized (intracellular rather than primarily on the cell surface (30)). In WT mice, hepatic overexpression of PDZ1-Tg results in a dominant negative effect with an increase in plasma cholesterol similar to that seen in PDZK1-KO mice (30).
We previously proposed several potential mechanisms by which binding of full-length PDZK1 to SR-BI might influence both SR-BI protein abundance and intracellular localization/function, whereas binding of the truncated PDZ1 protein might partially restore the abundance of SR-BI, which is nevertheless mislocalized and nonfunctional (30). PDZ1-mediated PDZK1 binding to the C terminus of SR-BI might inhibit degradation of hepatic SR-BI via one or more mechanisms. Bound PDZK1 or PDZ1 might directly prevent access of SR-BI to proteolytic pathways (e.g. blocking ubiquitination of the C terminus of SR-BI and degradation by proteasomes). PDZK1 might indirectly prevent proteolysis by preventing delivery of SR-BI to sites of degradation by controlling the intracellular trafficking of SR-BI (e.g. promoting stable cell surface localization by directing postmedial Golgi sorting to the cell surface, inhibiting endocytosis from the cell surface, or promoting normal endocytic recycling back to the cell surface). The differences in the effects of full-length PDZK1 and truncated PDZ1 suggested that PDZ domains of PDZK1 other than PDZ1 and/or the C-terminal non-PDZ region of PDZK1 (residues 462-519) are necessary for normal control of hepatic SR-BI. However, the roles of these other domains of PDZK1 are not well understood.
In the current study, we examined the effects on hepatic SR-BI protein abundance, localization, and function in WT and PDZK1 KO mice of hepatic expression of these other portions of the PDZK1 protein using four transgenes encoding C-terminal truncation mutants of PDZK1 (Fig. 1A, right). The results show that the C-terminal 57 residues are not required for normal PDZK1-dependent control of SR-BI abundance, localization, and function in mice when proteins encoded by these truncated cDNAs missing all or a portion of this region are overexpressed by ≥2.8-fold in hepatocytes in PDZK1 KO mice (pTEM-Tg and PDZ1.2.3.4-Tg). This C-terminal region includes a putative PDZ-binding sequence (517TEM) at the C terminus as well as phosphorylation sites (in the C-terminal 15 amino acids of rat PDZK1), which can influence PDZK1/SR-BI interaction in vitro and appear to be subject to regulation in vivo (32, 38). It is possible that the C-terminal region of PDZK1 confers subtle control or additional modes of regulation over SR-BI not explored by the experiments performed here.
PDZ1.2 comprises the N terminus and PDZ1 and PDZ2 domains of PDZK1 (Fig. 1A). Up to 5.5-fold overexpression of PDZ1.2 was unable to restore cell surface expression or function of SR-BI in PDZK1-KO mice. It did, however, increase the steady-state levels of the protein from <5% of WT in nontransgenic PDZK1 KO mice to 12% of WT in PDZK1-KO [PDZ1-Tg] mice. This receptor protein was distributed intracellularly, presumably associated with membranes in the cytoplasm. This phenotype is similar to that of PDZK1 KO mice with 24-fold overexpression of PDZ1 in which hepatic SR-BI levels were 24% of WT and SR-BI was similarly mislocalized intracellularly (30). Presumably the mechanisms underlying the similar phenotypes associated with PDZ1 and PDZ1.2 hepatic expression are comparable (see above) with the higher level of PDZ1 overexpression responsible, at least in part, for the high level of mislocalized, intracellular SR-BI. Expression of a truncated protein with three PDZ domains, PDZ1.2.3 in PDZK1 KO mice (Fig. 1A), even at relatively low levels (17% of WT) partially restored the abundance of SR-BI (∼30% of wild type), much of which appeared to be in the cytoplasm as is the case for PDZ1 and PDZ1.2. However, unlike the shorter proteins, PDZ1.2.3 was able to mediate the stable expression of a small amount of SR-BI on the cell surface. As a consequence there was partially restored SR-BI function in PDZK1 KO [PDZ1.2.3-Tg] mice as demonstrated by a small, but statistically significant, decrease in abnormally high plasma cholesterol levels (pooled data from two founders) relative to nontransgenic PDZK1 KO mice. These results suggest that the third PDZ domain of PDZK1, when expressed in the context of the first two PDZ domains, can influence steady-state SR-BI cell surface localization. It is possible that an unknown cellular component that binds to the PDZ3 domain of PDZK1 is involved in transporting SR-BI to the cell surface, maintaining stable SR-BI cell surface localization, and/or preventing abnormally rapid removal of SR-BI from the cell surface. As noted above, ≥2.8-fold hepatic overexpression of PDZ1.2.3.4-Tg resulted in virtually complete restoration of SR-BI protein abundance, cell surface expression, and function, establishing the importance of the fourth PDZ domain of PDZK1. Thus, the PDZ3 and PDZ4 domains of PDZK1 apparently play important roles in mediating the effects of PDZK1 on SR-BI stability and intracellular localization. Unlike PDZ1-Tg overexpressed in WT mice, the overexpression of PDZ1.2-Tg and PDZ1.2.3-Tg did not produce dominant negative phenotypes. This may be due to lower expression levels of PDZ1.2-Tg and PDZ1.2.3-Tg than PDZ1-Tg in these mice.
Additional studies will be needed to define precisely the roles that PDZ domains 2-4 play in normal PDZK1 function. There are several distinct, but not mutually exclusive, possibilities. For example, one or more of these domains may 1) facilitate intramolecular protein folding, 2) serve as spacers between the PDZ1 and PDZ4 domains, 3) bind to other proteins that promote stable expression of SR-BI at the cell surface, or 4) participate in intramolecular interactions possibly leading to dimerization/oligomerization of PDZK1 essential for SR-BI function as was suggested for some other PDZ domain-containing proteins (39).
In summary, PDZK1 appears to affect hepatic SR-BI protein levels through binding to the C terminus of SR-BI and consequently influencing the abundance and intracellular localization of SR-BI. The influence of PDZK1 on SR-BI is due, at least in part, to interactions involving its third and fourth PDZ domains, possibly with other cellular components. Future studies will be required to identify these putative cellular binding partners and the mechanism(s) by which they cooperate with PDZK1 to control the function of SR-BI and thus HDL metabolism.
Note Added in Proof—Zhu et al. (Zhu, W., Saddar, S., Seetharam, D., Chambliss, K. L., Longoria, C., Silver, D. L., Yuhanna, I. S., Shaul, P. W., and Mineo, C. (2008) Circ. Res. 29, 480-487) reported that HDL activation of Src in cultured bovine aortic endothelial cells, which is mediated by SR-BI and PDZK1, is inhibited by overexpression of a hemagglutinin-tagged truncated form of PDZK1 (residues 1 to 240) that is similar to PDZ1.2 (residues 1-220) in this report. For additional studies of PDZK1/SR-BI-mediated signaling in endothelial cells see Kimura et al. (Kimura, T., Tomura, H., Mogi, C., Kuwabara, A., Damirin, A., Ishizuka, T., Sekiguchi, A., Ishiwara, M., Im, D. S., Sato, K., Murakami, M., and Okajima, F. (2006) J. Biol. Chem. 281, 37457-37467).
Acknowledgments
We thank Joel Lawitts from the Beth Israel-Deaconess Medical Center transgenic facility. We also thank Hiroyuki Arai for generously providing reagents and Harvey Lodish and David Sabatini for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant HL077780 (to O. K.); Grants HL64737, HL52212, and HL66105 (to M. K.); and Clinical Translational Science Award Grant 1UL1RR025758-01 (to the Massachusetts Institute of Technology). This work was also supported by Fondo Nacional de Desarrollo Científico y Tecnológico Grant 1070634 (to A. R.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SR-BI, scavenger receptor class B, type I; HDL, high density lipoprotein; WT, wild-type; KO, knock-out; FPLC, fast performance liquid chromatography; COP, coat protein; FVB/N, friend leukemia virus strain B NIH.
References
- 1.Rigotti, A., Miettinen, H., and Krieger, M. (2003) Endocr. Rev. 24 357-387 [DOI] [PubMed] [Google Scholar]
- 2.Glass, C., Pittman, R., Weinstein, D., and Steinberg, D. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 5435-5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reaven, E., Chen, Y., Spicher, M., and Azhar, S. (1984) J. Clin. Investig. 74 1384-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein, Y., Dabach, Y., Hollander, G., Halperin, G., and Stein, O. (1983) Biochim. Biophys. Acta 752 98-105 [DOI] [PubMed] [Google Scholar]
- 5.Acton, S., Rigotti, A., Landschulz, K. T., Xu, S., Hobbs, H. H., and Krieger, M. (1996) Science 271 518-520 [DOI] [PubMed] [Google Scholar]
- 6.Acton, S. L., Scherer, P. E., Lodish, H. F., and Krieger, M. (1994) J. Biol. Chem. 269 21003-21009 [PubMed] [Google Scholar]
- 7.Fu, T., Kozarsky, K., and Borensztajn, J. (2003) J. Biol. Chem. 278 52559-52563 [DOI] [PubMed] [Google Scholar]
- 8.Out, R., Hoekstra, M., de Jager, S., de Vos, P., Van der Westhuyzen, D. R., Webb, N. R., Van Eck, M., Biessen, E., and Van Berkel, T. (2005) J. Lipid Res. 46 1172-1181 [DOI] [PubMed] [Google Scholar]
- 9.Out, R., Kruijt, J., Rensen, P., Hildebrand, R., de Vos, P., Van Eck, M., and Van Berkel, T. (2004) J. Biol. Chem. 279 18401-18406 [DOI] [PubMed] [Google Scholar]
- 10.Van Eck, M., Hoekstra, M., Out, R., Bos, I., Kruijt, J., Hildebrand, R., and Van Berkel, T. (2008) J. Lipid Res. 49 136-146 [DOI] [PubMed] [Google Scholar]
- 11.Ji, Y., Jian, B., Wang, N., Sun, Y., Moya, M., Phillips, M., Rothblat, G., Swaney, J., and Tall, A. (1997) J. Biol. Chem. 272 20982-20985 [DOI] [PubMed] [Google Scholar]
- 12.Jian, B., De la Llera-Moya, M., Ji, Y., Wang, N., Phillips, M., Swaney, J., Tall, A., and Rothblat, G. (1998) J. Biol. Chem. 273 5599-5606 [DOI] [PubMed] [Google Scholar]
- 13.Braun, A., Zhang, S., Miettinen, H., Ebrahim, S., Holm, T., Vasile, E., Post, M. J., Yoerger, D., Picard, M., Krieger, J., Andrews, N., Simons, M., and Krieger, M. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 7283-7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rigotti, A., Trigatti, B. L., Penman, M., Rayburn, H., Herz, J., and Krieger, M. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 12610-12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trigatti, B., Rayburn, H., Vinals, M., Braun, A., Miettinen, H., Penman, M., Hertz, M., Schrenzel, M., Amigo, L., Rigotti, A., and Krieger, M. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 9322-9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Eck, M., Twisk, J., Hoekstra, M., Van Rij, B., Van der Lans, C., Bos, I., Kruijt, J., Kuipers, F., and Van Berkel, T. (2003) J. Biol. Chem. 278 23699-23705 [DOI] [PubMed] [Google Scholar]
- 17.Yesilaltay, A., Morales, M., Amigo, L., Zanlungo, S., Rigotti, A., Karackattu, S., Donahee, M., Kozarsky, K., and Krieger, M. (2006) Endocrinology 147 1577-1588 [DOI] [PubMed] [Google Scholar]
- 18.Ikemoto, M., Arai, H., Feng, D., Tanaka, K., Aoki, J., Dohmae, N., Takio, K., Adachi, H., Tsujimoto, M., and Inoue, K. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 6538-6543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kocher, O., Comella, N., Tognazzi, K., and Brown, L. F. (1998) Lab. Investig. 78 117-125 [PubMed] [Google Scholar]
- 20.Kocher, O., Yesilaltay, A., Cirovic, C., Pal, R., Rigotti, A., and Krieger, M. (2003) J. Biol. Chem. 278 52820-52825 [DOI] [PubMed] [Google Scholar]
- 21.Yesilaltay, A., Kocher, O., Rigotti, A., and Krieger, M. (2005) Curr. Opin. Lipidol. 16 147-152 [DOI] [PubMed] [Google Scholar]
- 22.Eckhardt, E. R., Cai, L., Shetty, S., Zhao, Z., Szanto, A., Webb, N. R., and Van der Westhuyzen, D. R. (2006) J. Biol. Chem. 281 4348-4353 [DOI] [PubMed] [Google Scholar]
- 23.Eckhardt, E. R., Cai, L., Sun, B., Webb, N. R., and Van der Westhuyzen, D. R. (2004) J. Biol. Chem. 279 14372-14381 [DOI] [PubMed] [Google Scholar]
- 24.Hung, A., and Sheng, M. (2002) J. Biol. Chem. 277 5699-5702 [DOI] [PubMed] [Google Scholar]
- 25.Webb, N. R., Connell, P. M., Graf, G. A., Smart, E. J., De Villiers, W. J. S., De Beer, F. C., and Van der Westhuyzen, D. R. (1998) J. Biol. Chem. 273 15241-15248 [DOI] [PubMed] [Google Scholar]
- 26.Webb, N. R., De Villiers, W. J. S., Connell, P. M., De Beer, F. C., and Van der Westhuyzen, D. R. (1997) J. Lipid Res. 38 1490-1495 [PubMed] [Google Scholar]
- 27.Zhang, X., Moor, A., Merkler, K., Liu, Q., and McLean, M. (2007) Endocrinology 148 5295-5304 [DOI] [PubMed] [Google Scholar]
- 28.Silver, D. L. (2002) J. Biol. Chem. 277 34042-34047 [DOI] [PubMed] [Google Scholar]
- 29.Yesilaltay, A., Kocher, O., Pal, R., Leiva, A., Quinones, V., Rigotti, A., and Krieger, M. (2006) J. Biol. Chem. 281 28975-28980 [DOI] [PubMed] [Google Scholar]
- 30.Fenske, S., Yesilaltay, A., Pal, R., Daniels, K., Rigotti, A., Krieger, M., and Kocher, O. (2008) J. Biol. Chem. 283 22097-22104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, P., Wang, J., Xiao, Y., Murray, J., Novikoff, P., Angeletti, R., Orr, G., Lan, D., Silver, D. L., and Wolkoff, A. (2005) J. Biol. Chem. 280 30143-30149 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura, T., Shibata, N., Nishimoto-Shibata, T., Feng, D., Ikemoto, M., Motojima, K., Iso-o, N., Tsukamoto, K., Tsujimoto, M., and Arai, H. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 13404-13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gisler, S. M., Pribanic, S., Bacic, D., Forrer, P., Gantenbein, A., Sabourin, L., Tsuji, A., Zhao, Z., Manser, E., Biber, J., and Murer, H. (2003) Kidney Int. 64 1733-1745 [DOI] [PubMed] [Google Scholar]
- 34.Simonet, W., Bucay, N., Lauer, S., and Taylor, J. (1993) J. Biol. Chem. 268 8221-8229 [PubMed] [Google Scholar]
- 35.Palmiter, R., and Brinster, R. (1986) Annu. Rev. Genet. 20 465-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kocher, O., Pal, R., Roberts, M., Cirovic, C., and Gilchrist, A. (2003) Mol. Cell. Biol. 23 1175-1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo, Q., Penman, M., Trigatti, B., and Krieger, M. (1996) J. Biol. Chem. 271 11191-11196 [DOI] [PubMed] [Google Scholar]
- 38.Kocher, O., Comella, N., Gilchrist, A., Pal, R., Tognazzi, K., Brown, L. F., and Knoll, J. H. M. (1999) Lab. Investig. 79 1161-1170 [PubMed] [Google Scholar]
- 39.Gomperts, S. N. (1996) Cell 84 659-662 [DOI] [PubMed] [Google Scholar]