Abstract
Effective clearance of mucus is a critical innate airway defense mechanism, and under appropriate conditions, can be stimulated to enhance clearance of inhaled pathogens. It has become increasingly clear that extracellular nucleotides (ATP and UTP) and nucleosides (adenosine) are important regulators of mucus clearance in the airways as a result of their ability to stimulate fluid secretion, mucus hydration, and cilia beat frequency (CBF). One ubiquitous mechanism to stimulate ATP release is through external mechanical stress. This article addresses the role of physiologically-relevant mechanical forces in the lung and their effects on regulating mucociliary clearance (MCC). The effects of mechanical forces on the stimulating ATP release, fluid secretion, CBF, and MCC are discussed. Also discussed is evidence suggesting that airway hydration and stimulation of MCC by stress-mediated ATP release may play a role in several therapeutic strategies directed at improving mucus clearance in patients with obstructive lung diseases, including cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD).
1. Introduction
Mucus clearance is a term that refers to a coordinated integration of ion transport, water flow, mucin secretion, cilia action, and cough, resulting in the continuous flow of fluid and mucus on airway surfaces. Functionally, mucus clearance is an innate process involved in guarding the lung against inhaled bacteria, viruses, and other noxious particles. In addition to supporting mechanical clearance, the mucus lining the airways possess anti-bacterial agents such as lysozyme and lactoferrin (Boyton et al., 2002), migratory cells such as macrophages, and signaling molecules such as cytokines, to suppress microbial proliferation during the clearance process (Knowles et al., 2002). Key to removing all of the trapped debris is the maintenance is the rate of cilia-mediated mucus clearance, or mucociliary clearance (MCC). While it is well known that this process is undoubtedly dependent on the rate of ciliary beating (Satir et al., 1990), it is clear that the rate of clearance is also strongly influenced by the mucus hydration state, and hence the viscoelastic properties of the mucus (Puchelle et al., 1995; Winters et al., 1997; Tarran et al., 2001). In general, the more hydrated the mucus, the more efficiently it is cleared from the lungs.
The airway surface liquid (ASL) layer lining the airway surfaces is crucial for mediating mucociliary clearance rates (Boucher, 2002). More importantly, the clearance of mucus from airway surfaces requires the coordinated interaction of two separate layers that together comprise the ASL. As shown in Fig. 1, the first layer is the mucus layer which contains mucins secreted from goblet cells and glands (Rubin, 2002). The mucus layer is designed to bind and entrap virtually all of the particles deposited on the airway surface during normal breathing. In this system, the viscoelastic properties of the overlying mucus layer facilitate conversion of energy from beating cilia into vectorial mucus transport, facilitated by cough during stimulating irritation. As shown in Fig. 1., the mucus layer represents an unrestrained, tangled gel generated by the high-molecular-weight secreted, gel-forming, mucins (muc-5ac, muc-5b) that occupy this region (Thornton et al., 2004). The viscoelastic properties, and hence transportability, of this layer are determined by both the composition of the mucin macromolecules and, importantly, by the “hydration” state of this layer (Voynow, 2002; Boucher, 2003). As discussed below, the hydration state of the airways reflects the balance between the activities Na+ absorption and Cl− secretion mediated by the epithelial sodium channel (ENaC) and the, cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel, respectively (Tarran et al., 2005). While the mucus layer is a viscoelastic gel with the major structural components being large mucin glycoproteins, normal mucus represents only about 1% mucins, with the balance being salt (1%), other proteins (1%), and water (97%) (Matsui et al., 2006).
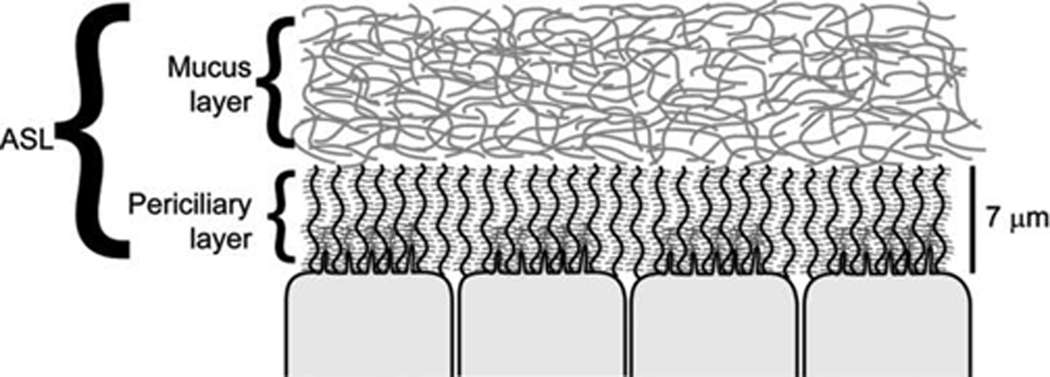
Fig. 1. Representation of the two layers that compose the airway surface layer (ASL).
The overlying mucus layer, composed of gel-forming mucins (muc-5b and muc-5ac, shown here as entangled strands) secreted by glands and superficial goblet cells, is a highly viscoelastic material that traps inhaled particles for clearance. The lower, or periciliary layer (PCL), is a grafted-gel structure composed of cell surface tethered mucins (muc-1 and muc-4) and glycolipids within the domain around the cilia and microvilli.
The second layer, underlying the mucus layer, is the periciliary layer (PCL) layer (Fig. 1). This layer was previously thought (Tarran et al., 2001) to simply be a thin (~7 µm), low-viscosity aqueous layer that acts as a lubricant layer for cilia beating and the movement of the mucus layer over the epithelial surface. However, more recent hypotheses have suggested that the PCL is also a gel layer; in this case, a “grafted polyanionic gel” (reviewed in Randell [2006]). Here, the cell surface of the human airways contains large membrane-bound glycoproteins as well as tethered mucins (muc-1, muc-4, and muc-16). Not only does such a structure provide an efficient lubricating layer for cilia to beat, but also serves as a barrier to restrict access of particles from accessing the cell surface directly due to the close approximation of these long-branched carbohydrate molecules (Randell et al., 2006). As with the overlying mucus layer, the hydration state of the PCL-gel layer reflects the balance of Na+ and Cl− ion transport activities (Tarran et al., 2005).
The rate of mucus clearance from the airways is dynamic and can, under appropriate conditions, be stimulated to increase clearance. This has been demonstrated in a number of studies measuring the basal rates of mucus clearance in healthy subjects, as well as the changes that occur in disease and in response to inhalation of exogenous toxicants and drugs. For example, it has been shown that the rate of mucus clearance can be increased by ~3 fold over basal levels in response to inhalation of exogenous agonists (Wanner et al., 1996). Despite a large body of pharmacologic data, it has only been recent that studies have been undertaken to understand how the mucociliary clearance apparatus is endogenously regulated at the microscopic level, i.e., how the hydration status of the airways, ciliary beat frequency (CBF), and mucin secretion rates are coordinated to effect efficient clearance under both basal and stimulated conditions. In part, based on studies designed to explore the pathogenesis of obstructive lung diseases, such as chronic obstructive pulmonary disease (COPD) and cystic fibrosis (CF), much attention has focused on understanding how these mechanisms that provide "innate" airways defense and how they are modulated in airways diseases characterized by chronic bacterial infection.
Key to this work are the findings from a number of studies that which demonstrate that endogenous factor are responsible for accelerating mucus clearance in reaction to airway stresses exposure as part of a "lung defense" response. As will be discussed throughout this review, a fundamental finding of this work was that the release of endogenous 5' nucleotide triphosphates, such as ATP, along with its metabolite adenosine, interact with airway epithelial purinergic receptors, to serve as major autocrine regulators of mucus clearance rates, and hence innate airways defense under basal, as well as, in response to inhaled toxins/irritants. It has also become apparent that the oscillatory motion generated during breathing is an important aspect of airway as well as lung physiology. While it has long been known that normal tidal breathing interspersed with sighing is important for maintaining alveolar integrity (Dietl et al., 2001), recent studies provide a mechanism that links the oscillatory motion of pulmonary ventilation to mucus clearance (Tarran 2005; Button, 2007), together suggesting that the mechanical stresses generated by tidal breathing are necessary for maintaining proper airways health.
2. Airway ion transport and ASL regulation
Airway epithelia have low transepithelial electrical resistances (Boucher, 1994) and are highly water permeable (Farinas et al., 1997; Matsui et al., 2000; Crews et al., 2001). These features suggest that large, osmotic gradients cannot be maintained across airway epithelia. This property initially appears disadvantageous during vectoral ion/water transport since extra energy is expended to move ions/water against a continual backflux. However, leaky epithelia, such as the airways, are capable of both absorbing fluid (by regulating ENaC-mediated Na+ absorption) (Spring, 1999) and secreting fluid (by regulating CaCC- and CFTR-mediated Cl− secretion) (Boucher, 1994). As discussed below, this capability allows the airway epithelia to finely tune the hydration state of the ASL (Tarran et al., 2001) to effect efficient mucus clearance (Kilburn, 1968).
Since it has been shown that the NaCl concentration within the ASL is principally isotonic with plasma (Knowles et al., 1997), one should consider the mass of NaCl in the ASL rather than its concentration as being the determining factor of fluid volume and composition. For example, if more salt is secreted into the ASL by the epithelium, water will follow passively and the ASL volume will rapidly increase to maintain isotonicity. Conversely, if NaCl is absorbed, water also follows passively (in the opposite direction) resulting in a decrease in the hydration of the surface of the airways. Such a result predicts that the mass of NaCl within the ASL determines the hydration state of the airway surface. Data support the hypothesis that this property permits the airway epithelia to optimize ASL hydration state by modifying the activities of apical ion channels to continuously switch between secretory and absorptive phenotypes (Tarran et al., 2001; Tarran et al., 2005).
3. Nucleotides and ion transport
Despite the importance of understanding the regulation of ASL hydration on lung health, key aspects of its physiology remain unexplored. While much is known about the processes that mediate transepithelial ion transport, including ion channels, pumps, and co-transporters, significantly less is known about the “signals” that coordinate the activity of this complex system to reciprocally modulate both Na+ absorption and Cl− secretion, to provide the proper balance of the ASL hydration. Airway epithelia appear to be disconnected from effects of systemic hormones that regulate ion transport rates in other epithelia. For example, airway epithelia are resistant to the actions of mineralocorticoid and steroid hormones and vasopressin that regulate Na+ transport in other epithelia (Grubb et al., 1998). Rather, evidence supports the notion that airway epithelia largely respond to local signals generated on airway extracellular surfaces to maintain ASL hydration state sufficient for basal mucus clearance, as well as, report the stresses on airway surfaces that require an adjustment of ASL volume and mucus clearance (Tarran et al., 2006).
It has become increasingly clear that ATP is involved in many important extracellular functions in addition to its well-established role in cell metabolism (Schwiebert et al., 2003). For example, there is a growing body of evidence for extracellular ATP regulation of phenomena as diverse as vascular tone (Yamamoto et al., 2006), cardiac function (Pelleg et al., 1996), renal epithelial transport (Komlosi et al., 2005), and fast synaptic transmission in the central nervous system (North et al., 2006). One dominating theme for extracellular nucleotides is that they have been shown to be important regulators of ion transport in epithelial tissues as a result of their ability to activate cell surface receptors. In particular, a number of studies have suggested that extracellular purine/pyrimidine nucleotides and nucleosides are luminally active regulators of airway epithelial ion transport, and thus, regulation of ASL hydration state and mucus clearance rates. First, it has been shown that exogenous application of ATP, UTP, and/or adenosine (ADO) produces coordinate regulatory effects on airway epithelia ion transport, including the inhibition of Na+ absorption (Devor et al., 1999; Devor et al., 2000; Mall et al., 2000) and activation of Cl− secretion (Mason et al., 1991; Clarke et al., 1992). Second, In vivo studies have demonstrated the stimulatory effects of nucleotides/nucleosides, including the effects of topical UTP (Knowles et al., 1991) and ADO on nasal bioelectrics as assessed by voltrometry (Clancy et al., 1999; Hentchel-Franks et al., 2004). Further, inhaled UTP has been shown to stimulate both mucus clearance rates, as assessed by particle clearance (Bennett et al., 1996; Kellerman, 2002a), and sputum production rates (Kellerman et al., 2002b).
The mechanisms of action of these agents are diverse, but systematic work by many investigators has elucidated many of these pathways in the lung, summarized in Fig. 2. In the lungs, G-protein coupled receptors that sense nucleotides on the apical membrane of superficial airway epithelial cells have been identified and include P2Y2 (stimulated by ATP and UTP) and P2Y6 (stimulated by UDP) (Lazarowski et al., 2001). The observation that P2Y2 receptor activation not only initiates Cl− secretion (Lazarowski et al., 2003), but also reciprocally inhibits Na+ absorption (Devor et al., 1999; Mall et al., 2000), led to the hypothesis that the capacity of these molecules to finely tune ASL hydration state, and thus, mucus clearance.
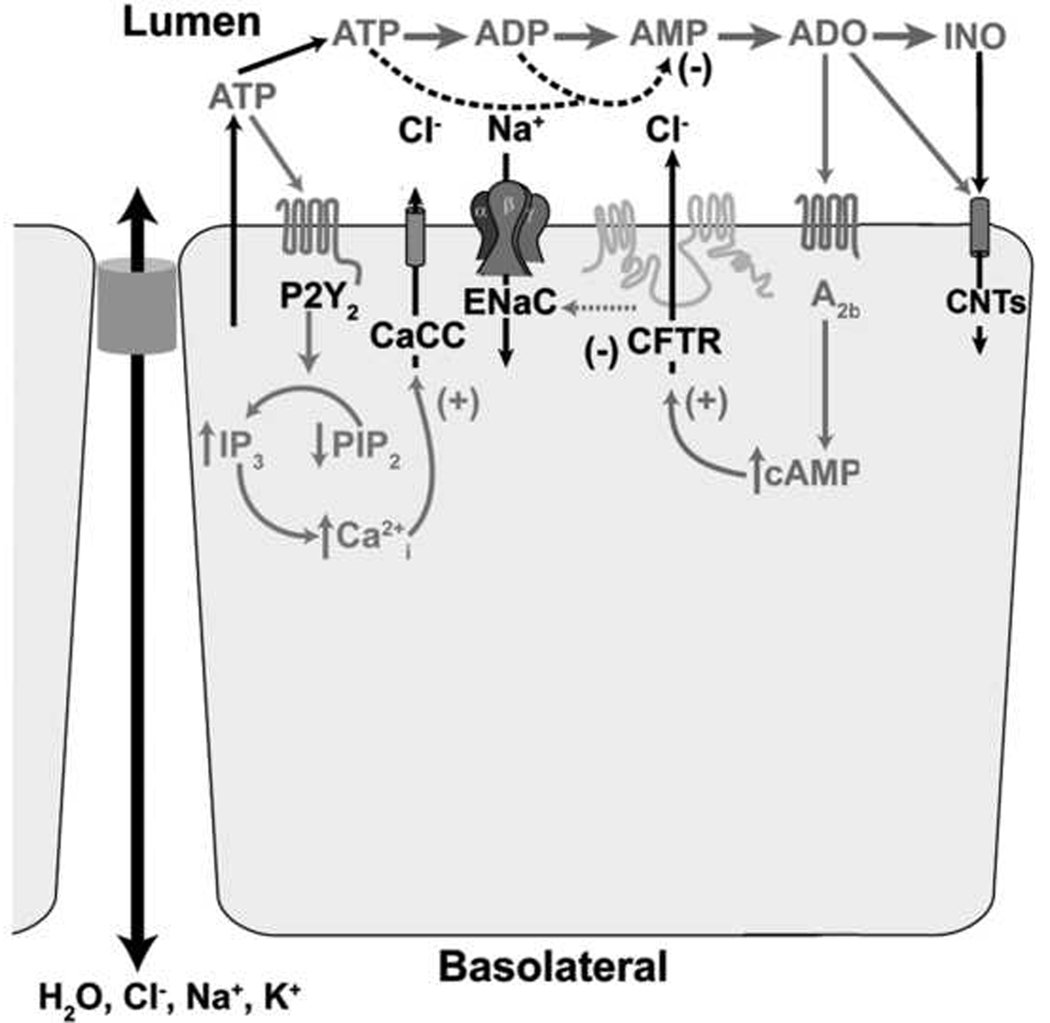
Fig. 2. Regulation of airway ion transport processes by extracellular nucleotides and nucleosides.
Autocrine/paracrine actions of nucleotides in the airways epithelial cells. ATP release and metabolism to adenosine (ADO) promotes activation of CaCC and CFTR channels and inhibition of ENaC via activation of P2Y2 receptors (ATP) and A2b receptors.
Activation of these receptors by ATP, UTP, or the hydrolysis-resistant analogue ATP-γS, results in the activation of a heterotrimeric G protein (Gq)that activates the production of the IP3 via phospholipase C. Activation of IP3 receptors in the endoplasmic reticulum (ER) results in the subsequent intracellular calcium (Ca2+i) mobilization. As a result, this increase in [Ca2+i] results in the stimulation of Cl− secretion by the activation of the alternate Ca2+-activated Cl− channel (CaCC) which is present in the apical membrane of human airway epithelia (Paradiso et al., 2001). Furthermore, activation of P2Y2 receptors by ATP can also result in the initiation of Cl− secretion via activation of CFTR by a PKC-dependent mechanism (Chang et al., 1993; Jia et al., 1997). In contrast, ENaC has been shown to be inactivated by purines (Devor et al., 1999), likely via depletion of PIP2, rather than by direct actions of IP3/Ca2+ (Yue et al., 2002; Kunzelmann et al., 2005). The net effect of these processes is the stimulation of fluid secretion in the airways, hydrating both the PCL and the overlying mucus layer, facilitating its clearance. It should be pointed out, as discussed below, that the increase in Ca2+i by activation of P2Y2 receptors can modulate the activity of other cellular processes in addition to changes in ion transport, including activation of mucin secretion (Lethem et al., 1993) and increasing the rate of ciliary beating (Yoshitsugu et al., 1993; Winters et al., 2007). Importantly, studies have demonstrated that the net result of this activation by exogenous luminal ATP is an increased mucus clearance rates (Bennett et al., 2001).
In addition to ATP, ADO has been shown to be a potent regulator of ASL hydration status. Studies have shown that addition of exogenous ADO to the lumens of human airway epithelia produces large effects on ion transport (Lazarowski et al., 1992). In the airway lumen, ADO is formed as a metabolite of the released ATP by specific extracellular enzymes. The predominate adenosine receptor in the apical membrane of the superficial airway epithelial cell is the A2b purinoceptor (Lazarowski et al., 1992; Clancy et al., 1999). Like ATP, this receptor activates a heterotrimeric G protein (Gs) that activates the production of intercellular cAMP via adenylate cyclase. In airway epithelia, ADO-mediated activation of the A2b purinoceptor produces coordinate regulatory effects on ion transport, including the inhibition of Na+ absorption by inhibiting ENaC (Stutts et al., 1995; Devor et al., 2000) and activation of Cl− secretion via activation of CFTR (Mason et al., 1991; Clarke et al., 1992), required for ASL volume homeostasis. The role of ADO in regulating ASL hydration is emphasized by studies which showed that removal of extracellular ADO, or inhibition of the A2b purinoceptor, resulted in a failure of the airway epithelia to regulate ASL height to levels sufficient for cilia beating, resulting in cessation of mucus clearance in human airway epithelial cultures (Lazarowski et al., 2004; Tarran et al., 2005).
The importance of nucleotides and nucleosides in the ASL is underscored the tight regulation of their concentrations via the action of a series of ecto-nucleotidases and transporters on the cell surface. Once ATP is released on epithelial surfaces, it is rapidly metabolized by a complex extracellular (ecto) enzyme system that can dephosphorylate and transphosphorylate purine and pyrimidine molecules (Picher et al., 2000; Picher et al., 2003; Davis et al., 2006). Members of several ectonucleotidase families have been identified on the surface of human airway epithelia by RNAse protection assays. These include nucleoside triphosphate diphosphohydrolases [NTPDases], nucleotide pyrophosphatases/phosphodiesterases [NPPs], non-specific alkaline phosphatase [AP], ecto adenylate kinase [ecto AK] (Picher et al., 2003), and nucleoside diphosphate kinase [NDPK] (Lazarowski et al., 1997). Based on their substrate affinities, the likely candidates for the regulation of ASL ATP levels are NTPDase1, NPPs, and ecto AK (Picher et al., 2000). Adenosine in the ASL is produced from AMP by ecto 5’-nucleotidase and alkaline phosphatase (Picher et al., 2000), and converted to inosine by. Finally, adenosine is converted to inosine by adenosine deaminase (ADA) and both are scavenged from the airway surface by concentrative nucleoside transporters [CNTs] (Hirsh et al., 2007). Both the basal extracellular nucleotide/nucleoside concentrations and importantly, the magnitude of the response of the epithelium to stimulated release of nucleotides, will depend on the enzymes that regulate ATP levels under these conditions. The net activities of these three processes, i.e., ATP release, metabolism, and uptake, all co-linear processes and interrelated, determine the net nucleotide and nucleoside “mass” on airway surfaces.
A simplified schema describing the elements that participate in acute and chronic ASL volume regulation is shown in Fig. 2. A general feature of the system is its complexity, with multiple redundant and interacting pathways. These pathways include: 1) ATP/UTP release pathways, as discussed later in this review, are so poorly defined mechanistically that it is depict as a simple arrow; 2) multiple enzymes on the extracellular surface that dephosphorylate, transphosphorylate, rephosphorylate, and ultimately recycle the released nucleotides; 3) at least three apical membrane purinoceptors that detect nucleotides and nucleosides in ASL, including P2Y2 and P2Y6 receptors, that sense ATP/UTP and UDP, respectively, and couple through Gq-PLCβ and the A2b receptor that senses adenosine and couples through Gs-adenylate cyclase; and 4) the apical membrane ion channels, including CFTR, ENaC, and CaCC, which regulate the ASL hydration state of the airways.
4. Stress-stimulated ATP release
As discussed throughout this issue, the lung is a unique organ in that it is subjected to complex physical forces during breathing, coughing and vascular perfusion, which all likely contribute to the regulation of lung function (Schumacker, 2002). Of import to this review, the cells lining the airway surfaces are subjected to oscillatory stresses during tidal respiration. It has long been appreciated that oscillatory motion of the lungs represents a key feature of pulmonary health, as demonstrated by the observation that normal breathing, coupled with intermittent large volume breaths (sighs), is important for maintaining alveolar health and patency (Dietl et al., 2001). Studies also suggest that this oscillatory motion is important in lung development (Moessinger et al., 1990; Liu et al., 1999), as evidenced by breathing movements observed as early as 10 weeks gestation in the human fetus (Kitterman, 1996). However, in extreme conditions, such as positive pressure mechanical ventilation or during severe bronchoconstriction, cells in the lung are compressed and/or stretched to supranormal values, leading to pathological outcomes (Bramley et al., 1994; Tschumperlin et al., 2000).
By virtue of their interfacial position in the lungs, the epithelial cells lining the airway are ideally situated to act as sensors and modulators of breathing-dependent changes in mechanical stress (such as airflow, stretching, and pressure). Despite clear evidence that nucleotides are important regulators of airway surface hydration and ultimately MCC rates, information regarding the tonic release of such nucleotides in airway cells has been elusive. The role of mechanical forces in pulmonary health raises the possibility of relationships between the forces generated by tidal breathing, ASL hydration, and the rates of mucociliary clearance. As a mechanism to link these functions, emerging evidence suggests that the mass of nucleotides and nucleosides on airway surfaces is regulated by the rate of ATP release, triggered by breathing-induced mechanical stress. Consistent with this thesis are previous studies which have reported that ATP is released from human airway epithelia subjected to physical forces. This includes forces generated by mechanical deformation (Kallok et al., 1983; Grygorczyk et al., 1997; Homolya et al., 2000; Knight et al., 2002), fluid shear stress (Grierson et al., 1995; Guyot et al., 2002; Lazarowski et al., 2004; Tarran et al., 2005), compression/stretch (Basser et al., 1989; Button et al., 2007), osmotic shock (Mitchell et al., 1998; Okada et al., 2006), or by simply changing of the media of cultured cells (Lazarowski et al., 2000).
Two key forces on the airway epithelial surfaces relevant to tidal breathing are airflow-induced shear stress and transepithelial pressure gradients. During normal breathing, airflow across the surface of the airway epithelium produces a wall shear stress. This physical stress occurs during inspiration and, in the opposite direction, during expiration. Interestingly, it has been shown that airway wall shear stress varies little with airway generation in the lung (Fredberg et al., 1978; Tarran et al., 2005), on the order of 0.45 dynes·cm2. This constancy occurs because as linear airflow velocities decrease dramatically in the deeper lung, airway diameters also decrease proportionately. Thus, the shear rate, which is roughly the velocity divided by the diameter, varies little and wall shear stress is nearly conserved across all diameters of airway. However, in the nasal cavity, wall shear stresses during normal breathing have been estimated to reach as high as 3 dynes·cm2, a value that is on the order of the wall shear stress exerted on the endothelia cells of large arteries (Elad et al., 2006).
Another mechanical stress that is exerted on the airway epithelia during normal respiration are the trans-airway pressure gradients. During each cycle of breathing, the pressure gradient fluctuates below and above atmospheric pressure. Commonly, during expiration a transmural pressure gradient of ~8.5 cmH2O is generated (Levitzky, 1991). During forced expiration, such as during exercise, transmural pressure gradients generated within the airways can approach ~20 cmH2O in the proximal airways. As discussed below, these forces are also generated during pathological conditions, such as the pressures generated on the airways generated by bronchoconstriction associated with asthma (Ressler et al., 2000).
Mechanical stresses on airways are even more extreme during cough (Leith, 1985). In the initial, “compression phase” of a cough, the combination of closure of the glottis with the rapid onset expiratory muscle activity produces very rapid changes (600–1600 cmH2O·s−1) in subglottic transpulmonary pressures, reaching sustained pressures as high as 200 cmH2O. During the expiration phase of coughing, this pressure abruptly falls, producing peak air flow rates of greater than 500 l·min−1 (Leith, 1985). Theoretical modeling of cough suggests that the level of wall surface shear stress under these conditions can reach as high as 1700 dynes·cm−2 (Basser et al., 1989).
5. Mechanical stress mechanotransduction and ATP release
While it is known that external mechanical stress represents a ubiquitous mechanism to stimulate ATP release, the underlying sensors and response mechanisms whereby mechanical shear stresses are recognized and transduced into cellular effects by airway epithelia remain poorly understood. However, force transduction in endothelia has been extensively studied, and it is possible to speculate about the nature of shear stress sensing in airways based on this research. For example, it is widely known that endothelial cells align their cytoskeletons in response to fluid shear stress (Chrzanowska-Wodnicka et al., 1992; Galbraith et al., 1998). These studies have shown that in vivo, the morphology of endothelial cells are randomly oriented in areas of low shear stress, but are elongated and aligned in the direction of blood flow in regions exposed to high levels of shear stress. The response to shear by cytoskeletal organization has been linked with the activation of several intracellular GTPases, such as Rho A, which activities are transduced into downstream cell signaling pathways (Dudek et al., 2001). A change in the activity of another GTPase, Rac-1, also occurs during shear stress resulting in the alteration of the expression of a number of key signal transduction proteins including NFkappaB (Tzima et al., 2002).
A number of ion channels have also been to shown to be mechanosensitive, including a class of stretch activated channels which are ubiquitously expressed in most cells (Hamill, 2006). Although little is known about these channels, it has been proposed that these channels are directly coupled to the cytoskeleton, where physical forces are transmitted to these channels resulting in an increase in channel open probability and cation influx (reviewed in Sackin [1995]) leading to activation of downstream signaling pathways. Recently, several types of Transient receptor potential (TRP) channels from multiple subfamilies have been suggested to act as mechanosensitive Ca2+ channels in a wide variety of cell types and organisms (Clapham, 2003). The epithelial sodium channel (ENaC) has also been suggested to be shear-sensitive in renal epithelia (Benos, 2004). However, it should be noted that these reports demonstrate that ENaC is commonly activated by shear stress, which is the opposite of the effects of shear stress on ENaC-activation reported in airway epithelia (Tarran et al., 2005). While the mechanism of mechanosensitivity of all of these channels are not known, and likely vary between different channels, it is likely that they include changes in the tension changes in the actin cytoskeleton, changes in the curvature of the lipid bilayer surrounding the ion channel, or signaling events such as protein phosphorylation/ dephosphorylation and lipid metabolism.
Finally, primary cilia have been also shown to act as flow sensors in renal epithelia where direct mechanical force applied to these cilia results in Ca2+ influx (Praetorius et al., 2001; Praetorius et al., 2003). However, while it cannot exclude the possibility that primary cilia may transduce shear stress in the airways, any primary cilia in airway epithelia likely involve well-differentiated, ciliated, cells and would be surrounded by continuously beating motile cilia, which could likely obfuscate the shear signals generated during tidal breathing. Similarly, it has been demonstrated that endothelial cells from rats require an intact glycocalyx for stress-mediated cytoskeletal reorganization (Thi et al., 2004). However, the glycocalyx in airway epithelia is complex, with components on both the cell surface and the cilia, which would, again, make transmission of the shear signal complex in the well-ciliated airway epithelia.
Little is also known about the mechanisms and pathways involved in release of nucleotides under both basal and stimulated conditions. Two broad models for epithelial ATP release have been proposed. In one model, intracellular ATP is released by ATP-permeable channels (Braunstein et al., 2001; Schwiebert, 2001; Okada et al., 2004), as suggested by both patch-clamp (Strange et al., 1996) and atomic-force microscopy (Schneider et al., 1999) studies. As the cytoplasm contains millimolar concentrations of ATP, this is a realistic possibility. For epithelia and other cells subjected to mechanical stress, it was also proposed that other members of the ATP-binding cassette protein family could transport ATP directly (reviewed in Schwiebert [1999]). However, whether one of these family members, CFTR, contributes to ATP release remains controversial. It has been suggested that CFTR can either conduct ATP directly (Reisin et al., 1994; Schwiebert et al., 1995; Pasyk et al., 1997) or facilitate its release by regulating a separate ATP release pathway (Sugita et al., 1998; Braunstein et al., 2001). However, other studies (Reddy et al., 1996; Grygorczyk et al., 1997; Watt et al., 1998), including our own (Button et al., 2007), have failed to find a link between CFTR and ATP release.
The second model for ATP release involves rapid exocytosis of ATP-containing vesicles. Such a pathway is analogous to the exocytic pathways seen in endothelial and chromaffin cells (Bankston et al., 1996). Evidence for this mode of ATP release has recently been provided in endothelia (Bodin et al., 2001), biliary epithelia (Gatof et al., 2004) and bladder epithelia (Knight et al., 2002). In these cases, ATP release was prevented by monensin, an inhibitor of post-golgi vesicular formation, and by brefeldin A, which inhibits transport from the endoplasmic reticulum to the Golgi apparatus. Boudreault and Grygorczyk (2004) reported that lowering the temperature of A549 lung epithelial cells to 10°C nearly abolished cell swelling-induced ATP release. Finally, it has been recently reported that ATP release in Calu-3 cells, an airway epithelial cell line, is via an exocytotic pathway (Kreda et al., 2007). Interestingly, they demonstrated that in these cells, which poses goblet cell-like mucin granules, stimulation of ATP release, by increasing intracellular calcium with ionomycin, was directly coordinated with mucin secretion, suggesting that ATP and mucus can be exocytosed from the same vesicle. Such coordinated co-release of ATP and mucins imply that mucin-secreting cells, such as airway goblet cells, can generate the signals to initiate fluid secretion, and thus mucin hydration. This observation provides a mechanism for effective mucin dispersion, i.e. well hydrated mucins, in the ASL upon appropriate stimulation.
6. Techniques to produce oscillatory mechanical stress in airway epithelia
While it has been shown that ATP release represents a common response to a wide array physical stimulation, a key issue has been to measure the effect of stresses in the physiologically-relevant range, generated by stresses similar to those exerted on airway epithelial cells during normal breathing. To this end, our laboratory has designed two approaches to deliver mechanical stress to airway epithelial surfaces in a quantitative/controllable manner, mimicking forces exerted on the airway epithelia during normal breathing and test their effect on key regulators of mucus clearance, including ATP release, ASL hydration state, and cilia beat frequency. In the first approach, a system has been developed to subject human airway epithelial cultures to oscillatory rotational shear stress mimicking the inhale and exhale airflow-induced shear stress observed in vivo. This device, shown in Fig. 3A, accelerates/decelerates human airway epithelial cultures inside a highly humidified incubator to generate “thin-film” liquid flow over airway epithelial surfaces whilst avoiding airflow-induced dehydration of the ASL (Tarran et al., 2005). The change in relative velocities of ASL and the underlying epithelium caused by this oscillatory motion generates a shear stress over the apical surface with profiles similar to airflow-induced shear stress generated during tidal breathing (Tarran et al., 2005; Elad et al., 2006; Even-Tzur et al., 2006). The acceleration rate is controlled so that the magnitude of shear stress at the epithelial surface can be varied over a wide range of shear stress (0.01 to 10 dynes·cm−2) at 28 cycles per minute (CPM), a rate approximating the combined number of both inhalations and exhalations in one minute.
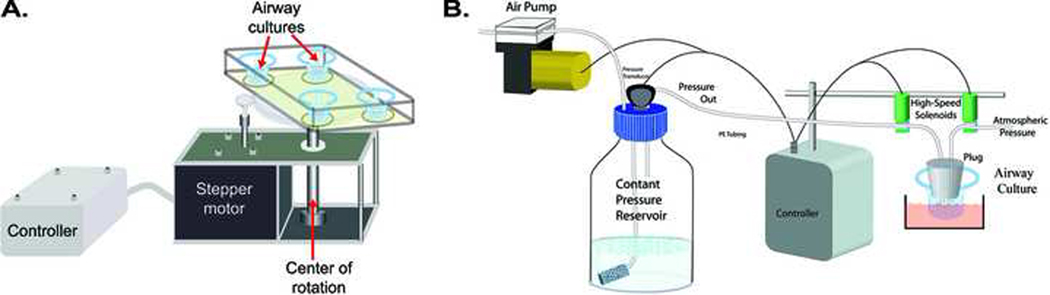
Fig. 3. Approaches used to deliver mechanical stress to airway epithelial surfaces in a quantitative/controllable manner.
(A) Shear stress device. To generate shear stress, 4 cultures were rotated in a stop-go fashion for various times inside a highly humidified incubator. The device controlled via a stepper motor connected to a microprocessor based controller. Shear stress, over the range from .005 to 10 dynes·cm−2 could be produced by varying the rate of acceleration of the stepper motor (Tarran et al., 2005). (B) System used to produce cyclic compressive stress (CCS) on human airway cultures. Here, a microprocessor controlled the pressure amplitude and timing of the transepithelial pressure to the apical surface of the cultured human airway epithelial cells using high-speed solenoids (Button et al., 2007). Pressure could be delivered under oscillatory or static (non-oscillatory) conditions over a range of pressures (1 to 100 cmH2O).
In a second approach, our laboratory has developed a device to deliver cyclic compressive stress (CCS) mimicking the oscillatory transmural airway pressures of tidal breathing (Levitzky, 1991), by cyclically applying apical-to-basolateral transepithelial pressure gradients (Button et al., 2007). This system, Fig. 3B, is capable of delivering compressive stress to cultures over a wide range of pressure (0 to 100 cmH2O) over a range of frequencies and duty cycles (Button et al., 2007). In this system, a novel, computer-controlled device, is used to impart oscillatory positive transepithelial pressures at frequencies consistent with normal tidal breathing, to well-differentiated human airway epithelial cultures. While this system does not recapitulate the substantial elongation/stretching of the epithelium manifested in vivo during tidal breathing, the cell compression utilized in this system does expose cells to transepithelial pressure gradients generated during tidal breathing in vivo. In the experiments described below, cyclic compressive stress was administered by subjecting cultures to a 3-second cycle composed of 2 sec at atmospheric pressure (0 cmH2O transepithelial) and 1 sec at a given positive pressure, at a frequency of 20 cycles CPM.
7. Human airway cultures under “thin-film” conditions
The culture of primary airway epithelial cells at air liquid interface on porous culture substrates has become the gold-standard technique for study human and animal airway epithelia. These cultures recapitulate the mixed mucus-secretory and ciliated surface phenotype expressed in vivo (Matsui et al., 1998), and has been extraordinarily useful in elucidating many links between ion transport regulation and ASL volume homeostasis (Matsui et al., 1998; Tarran et al., 2001; Button et al., 2007). The advantage of this system is that it utilizes well-differentiated human bronchial epithelial cultures maintained under air-liquid interface conditions. Specifically, these culture produces the two-phase mucus transport system on its surface, and, via the coordinated beating of cilia, maintains rotational mucus transport within cell culture dishes that approximates rates observed in vivo (Matsui et al., 1998). The PCL and mucus layers can be labeled so that measurements of ASL hydration state and mucus transport rates can be made with confocal microscopy and epifluorescence microscopy, respectively (Matsui et al., 1998).
The established body of literature regarding airway ion transport is largely based on experiments performed with large volumes of Ringer/saline bathing epithelial mucosal surfaces. Under these conditions, endogenously-produced ASL and its constituents (such as ATP) are grossly diluted and may even be washed away entirely. Thus, signaling molecules that are secreted into the ASL are lost, which will alter epithelial ion transport rates (Tarran et al., 2006). Therefore, a culture system that allows the epithelium to generate and maintain an endogenous “thin film” of ASL on airway surfaces has been instrumental in studying the effect of oscillatory stress on ATP, ASL hydration, and mucociliary clearance homeostasis.
8. Regulation of ASL volume and mucus clearance by stress-mediated ATP release
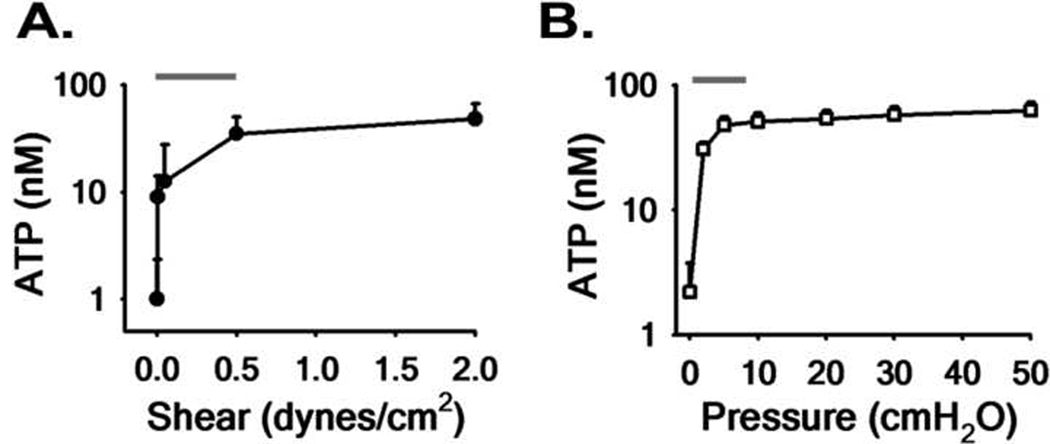
The effect of physiologically relevant levels of mechanical stress on stimulating ATP release by human airway epithelial cell cultures has been investigated during both oscillatory shear (Tarran et al., 2005) and compressive stress (Button et al., 2007). In these studies, well-differentiated human airway epithelial cultures under physiological air-liquid interface conditions, were subjected to a range of oscillatory shear and compressive stresses for 30 min prior to quantitation of apical [ATP], using a luciferin–luciferase bioluminescence based approach (Lazarowski et al., 2004). The data summarizing this work is shown in Fig. 4. These studies showed that ATP release from human airway epithelial cells was highly sensitive to small changes in both oscillatory shear stress (Fig. 4A) and compressive stress (Fig. 4B). The unexpected finding was that ATP release in both these maneuvers was most sensitive in the relevant physiological range of stress amplitudes (up to 0.5 dynes·cm2 for shear stress and 8.5 cmH2O for compressive stress). At higher amplitudes, the change in [ATP]/Δ unit stress was less steep, suggesting that ATP release is “tuned” for maximal ATP release over the physiological range of mechanical stresses.
Fig. 4. Release of ATP during physiological stress.
Relationship between magnitude of oscillatory shear stress (A) and compressive stress (B) vs. apical ATP concentration at steady-state (at 30 min). Lines above denote the physiological range of shear (Tarran et al., 2005) and compressive stress (Button et al., 2007) during tidal breathing (see text).
In these experiments, the “mass” of nucleotides and nucleosides on airway surfaces is directly proportional to distribution of released nucleotide species, metabolism, and uptake. Despite the rapid metabolism of ATP by ecto-enzymes, sufficient ATP is released to increase ASL ATP concentrations under both shear and compressive stress, reaching levels that are predicted to be sufficient to induce Cl− secretion via P2Y2 receptor-mediated ion channel activation (Tarran et al., 2005). It should be noted that because intracellular ATP levels are on the order of several millimolar, sufficient ATP is present to allow for constant release during oscillatory stress without drastically changing intracellular ATP levels, as extracellular ATP is activating purinoceptors at ~1000-fold less in concentration.
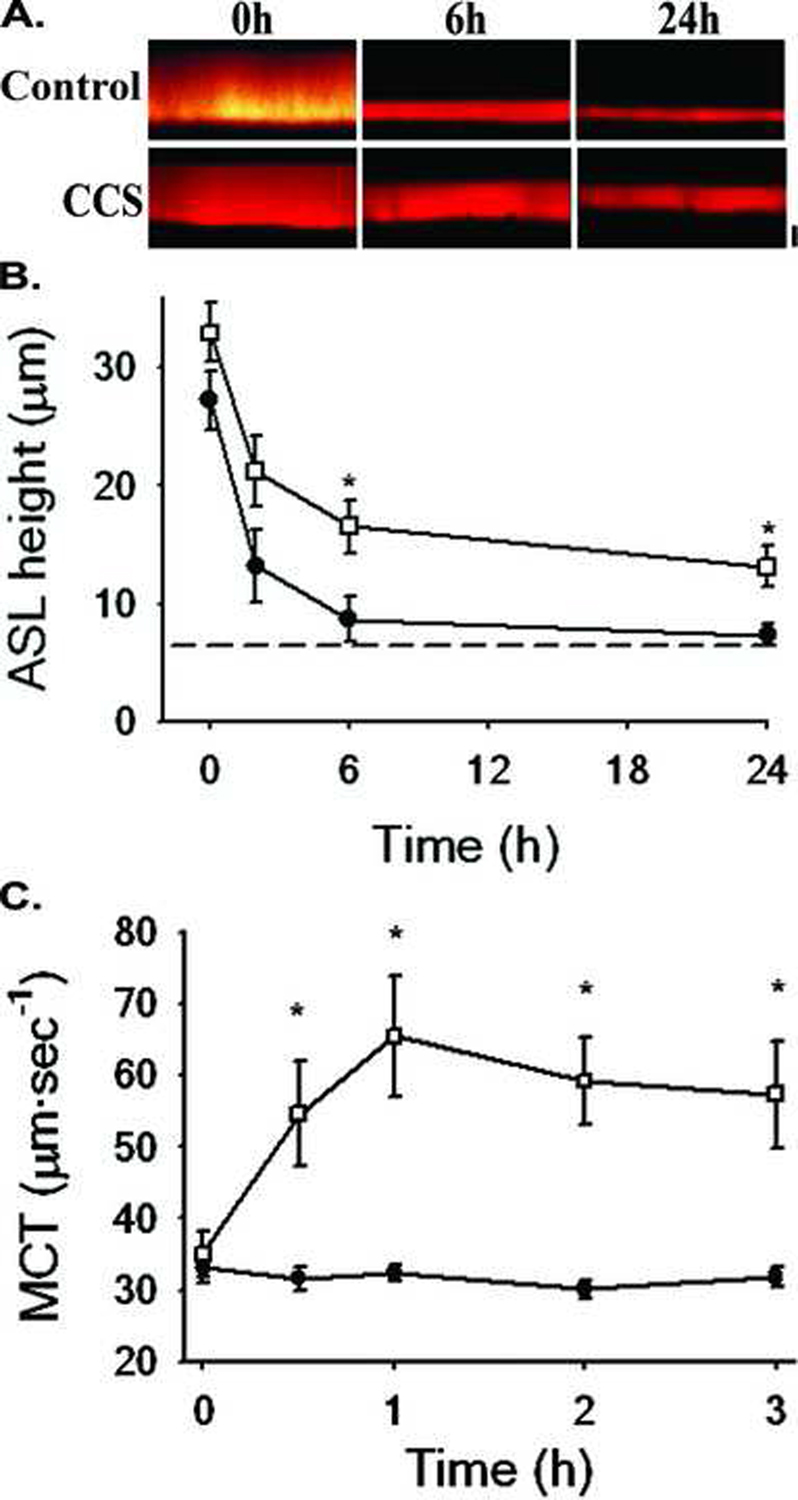
To investigate the effect of stress-mediated ATP release on activation of apical purinoceptors and ion transport mechanisms, the effect of oscillatory stress on ASL volume homeostasis was assessed. In these studies, the ASL volume, i.e. the amount of fluid on the surface of the epithelium, was measured in human airway epithelial cultures using XZ confocal microscopy with a cell-impermeant fluorescent probe (Texas Red-dextran, 10k MW) as a marker of the ASL fluid (Matsui et al., 1998). Figs. 5a&b summarizes the results of experiments performed in normal human airway epithelial cultures to assess the regulation of ASL height under control and oscillatory compressive stress (CCS) conditions. Typically, these experiments are initiated by adding a small volume of “excess” fluid, containing Texas Red-dextran, to the airway surface (Fig. 5A) and evaluating how the epithelium regulates ASL volume over time in response to this challenge (Fig. 5B). In airway epithelium under control conditions, the added liquid is absorbed until a steady state height of 7 µm is achieved. Under these experimental conditions, this 7-µm layer represents the fully hydrated PCL environment in which cilia are able to fully extend and beat (Matsui, 1998).
Fig. 5. Oscillatory stress stimulates ASL hydration and mucociliary transport rates.
(A) Representative XZ confocal images of ASL at 0, 6, and 48 h after mucosal addition of 30 µl of “excess” fluid containing Texas-red dextran to the apical side of normal human airway epithelial cultures under control conditions and undergoing CCS (20 cmH2O, 20 CPM). Bar=10µm. (B) Time-course of changes in ASL height after fluid absorption under control (●) or CCS (□, 20 cmH2O, 20 CPM) conditions. Note: dotted line denotes the length of the outstretched cilia. (C) Mucociliary transport rates before (T=0) and during control (●) or CCS (□, 20 cmH2O, 20 CPM) conditions. In these experiments, MCT rate was determined by the movement of 1 µm florescent beads added to cultures as previously described (Matsui et al., 1998).
ATP and adenosine measurements in ASL, under static conditions, revealed ATP levels below those expected to activate P2Y2 receptors (< 1 nM), whereas adenosine was in the concentration range expected to activate A2b receptors (~ 200 nM), sufficient to activate CFTR (Tarran et al., 2005). This was verified (Tarran et al., 2005) in experiments where addition of exogenous adenosine deaminase, which metabolically removes adenosine (Zimmermann, 2000), or 8-SPT to inhibit A2b purinoceptors, resulted in unrestrained Na+ absorption and ASL collapse. Together, these results suggest that under static conditions, ATP released onto the airway surface, estimated at a rate ~300–400 fmoles/cm2/min (Lazarowski et al., 2000; Button et al., 2007), is predominantly converted enzymatically into adenosine, which stimulates the A2b receptor, in turn activating CFTR, which serves as both a Cl− secretory channel and an inhibitor of ENaC under steady-state conditions. However, the addition of excess fluid to the surface of the epithelium dilutes the basally released ATP and metabolically-produced ADO. Here, A2b is inactivated, resulting in the inactivation of CFTR and activation of ENaC, resulting in absorption of the excess fluid. As the ASL reaches ~7 µm, ATP/ADO levels reach levels that will terminate the absorption of fluid. The net effect of this system is to balance absorption and secretion to maintain an adequate volume of liquid for efficient mucus transport on the airway surface.
In studies of human airway epithelial cultures under oscillatory compressive stress, steady state ASL height in response to addition of the excess fluid was significantly increased (to ~15 µm) compared to control cultures (~7 µm) (Fig. 5B). The relative importance of ATP and adenosine signaling to the alteration of ASL volume under oscillatory stress conditions was revealed by the inhibition of this increase in ASL volume in response to pre-treating cultures with enzymes that cleave ATP (apyrase) and ADO (adenosine deaminase) (Button et al., 2007). It has also been shown that under oscillatory motion conditions, 8-SPT, an inhibitor of A2b purinoceptors (Hirsh et al., 2007), significantly reduces ASL height, suggesting that ADO is the most important regulator of ASL homeostasis via A2b-mediated activation of CFTR activity, and thus ASL hydration (Tarran et al., 2005). However, these studies also showed that addition of apyrase, to prevent ATP accumulation, in the presence of 8-SPT further reduced ASL height, suggesting that CaCC-mediated fluid secretion was also active under oscillatory stress. In keeping with this schema, they showed that addition of the specific CFTR antagonist CFTRinh-172 (Thiagarajah et al., 2004) greatly reduced ASL height, with the remainder of ASL height being inhibited by DIDS, to inhibit CaCC. Nevertheless, mechanistically, the change in ASL height with increased mechanical stress reflects the increased concentration of both ATP and ADO in the ASL, which, via P2Y2 and A2b receptor-dependent mechanisms, inhibited Na+ absorption, and activated CFTR- and CaCC-mediated Cl− secretion.
A key finding of these studies was that oscillatory mechanical stress rebalanced ASL volume at higher plateaus in human epithelial cultures, compared to static controls. Measurement of ASL volume homeostasis revealed that the initial absorption rate of the added liquid was reduced during oscillatory stress and was followed by a plateau level that was significantly higher than controls. This finding emphasizes that there is no fixed set point for ASL volume, rather that the final ASL volume in the presence of a fixed rate of ATP release/metabolism/uptake will vary with ion channel density, e.g., CFTR and ENaC, and purinoceptor density. It is further speculated that this capacity allows for normal airway epithelium to respond to airway stresses with differing levels of ASL hydration state. It is worth noting, however, that these experiments were performed in the absence of mucus. In vivo, we speculate that ASL in excess of the ~7 µm would be transferred to the overlaying mucus layer, resulting in the increased hydration state of the mucus and accelerated clearance.
A key component of mucociliary clearance is the coordinated activity of cilia beating within the well-hydrated PCL-gel layer. As discussed in detail in the section by Dr. William Davis, there are a number of intracellular signaling mediators that regulate cilia beat frequency (CBF). These include: cAMP, cGMP, nitric oxide, and Ca2+ mobilization (Satir et al., 1990; Salathe et al., 2000). In addition, extracellular ATP has been shown to be the most potent Ca2+-dependent stimulators of cilia activity (Evans et al., 1999; Lansley et al., 1999; Zhang et al., 2003). In additional to chemical stimulation of CBF, cilia beating has also been shown to be stimulated during physical mechanical perturbations (Lansley et al., 1999). Physiologically-relevant oscillatory compressive stress was investigated to test whether oscillatory compressive stress-mediated ATP release results in the stimulation of the rate of cilia beating in human airway epithelia cultures (Button et al., 2007). Measurements of ciliary beat in normal cultures by high-speed phase-contrast video microscopy indicated that oscillatory stress significantly increased CBF by ~50%, compared to static control cultures (Button et al., 2007). The agonist mediating oscillatory stress-induced increases in CBF could be ATP itself or its metabolic product adenosine, which has also been shown to increase CBF on human airway epithelia through A2b receptors (Morse et al., 2001) by a cAMP-independent signaling pathway (Zhang et al., 2003). Pre-treatment of human airway epithelial cultures with apyrase, to degrade released ATP completely inhibited the oscillatory compressive stress-induced stimulation of cilia beating activity (Button et al., 2007), suggesting that ATP-mediated purinoceptor activation represents the predominant mechanism stimulating CBF on mechanically-stimulated airway epithelia.
As noted above, the rate of mucus clearance in the airways directly depends on both the beat frequency of the cilia and the hydration status of the PCL and overlying mucus layer, via alterations in viscoelasticity. Because results of experiments imposing mechanical stress on human airway epithelial cells demonstrated that oscillatory stress stimulated net fluid secretion and cilia beat frequency, the impact of these mechanical stresses on mucociliary transport (MCT) rates were also assessed (Button et al., 2007). As described above, the human airway epithelial cultures utilized by our laboratory display rotational displacement of mucus, as evidenced by the coordinated movement of fluorescent microspheres in the ASL (Matsui et al., 1998; Button et al., 2007). In fact, normal cultures can maintain this transport for many days, making them useful for long-term study of mucociliary transport processes. In these studies, MCT rate was measured after 1h of continuous oscillatory compressive stress. In cultures undergoing oscillatory stress (Fig. 5C), the angular velocity of fluorescent microspheres, as a measure of their MCT rates, doubled (from 32.4 ± 1.1 to 65.4 ± 8.3 µm·sec−1) compared to control (atmospheric) conditions. Together, these results demonstrate that physiologically-relevant oscillatory stress can stimulate mucus clearance rates via an extracellular ATP-dependant process, and provide a mechanism for the airway epithelium to stimulate clearance of mucus by increasing the rate of ATP release, CBF, and MCC.
9. Differential effects of oscillatory versus non-oscillatory stress
In addition to oscillatory forces associated with tidal breathing, airway epithelia can be subjected to continuous, non-oscillatory, transmural pressures (Tschumperlin et al., 2006). One example is seen in the folding of the epithelial lining of the airway wall is a well-recognized process (Lambert et al., 1994) where bending of the epithelium and its substrate can give rise to long-term local shear deformations and pressure gradients (Wiggs et al., 1997). For example, during an asthmatic exacerbation, constriction of smooth muscles in the airway wall causes the overlying airway epithelium to fold into crevasses, producing continuous, non-oscillatory, positive transepithelial pressure on the superficial airway epithelia (Gunst et al., 1988; Ressler et al., 2000). More importantly, the magnitude of the resulting positive pressure exerted on airway epithelia estimated to be on the order of ~30 cmH2O (Ressler et al., 2000), which is significantly higher than pressure exerted during normal tidal breathing (~8.5 cmH2O) (Levitzky, 1991). Studies have demonstrated that such forces can result in physiological changes to the airway epithelia, including magnitude dependent changes in gene expression and signaling through ERK phosphorylation in both primary human bronchial epithelial cells and rat tracheal epithelia cells (Ressler et al., 2000; Tschumperlin et al., 2002).
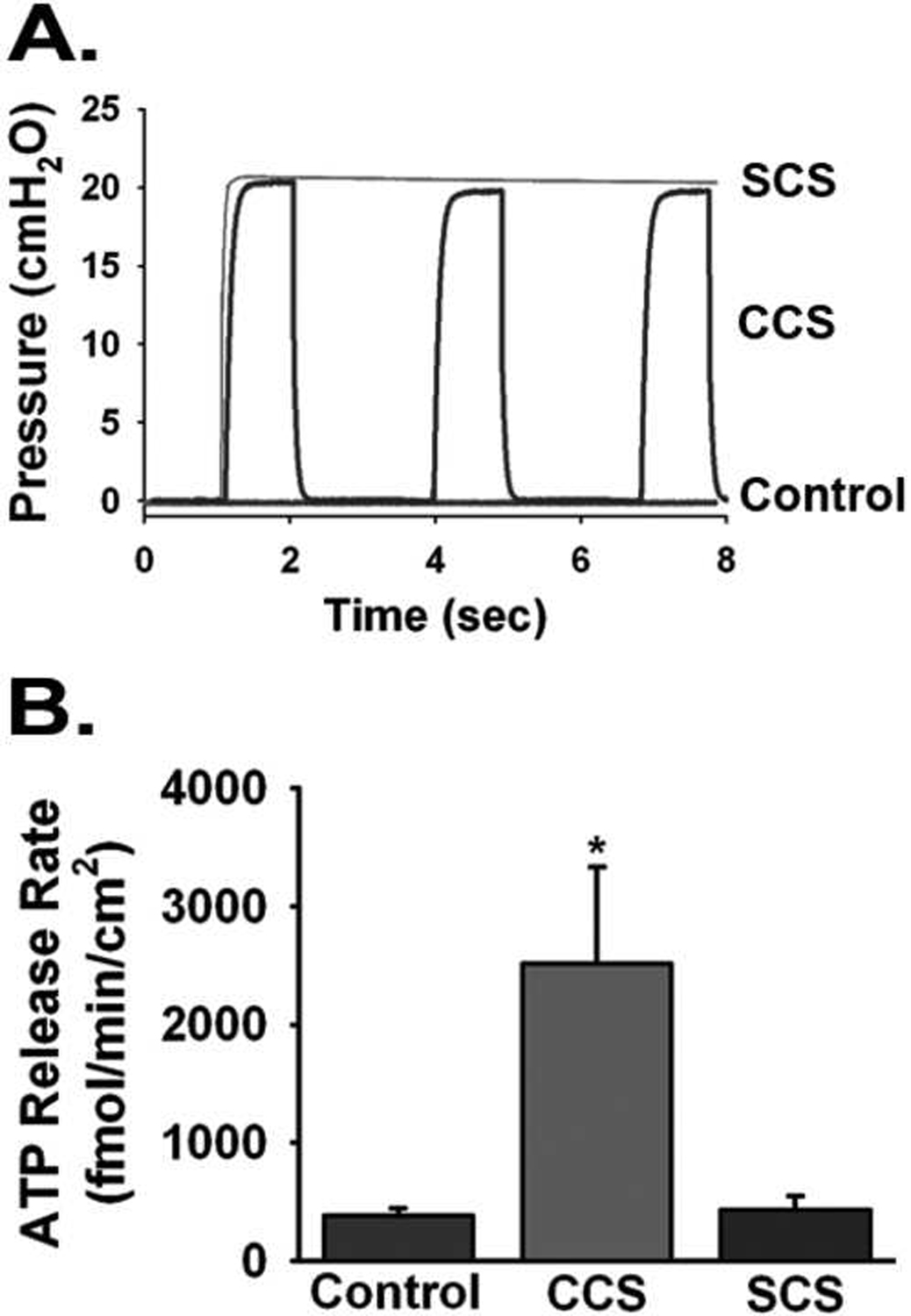
Experiments were performed to determine whether the non-oscillatory mechanical stress is capable of stimulation of nucleotide release. In these experiments, human airway epithelial cultures were subjected to a non-oscillatory, or static compressive stress (SCS), produced by applying a constant pressure of 20 cmH2O (Fig. 6A), mimicking the constant stress and pressure, experienced during bronchoconstriction. Steady-state apical ATP release rates were determined after subjecting human airway epithelial cultures to control (atmospheric pressure), CCS, or SCS for 30 min. It was observed that compared to oscillatory stress, the ATP release rates in cultures under non-oscillatory stress was not significantly elevated compared to cultures maintained at atmospheric pressures (Fig. 6B). These results suggest that sustained, non-oscillatory, stresses associated with bronchoconstriction do not stimulate ATP release, compared to CCS. Consistent with the notion that ATP release is an absolute requirement for stimulation in ASL hydration status and MCC rates, it was shown (Button et al., 2007) that non-oscillatory stress failed to have an effect on steady-state ASL height or MCT rates in human airway epithelial cultures. Such results emphasize the importance of the oscillatory nature of mechanical stress to stimulate ATP release and purinoceptor-mediated regulation of ion channels supporting airway hydration and mucus clearance. While the mechanism behind the requirement for oscillatory stress on stimulating ATP release is unknown, it is possible that either the processes involved in sensing and/or transducing the stress can adapt to the presence of sustained stress over time, or that changes in ATP release is triggered by the rate of change in stress over time, rather than the presence of constant stress.
Fig. 6. Differential effects of cyclic and constant stress on ATP release.
(A) Example of pressure tracings from cultures exposed to atmospheric pressure (control), oscillatory compressive stress (CCS) @ 20 cmH2O/ 20 CPM, and non-oscillatory compressive stress (SCS) @ 20 cmH2O. (B) Comparison of ATP release rates in cultures undergoing CCS and SCS for 30 min. Rates were determined by measuring ASL [ATP] over 30 min in cultures pre-treated with a cocktail of ATPase inhibitors (Button et al., 2007).
While numerous studies, including as our own, demonstrate the importance of extracellular ATP as an autocrine/paracrine signaling molecule in a variety of cell processes, it remains logical that cells must be able to protect themselves from overstimulation by the mechanical environment. In the lung, the corollary is that the airways must protect themselves from “flooding” of the airways that might be expected if ATP release were over-stimulated. For example, Matalon and co-workers have illustrated that respiratory syncytial virus (RSV) caused alveolar flooding in mice, and this effect was mimicked by exogenous nucleotides (Davis et al., 2004). Subsequent studies revealed an elevation in ATP concentration in bronchoalveolar lavage (BAL) from RSV-infected mice, relative to control animals (Davis et al., 2006). Selective removal of endogenous nucleotides prevented RSV-induced alveolar flooding, suggesting that RSV actions were mediated by released nucleotides (Kunzelmann et al., 2004). Finally, Rich and co-workers showed that increased intratracheal pressure, during high-pressure mechanical ventilation, raised airway ATP levels and caused lung edema (Rich et al., 2003).
One mechanism by which airway epithelia can protect themselves from abnormal ATP levels is by desensitization of purinoceptors (Gonzalez et al., 2005). However, based on data from human airway cultures under shear stress or compressive stress, we predict that cells also protect themselves by directly limiting ATP release during non-physiological stresses. First, in experiments using oscillatory shear and compressive stress, ATP release rates were most sensitive over the physiological range of stresses (Fig. 4). However, past this range of stress, release rates are less sensitive to higher stresses applied (shear stress or transmural pressures). Secondly, as noted above, non-oscillatory, stress does not stimulate ATP release rate, as compared to oscillatory stress (Fig. 6). Further studies will be needed to not only delineate the mechanism behind this phenomenon, but to understand the physiological importance on lung health.
10. Therapeutic implications of oscillatory motion in the airways
In addition to the in vitro data, above, there are in vivo data that suggest that stress-stimulated released nucleotides regulate components of the mucociliary system, e.g., ion transport, and mucociliary clearance. For example, studies of chest wall oscillatory ventilation in dogs revealed that mucus clearance doubled when chest-wall pressure oscillations were administered (King et al., 1983; Rubin et al., 1989). However, the authors could provide no simple explanation as to why vibration of the airway wall should increase mucus clearance.
Using an inhaled radionuclide technique to measure mucus clearance/retention, Bennett and colleagues (1990 and 1994) studied potential "gas liquid" interactions with mucus clearance in normal human subjects,. In the initial portion of this study, normal subjects performed an artificial cough. Subjects inspired to approximately 80% of total lung capacity (TLC). They then generated transthoracic pressures of ~80 cmH2O against a closed solenoid valve, following which, the valve opened and the patients exhaled at a maximum flow rate that simulated that of a natural cough. The authors found that this maneuver doubled mucus clearance rates. As a control for gas liquid interactions being the explanation for this response, the authors inverted the direction of maximal flow by having patients exhale to RV, at this point the solenoid valve was closed, and the patients were asked to try to inhale. Following the generation of a large transthoracic pressure, the valve was opened and a high inspiratory flow rate (that approximated the simulated cough expiratory flow rate) was generated. Interestingly, despite the inward net vector for maximal air flow, mucus clearance rates still doubled. While the authors had no explanation for this phenomenon, one can speculate that it reflects the changes in mechanical force-induced ATP release that is common in both maneuvers applied in their protocols.
Numerous studies have also shown that regular vigorous aerobic exercise stimulates deep breathing and can promote mucus clearance, by effectively mobilize secretions. This has been shown to slow the progression of lung disease in patients with cystic fibrosis (Schneiderman-Walker et al., 2000; Bradley et al., 2002). It has further been observed that that ATP is present at measurable levels under resting conditions in vivo from human nasal secretions from normal and CF donors (Donaldson et al., 2000). More importantly, studies have shown that mechanical forces exerted during exercise inhibits Na+ transport (Hebestreit et al., 2001) and initiated Cl− secretion (Alsuwaidan et al., 1994), providing a potential for stimulated ATP release to account for amelioration of the severity of obstructive lung disease by exercise.
It has been demonstrated that CF patients who undergo chest physical therapy regularly typically display improved lung function as compared to CF subjects who choose not to follow such regimes (Moorcroft et al., 1998; Prasad et al., 2002). While the mechanism of action of chest physical therapy remains unknown, it is believed that the motion generated during such therapy loosens and physically detaches mucus from the airway lumen (Pryor, 1999). In addition to chest physical therapy, there are also a number of therapeutic devices in use clinically that have been shown to enhance airway clearance in patients with obstructive lung diseases (Langenderfer, 1998). A number of these devices work based on the use of positive expiratory pressure (PEP) with premise of positive intra-airway pressure to maintain open airways and enhance airway clearance (reviewed in Marks [2007]). These include PEP-mask and CPAP, which deliver a continuous positive airway pressure between 10–20 cmH2O, during normal breathing. There is also a number of oscillating PEP devices in clinical use that produce cyclic airway pressure at high-frequency. These devices are either active mechanical devices, such as Percussionair-IPV™, that deliver high-velocity percussive air inflow, or passive devices such as the Flutter™, which uses a steel ball vibrating inside a cone, causing high-frequency air flow oscillation. In addition to PEP airway devices, high frequency chest wall oscillation (HFCWO) jackets, such as the VEST™, have been used to facilitate mucus clearance in patients with obstructive lung disease (Kempainen et al., 2007).
An in vitro study of the viscoelastic properties of mucous gel simulants subjected to oscillating airflow suggests that the oscillating airflow that these devices produce acts as a physical "mucolytic," reducing the viscoelasticity of the simulants and enhancing mucus clearance (Tomkiewicz et al., 1994). Such oscillatory physiotherapy has been shown to have a mucolytic impact on bronchial secretions including breakdown of high-molecular-weight DNA and a decrease in mucus viscoelasticity in cystic fibrosis (CF) sputum samples (App et al., 1998). The net effect is the ability to mobilize accumulated mucus toward larger airways where they it can be better removed by cough and other forceful clearance maneuvers.
While suggestive, a direct link between exercise, physiotherapy, and the use of these airway clearance devices, and ATP-mediated changes in ion transport and ASL secretion has yet to be demonstrated. However, based on data from in vitro models, it is theorized that exercise, chest percussion and use of these therapeutic devices increase ATP release which in turn promotes Cl− and ASL secretion, and thus, mucus rehydration (Button et al., 2007). An increased understanding of purine-regulated ion transport may direct future therapies that couple physical therapy and pharmacotherapy, with the former employed to stimulate ATP release and the latter simultaneously applied to prolong the duration of purine nucleotides/nucleosides in the airways, perhaps by inhibiting ATP breakdown by ecto-enzymes. Nevertheless, these studies emphasize the importance of regular exercise/therapy programs in patients with obstructive lung disease to promote a favorable balance of ion transport to facilitate mucus clearance. It is hoped that such a regime, combined with additional pharmacological therapies, can help maintain mucus clearance in these patients during viral exacerbations which can produce mucus stasis and plugging, leading to persistent bacterial infections (Boucher, 2007).
11. Systems integration approach to understanding mucus clearance
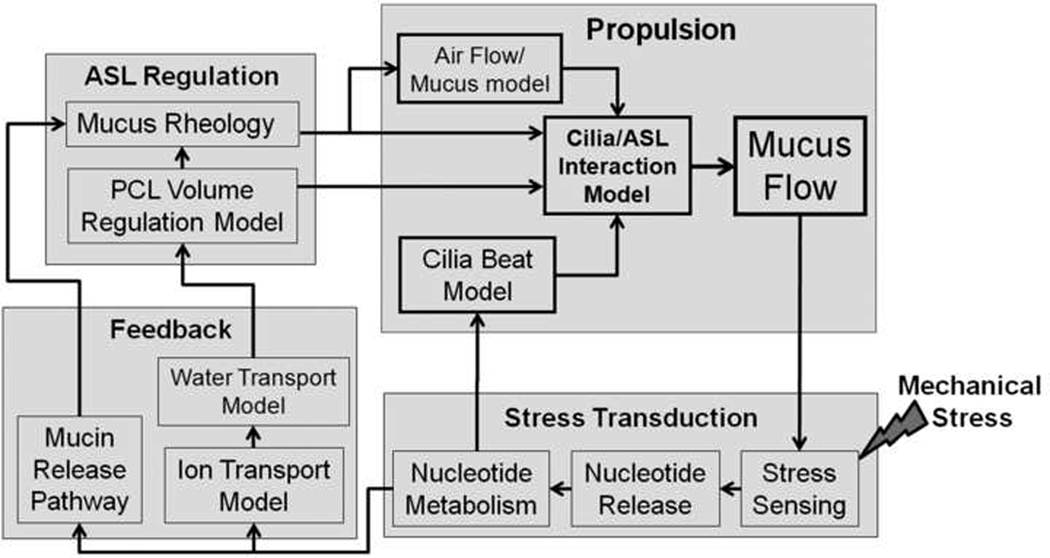
It has become clear that to fully understand how this complex system regulates ASL hydration and mucus clearance, mathematical models of these processes are necessary. Therefore, a diverse group of scientists has been assembled to combine experimental analysis with mathematical modeling to investigate how physical phenomena and the putative feedback mechanisms that regulate mucus height, viscoelasticity and cilia beat frequency interrelate to regulate mucus clearance. This group, called the UNC Virtual Lung Group, has developed a systems biology approach to understand how transport of mucus is regulated in the normal lung. This physics/engineering-based approach has generated computer algorithms that allow us to describe the normal processes and “control circuits” that regulate mucus clearance, and predict the consequences of failure of individual elements in the system.
The overall structure of this emerging systems biology approach to mucus clearance is illustrated in Fig. 7. This system is organized as a feedback loop of a sequence of the modeled systems involved in the regulation of mucus clearance. The systems involved are divided into four key modeling areas of focus: stress transduction, feedback, ASL regulation, and propulsion. The components of each of the modeled areas are comprised of a number of sensors, comparators, and effectors that subsequently alter the rate of clearance (mucus flow). By understanding how each of these components are related with one another and combine to affect ASL hydration state and, finally, mucus clearance rates. In the long term, the hope is to be capable of computing the predicted consequences of, for example, the stress-induced variations in ASL nucleotide/nucleoside levels on mucus hydration, mucus viscoelastic properties, and, ultimately, mucus clearance. Further, it is anticipated that these models will allow us to predict the most efficient means of rectifying a failure, e.g., will chronically increasing mechanical stress on airway surfaces of persons with cystic fibrosis increase nucleotide levels and adequately restore ASL hydration to levels sufficient to facilitate mucus clearance? Finally, in the future, it may be possible to use such a mathematical model-based approach to both design and test novel small molecule therapeutics for various airway diseases in silico.
Fig. 7. Model systems diagram of mucus clearance.
12. Summary
Mucus clearance is an essential innate immune protective mechanism in the airways. Recent advances have greatly increased our understanding of the structure and function of the mucus clearance apparatus, its homeostatic regulation in normal airways, and how both genetic and acquired diseases disrupt its function to ultimately degrade lung function. Further, recent studies culminated in the observations that physiologically relevant mechanical stresses can stimulate mucus clearance via increases in rates of ATP release into the luminal compartment, resulting in increases in ASL hydration. In this way, the lung can respond to stress-inducing stimuli on the intrapulmonary airways by stimulating secretion and accelerating clearance, thus promoting lung health. Unfortunately, our understanding of the mechanisms involved in regulating mucus clearance is far from complete. For example, are the viscoelastic properties of the mucus layer sensed by the airway epithelium from the forces generated by the cilia beating in thickened mucus? Second, does such a mechanism provide an additional feedback mechanism to sense/alter the hydration status of the ASL? Thus, considerable additional work addressing the processes involved, such as stress sensing, nucleotide regulation and PCL/mucus interaction, are clearly needed. However, we envision that a better understanding of how these processes are regulated, though individual researchers and collaborative groups such as the UNC Virtual Lung Group, will be of immense value in understanding and treating chronic lung diseases which exhibit mucus plugging, such as COPD and CF.
Acknowledgments
The authors thank their many colleagues at the UNC CF Center and the UNC Virtual Lung Group for sharing thoughts and data cited in this review. Work cited here was supported, in part, by grants from the Cystic Fibrosis Foundation (BUTTON04I0 and BUTTON06G0) and the National Institutes of Health (DK065988, EB002025, and HL077546). We also would like to thank Lisa Brown for providing graphics and editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsuwaidan S, Li Wan Po A, Morrison G, Redmond A, Dodge JA, McElnay J, Stewart E, Stanford CF. Effect of exercise on the nasal transmucosal potential difference in patients with cystic fibrosis and normal subjects. Thorax. 1994;49:1249–1250. doi: 10.1136/thx.49.12.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- App EM, Kieselmann R, Reinhardt D, Lindemann H, Dasgupta B, King M, Brand P. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy: flutter vs autogenic drainage. Chest. 1998;114:171–177. doi: 10.1378/chest.114.1.171. [DOI] [PubMed] [Google Scholar]
- Bankston LA, Guidotti G. Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem. 1996;271:17132–17138. doi: 10.1074/jbc.271.29.17132. [DOI] [PubMed] [Google Scholar]
- Basser PJ, McMahon TA, Griffith P. The mechanism of mucus clearance in cough. J Biomech Eng. 1989;111:288–297. doi: 10.1115/1.3168381. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Foster WM, Chapman WF. Cough-enhanced mucus clearance in the normal lung. J Appl Physiol. 1990;69:1670–1675. doi: 10.1152/jappl.1990.69.5.1670. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Olivier KN, Zeman KL, Hohneker KW, Boucher RC, Knowles MR. Effect of uridine 5'-triphosphate plus amiloride on mucociliary clearance in adult cystic fibrosis. Am J Respir Crit Care Med. 1996;153:1796–1801. doi: 10.1164/ajrccm.153.6.8665037. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Zeman KL. Effect of enhanced supramaximal flows on cough clearance. J Appl Physiol. 1994;77:1577–1583. doi: 10.1152/jappl.1994.77.4.1577. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Zeman KL, Foy C, Shaffer CL, Johnson FL, Regnis JA, Sannuti A, Johnson J. Effect of aerosolized uridine 5'-triphosphate on mucociliary clearance in mild chronic bronchitis. Am J Respir Crit Care Med. 2001;164:302–306. doi: 10.1164/ajrccm.164.2.2008094. [DOI] [PubMed] [Google Scholar]
- Benos DJ. Sensing tension: recognizing ENaC as a stretch sensor. Hypertension. 2004;44:616–617. doi: 10.1161/01.HYP.0000144467.43205.ed. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Human airway ion transport. Part one. Am J Respir Crit Care Med. 1994;150:271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol. 2004;561:499–513. doi: 10.1113/jphysiol.2004.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyton RJ, Openshaw PJ. Pulmonary defences to acute respiratory infection. Br Med Bull. 2002;61:1–12. doi: 10.1093/bmb/61.1.1. [DOI] [PubMed] [Google Scholar]
- Bradley J, Moran F. Physical training for cystic fibrosis. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002768. CD002768. [DOI] [PubMed] [Google Scholar]
- Bramley AM, Thomson RJ, Roberts CR, Schellenberg RR. Hypothesis: excessive bronchoconstriction in asthma is due to decreased airway elastance. Eur Respir J. 1994;7:337–341. doi: 10.1183/09031936.94.07020337. [DOI] [PubMed] [Google Scholar]
- Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Ismailov II, Benos DJ, Schwiebert LM, Fitz JG, Schwiebert EM. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem. 2001;276:6621–6630. doi: 10.1074/jbc.M005893200. [DOI] [PubMed] [Google Scholar]
- Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang XB, Tabcharani JA, Hou YX, Jensen TJ, Kartner N, Alon N, Hanrahan JW, Riordan JR. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho, rac and the actin cytoskeleton. Bioessays. 1992;14:777–778. doi: 10.1002/bies.950141110. [DOI] [PubMed] [Google Scholar]
- Clancy JP, Ruiz FE, Sorscher EJ. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2B receptor-coupled pathway. Am J Physiol. 1999;276:C361–C369. doi: 10.1152/ajpcell.1999.276.2.C361. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Boucher RC. Chloride secretory response to extracellular ATP in human normal and cystic fibrosis nasal epithelia. American Journal of Physiology. 1992;263:C348–C356. doi: 10.1152/ajpcell.1992.263.2.C348. [DOI] [PubMed] [Google Scholar]
- Crews A, Taylor AE, Ballard ST. Liquid transport properties of porcine tracheal epithelium. J Appl Physiol. 2001;91:797–802. doi: 10.1152/jappl.2001.91.2.797. [DOI] [PubMed] [Google Scholar]
- Davis IC, Lazarowski ER, Hickman-Davis JM, Fortenberry JA, Chen FP, Zhao X, Sorscher E, Graves LM, Sullender WM, Matalon S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am J Respir Crit Care Med. 2006;173:673–682. doi: 10.1164/rccm.200508-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L112–L120. doi: 10.1152/ajplung.00218.2003. [DOI] [PubMed] [Google Scholar]
- Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol. 2000;279:C461–C479. doi: 10.1152/ajpcell.2000.279.2.C461. [DOI] [PubMed] [Google Scholar]
- Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- Dietl P, Haller T, Mair N, Frick M. Mechanisms of surfactant exocytosis in alveolar type II cells in vitro and in vivo. News Physiol Sci. 2001;16:239–243. doi: 10.1152/physiologyonline.2001.16.5.239. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med. 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Elad D, Naftali S, Rosenfeld M, Wolf M. Physical stresses at the air-wall interface of the human nasal cavity during breathing. J Appl Physiol. 2006;100:1003–1010. doi: 10.1152/japplphysiol.01049.2005. [DOI] [PubMed] [Google Scholar]
- Evans JH, Sanderson MJ. Intracellular calcium oscillations regulate ciliary beat frequency of airway epithelial cells. Cell Calcium. 1999;26:103–110. doi: 10.1054/ceca.1999.0060. [DOI] [PubMed] [Google Scholar]
- Even-Tzur N, Elad D, Zaretsky U, Randell SH, Haklai R, Wolf M. Custom-designed wells and flow chamber for exposing air-liquid interface cultures to wall shear stress. Ann Biomed Eng. 2006;34:1890–1895. doi: 10.1007/s10439-006-9211-8. [DOI] [PubMed] [Google Scholar]
- Farinas J, Kneen M, Moore M, Verkman AS. Plasma membrane water permeability of cultured cells and epithelia measured by light microscopy with spatial filtering. J Gen Physiol. 1997;110:283–296. doi: 10.1085/jgp.110.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredberg JJ, Moore JA. The distributed response of complex branching duct networks. J Acoust Soc Am. 1978;63:954–961. doi: 10.1121/1.381775. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40:317–330. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Gatof D, Kilic G, Fitz JG. Vesicular exocytosis contributes to volume-sensitive ATP release in biliary cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G538–G546. doi: 10.1152/ajpgi.00355.2003. [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Weisman GA, Erb L, Seye CI, Sun GY, Velazquez B, Hernandez-Perez M, Chorna NE. Mechanisms for inhibition of P2 receptors signaling in neural cells. Mol Neurobiol. 2005;31:65–79. doi: 10.1385/MN:31:1-3:065. [DOI] [PubMed] [Google Scholar]
- Grierson JP, Meldolesi J. Shear stress-induced [Ca2+]i transients and oscillations in mouse fibroblasts are mediated by endogenously released ATP. J Biol Chem. 1995;270:4451–4456. doi: 10.1074/jbc.270.9.4451. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Effect of in vivo corticosteroids on Na+ transport across airway epithelia. Am J Physiol. 1998;275:C303–C308. doi: 10.1152/ajpcell.1998.275.1.C303. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Stropp JQ. Pressure-volume and length-stress relationships in canine bronchi in vitro. J Appl Physiol. 1988;64:2522–2531. doi: 10.1152/jappl.1988.64.6.2522. [DOI] [PubMed] [Google Scholar]
- Guyot A, Hanrahan JW. ATP release from human airway epithelial cells studied using a capillary cell culture system. J Physiol. 2002;545:199–206. doi: 10.1113/jphysiol.2002.030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP. Twenty odd years of stretch-sensitive channels. Pflugers Arch. 2006;453:333–351. doi: 10.1007/s00424-006-0131-0. [DOI] [PubMed] [Google Scholar]
- Hebestreit A, Kersting U, Basler B, Jeschke R, Hebestreit H. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. Am J Respir Crit Care Med. 2001;164:443–446. doi: 10.1164/ajrccm.164.3.2007168. [DOI] [PubMed] [Google Scholar]
- Hentchel-Franks K, Lozano D, Eubanks-Tarn V, Cobb B, Fan L, Oster R, Sorscher E, Clancy JP. Activation of airway cl- secretion in human subjects by adenosine. Am J Respir Cell Mol Biol. 2004;31:140–146. doi: 10.1165/rcmb.2004-0012OC. [DOI] [PubMed] [Google Scholar]
- Hirsh AJ, Stonebraker JR, van Heusden CA, Lazarowski ER, Boucher RC, Picher M. Adenosine deaminase 1 and concentrative nucleoside transporters 2 and 3 regulate adenosine on the apical surface of human airway epithelia: implications for inflammatory lung diseases. Biochemistry. 2007;46:10373–10383. doi: 10.1021/bi7009647. [DOI] [PubMed] [Google Scholar]
- Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Mathews CJ, Hanrahan JW. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J Biol Chem. 1997;272:4978–4984. doi: 10.1074/jbc.272.8.4978. [DOI] [PubMed] [Google Scholar]
- Kallok MJ, Lai-Fook SJ, Hajji MA, Wilson TA. Axial distortion of airways in the lung. J Appl Physiol. 1983;54:185–190. doi: 10.1152/jappl.1983.54.1.185. [DOI] [PubMed] [Google Scholar]
- Kellerman D, Evans R, Mathews D, Shaffer C. Inhaled P2Y2 receptor agonists as a treatment for patients with Cystic Fibrosis lung disease. Adv Drug Deliv Rev. 2002a;54:1463–1474. doi: 10.1016/s0169-409x(02)00154-0. [DOI] [PubMed] [Google Scholar]
- Kellerman DJ. P2Y(2) receptor agonists: a new class of medication targeted at improved mucociliary clearance. Chest. 2002;121:201S–205S. doi: 10.1378/chest.121.5_suppl.201s. [DOI] [PubMed] [Google Scholar]
- Kempainen RR, Williams CB, Hazelwood A, Rubin BK, Milla CE. Comparison of high-frequency chest wall oscillation with differing waveforms for airway clearance in cystic fibrosis. Chest. 2007;132:1227–1232. doi: 10.1378/chest.07-1078. [DOI] [PubMed] [Google Scholar]
- Kilburn KH. A hypothesis for pulmonary clearance and its implications. Am Rev Respir Dis. 1968;98:449–463. doi: 10.1164/arrd.1968.98.3.449. [DOI] [PubMed] [Google Scholar]
- King M, Phillips DM, Gross D, Vartian V, Chang HK, Zidulka A. Enhanced tracheal mucus clearance with high frequency chest wall compression. Am Rev Respir Dis. 1983;128:511–515. doi: 10.1164/arrd.1983.128.3.511. [DOI] [PubMed] [Google Scholar]
- Kitterman JA. The effects of mechanical forces on fetal lung growth. Clin Perinatol. 1996;23:727–740. [PubMed] [Google Scholar]
- Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Clarke LL, Boucher RC. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. New England Journal of Medicine. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [comment] [DOI] [PubMed] [Google Scholar]
- Knowles MR, Robinson JM, Wood RE, Pue CA, Mentz WM, Wager GC, Gatzy JT, Boucher RC. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komlosi P, Fintha A, Bell PD. Renal cell-to-cell communication via extracellular ATP. Physiology (Bethesda) 2005;20:86–90. doi: 10.1152/physiol.00002.2005. [DOI] [PubMed] [Google Scholar]
- Kreda SM, Okada SF, van Heusden CA, O'Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. Faseb J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Konig J, Sun J, Markovich D, King NJ, Karupiah G, Young JA, Cook DI. Acute effects of parainfluenza virus on epithelial electrolyte transport. J Biol Chem. 2004;279:48760–48766. doi: 10.1074/jbc.M409747200. [DOI] [PubMed] [Google Scholar]
- Lambert RK, Codd SL, Alley MR, Pack RJ. Physical determinants of bronchial mucosal folding. J Appl Physiol. 1994;77:1206–1216. doi: 10.1152/jappl.1994.77.3.1206. [DOI] [PubMed] [Google Scholar]
- Langenderfer B. Alternatives to percussion and postural drainage. A review of mucus clearance therapies: percussion and postural drainage, autogenic drainage, positive expiratory pressure, flutter valve, intrapulmonary percussive ventilation, and high-frequency chest compression with the ThAIRapy Vest. J Cardiopulm Rehabil. 1998;18:283–289. doi: 10.1097/00008483-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Lansley AB, Sanderson MJ. Regulation of airway ciliary activity by Ca2+: simultaneous measurement of beat frequency and intracellular Ca2+ Biophys J. 1999;77:629–638. doi: 10.1016/S0006-3495(99)76919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC. UTP as an extracellular signaling molecule. News Physiol Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. Journal of Biological Chemistry. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith DE. The development of cough. Am Rev Respir Dis. 1985;131:S39–S42. doi: 10.1164/arrd.1985.131.S5.S39. 1985. [DOI] [PubMed] [Google Scholar]
- Lethem MI, Dowell ML, Van Scott M, Yankaskas JR, Egan T, Boucher RC, Davis CW. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am J Respir Cell Mol Biol. 1993;9:315–322. doi: 10.1165/ajrcmb/9.3.315. [DOI] [PubMed] [Google Scholar]
- Levitzky MG. Pulmonary Physiology. New York, NY: McGraw-Hill, INC; 1991. [Google Scholar]
- Liu M, Tanswell AK, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol. 1999;277:L667–L683. doi: 10.1152/ajplung.1999.277.4.L667. [DOI] [PubMed] [Google Scholar]
- Mall M, Wissner A, Gonska T, Calenborn D, Kuehr J, Brandis M, Kunzelmann K. Inhibition of amiloride-sensitive epithelial Na(+) absorption by extracellular nucleotides in human normal and cystic fibrosis airways. Am J Respir Cell Mol Biol. 2000;23:755–761. doi: 10.1165/ajrcmb.23.6.4207. [DOI] [PubMed] [Google Scholar]
- Marks JH. Airway clearance devices in cystic fibrosis. Paediatr Respir Rev. 2007;8:17–23. doi: 10.1016/j.prrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. British Journal of Pharmacology. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J Clin Invest. 2000;105:1419–1427. doi: 10.1172/JCI4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, Taylor RM, 2nd, Superfine R, Rubinstein M, Iglewski BH, Boucher RC. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A. 2006;103:18131–18136. doi: 10.1073/pnas.0606428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Carre DA, McGlinn AM, Stone RA, Civan MM. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc Natl Acad Sci U S A. 1998;95:7174–7178. doi: 10.1073/pnas.95.12.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessinger AC, Harding R, Adamson TM, Singh M, Kiu GT. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest. 1990;86:1270–1277. doi: 10.1172/JCI114834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorcroft AJ, Dodd ME, Webb AK. Exercise limitations and training for patients with cystic fibrosis. Disabil Rehabil. 1998;20:247–253. doi: 10.3109/09638289809166735. [DOI] [PubMed] [Google Scholar]
- Morse DM, Smullen JL, Davis CW. Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am J Physiol Cell Physiol. 2001;280:C1485–C1497. doi: 10.1152/ajpcell.2001.280.6.C1485. [DOI] [PubMed] [Google Scholar]
- North RA, Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch. 2006;452:479–485. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol. 2004;124:513–526. doi: 10.1085/jgp.200409154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso AM, Ribeiro CM, Boucher RC. Polarized signaling via purinoceptors in normal and cystic fibrosis airway epithelia. J Gen Physiol. 2001;117:53–67. doi: 10.1085/jgp.117.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasyk EA, Foskett JK. Cystic fibrosis transmembrane conductance regulator-associated ATP and adenosine 3'-phosphate 5'-phosphosulfate channels in endoplasmic reticulum and plasma membranes. J Biol Chem. 1997;272:7746–7751. doi: 10.1074/jbc.272.12.7746. [DOI] [PubMed] [Google Scholar]
- Pelleg A, Katchanov G, Xu J. Purinergic modulation of neural control of cardiac function. J Auton Pharmacol. 1996;16:401–405. doi: 10.1111/j.1474-8673.1996.tb00063.x. [DOI] [PubMed] [Google Scholar]
- Picher M, Boucher RC. Biochemical evidence for an ecto alkaline phosphodiesterase I in human airways. Am J Respir Cell Mol Biol. 2000;23:255–261. doi: 10.1165/ajrcmb.23.2.4088. [DOI] [PubMed] [Google Scholar]
- Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5'-nucleotidase and nonspecific alkaline phosphatase. Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem. 2003;278:13468–13479. doi: 10.1074/jbc.M300569200. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–520. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- Prasad SA, Cerny FJ. Factors that influence adherence to exercise and their effectiveness: application to cystic fibrosis. Pediatr Pulmonol. 2002;34:66–72. doi: 10.1002/ppul.10126. [DOI] [PubMed] [Google Scholar]
- Pryor JA. Physiotherapy for airway clearance in adults. Eur Respir J. 1999;14:1418–1424. doi: 10.1183/09031936.99.14614189. [DOI] [PubMed] [Google Scholar]
- Puchelle E, de Bentzmann S, Zahm JM. Physical and functional properties of airway secretions in cystic fibrosis--therapeutic approaches. Respiration. 1995;62 Suppl 1:2–12. doi: 10.1159/000196486. [DOI] [PubMed] [Google Scholar]
- Randell SH, Boucher RC. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol. 2006;35:20–28. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science. 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, Cantiello HF. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- Ressler B, Lee RT, Randell SH, Drazen JM, Kamm RD. Molecular responses of rat tracheal epithelial cells to transmembrane pressure. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1264–L1272. doi: 10.1152/ajplung.2000.278.6.L1264. [DOI] [PubMed] [Google Scholar]
- Rich PB, Douillet CD, Mahler SA, Husain SA, Boucher RC. Adenosine triphosphate is released during injurious mechanical ventilation and contributes to lung edema. J Trauma. 2003;55:290–297. doi: 10.1097/01.TA.0000078882.11919.AF. [DOI] [PubMed] [Google Scholar]
- Rubin BK. Physiology of airway mucus clearance. Respir Care. 2002;47:761–768. [PubMed] [Google Scholar]
- Rubin EM, Scantlen GE, Chapman GA, Eldridge M, Menendez R, Wanner A. Effect of chest wall oscillation on mucus clearance: comparison of two vibrators. Pediatr Pulmonol. 1989;6:122–126. doi: 10.1002/ppul.1950060213. [DOI] [PubMed] [Google Scholar]
- Sackin H. Stretch-activated ion channels. Kidney Int. 1995;48:1134–1147. doi: 10.1038/ki.1995.397. [DOI] [PubMed] [Google Scholar]
- Salathe M, Lieb T, Bookman RJ. Lack of nitric oxide involvement in cholinergic modulation of ovine ciliary beat frequency. J Aerosol Med. 2000;13:219–229. doi: 10.1089/jam.2000.13.219. [DOI] [PubMed] [Google Scholar]
- Satir P, Sleigh MA. The physiology of cilia and mucociliary interactions. Annu Rev Physiol. 1990;52:137–155. doi: 10.1146/annurev.ph.52.030190.001033. [DOI] [PubMed] [Google Scholar]
- Schneider SW, Egan ME, Jena BP, Guggino WB, Oberleithner H, Geibel JP. Continuous detection of extracellular ATP on living cells by using atomic force microscopy. Proc Natl Acad Sci U S A. 1999;96:12180–12185. doi: 10.1073/pnas.96.21.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman-Walker J, Pollock SL, Corey M, Wilkes DD, Canny GJ, Pedder L, Reisman JJ. A randomized controlled trial of a 3-year home exercise program in cystic fibrosis. J Pediatr. 2000;136:304–310. doi: 10.1067/mpd.2000.103408. [DOI] [PubMed] [Google Scholar]
- Schumacker PT. Straining to understand mechanotransduction in the lung. Am J Physiol Lung Cell Mol Physiol. 2002;282:L881–L882. doi: 10.1152/ajplung.00043.2002. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM. ABC transporter-facilitated ATP conductive transport. Am J Physiol. 1999;276:C1–C8. doi: 10.1152/ajpcell.1999.276.1.C1. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol. 2001;28:340–350. doi: 10.1046/j.1440-1681.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- Spring KR. Epithelial Fluid Transport--A Century of Investigation. News Physiol Sci. 1999;14:92–98. doi: 10.1152/physiologyonline.1999.14.3.92. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Sugita M, Yue Y, Foskett JK. CFTR Cl- channel and CFTR-associated ATP channel: distinct pores regulated by common gates. Embo J. 1998;17:898–908. doi: 10.1093/emboj/17.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol. 2001;118:223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127:591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: a "bumper-car" model. Proc Natl Acad Sci U S A. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajah JR, Song Y, Haggie PM, Verkman AS. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. Faseb J. 2004;18:875–877. doi: 10.1096/fj.03-1248fje. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- Tomkiewicz RP, Biviji A, King M. Effects of oscillating air flow on the rheological properties and clearability of mucous gel simulants. Biorheology. 1994;31:511–520. doi: 10.3233/bir-1994-31501. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Drazen JM. Chronic effects of mechanical force on airways. Annu Rev Physiol. 2006;68:563–583. doi: 10.1146/annurev.physiol.68.072304.113102. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Shively JD, Swartz MA, Silverman ES, Haley KJ, Raab G, Drazen JM. Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am J Physiol Lung Cell Mol Physiol. 2002;282:L904–L911. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, chwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. Embo J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA. What does mucin have to do with lung disease? Paediatr Respir Rev. 2002;3:98–103. doi: 10.1016/s1526-0550(02)00007-0. [DOI] [PubMed] [Google Scholar]
- Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Watt WC, Lazarowski ER, Boucher RC. Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J Biol Chem. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- Wiggs BR, Hrousis CA, Drazen JM, Kamm RD. On the mechanism of mucosal folding in normal and asthmatic airways. J Appl Physiol. 1997;83:1814–1821. doi: 10.1152/jappl.1997.83.6.1814. [DOI] [PubMed] [Google Scholar]
- Winters SL, Davis CW, Boucher RC. Mechanosensitivity of mouse tracheal ciliary beat frequency: roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am J Physiol Lung Cell Mol Physiol. 2007;292:L614–L624. doi: 10.1152/ajplung.00288.2005. [DOI] [PubMed] [Google Scholar]
- Winters SL, Yeates DB. Roles of hydration, sodium, and chloride in regulation of canine mucociliary transport system. J Appl Physiol. 1997;83:1360–1369. doi: 10.1152/jappl.1997.83.4.1360. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med. 2006;12:133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- Yoshitsugu M, Rautiainen M, Matsune S, Nuutinen J, Ohyama M. Effect of exogenous ATP on ciliary beat of human ciliated cells studied with differential interference microscope equipped with high speed video. Acta Otolaryngol. 1993;113:655–659. doi: 10.3109/00016489309135880. [DOI] [PubMed] [Google Scholar]
- Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Sanderson MJ. Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP. J Physiol. 2003;546:733–749. doi: 10.1113/jphysiol.2002.028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]