Summary
Stomata and pavement cells are produced by a series of asymmetric divisions and progressive fate transitions within a stem cell lineage. In Arabidopsis, this process is regulated so that new lineages can be inserted between previously differentiated cells while maintaining stomatal spacing. The small peptide EPIDERMAL PATTERNING FACTOR 1 may be a positional signal secreted by stomatal precursors to modulate behavior of nearby cells. Signal-receiving cells may use TOO MANY MOUTHS and ERECTA family receptors and a MAPK pathway to regulate initiation of new lineages, promote asymmetric division, and control the plane of spacing divisions. Cell fate transitions are controlled by bHLH, MYB and MADS-box transcription factors, and there is evidence of miRNA regulation. These results provide insight into positive and negative influences on stomatal cell transitions and suggest points of potential environmental regulation.
Introduction
Stomata are composed of two guard cells surrounding a pore in the epidermis. The guard cell pair acts as a gate to balance gas exchange against loss of water vapor. In Arabidopsis, as in almost all plants, stomata are spaced by at least one intervening epidermal pavement cell [1–3]. This simple distribution pattern is remarkable in that it is maintained throughout the course of organ development, often in circumstances that require new stomata to be inserted between other terminally differentiated cells. Proliferative activity, including stomatal installation, must also be tightly coordinated with growth and cell division in other cell layers [4], and must be able to respond to environmental conditions that influence stomatal density and index [5]. The essence of this process involves appropriate regulation of a population of adult stem cells that persist within the epidermis until the organ is complete and all cells terminally differentiate. Correct stomatal patterning involves several events of fundamental interest, including the establishment of diverse cell types through asymmetric division, control of the timing and orientation of cell divisions, progressive cell fate selection within a cell lineage, and local and global cell-cell communication. This review integrates recent findings with our current understanding of the positive and negative influences on stomatal stem cell behavior during epidermal development. In particular, the recent discovery of several broadly expressed and cell-autonomous determinants that positively regulate transitions in the stomatal cell lineage, as well as the identification of new components of the extrinsic signaling pathway for patterning stomata, will be discussed.
Extrinsic signaling in stomatal lineage regulation
Stomata are correctly patterned by cell-cell signaling that reads positional information to direct the behavior of stomatal stem cells [2,6,7]. Two classes of presumptive cell surface receptors, the LRR-receptor like protein TOO MANY MOUTHS (TMM) and three ERECTA family (ERf) LRR-receptor kinases (ERECTA, ERL1, ERL2), participate in stomatal signaling [2,6,8–10]. In the most basic terms, signaling controls several related but discrete events; multipotent epidermal stem cells (MESCs; see Figure 1) are restrained from initiating a new stomatal lineage close to another stomatal precursor, or if a MESC divides, the plane of division is oriented relative to nearby precursors to maintain spacing [1,11,12]. A final activity lies in regulating fate progression within the stomatal lineage, in which case complex combinatorial interactions between the four receptors positively or negatively regulate entry into the pathway and meristemoid self-renewal versus differentiation as a GMC [9]. For example, both ER and ERL2 often promote fate progression, while ERL1 seems to promote meristemoid maintenance/self-renewal.
Figure 1.
Schematic representation of stomatal biogenesis.
Our current understanding of the biogenesis of stomata in Arabidopsis suggests that a stomatal lineage forms when a cell from a pool of multipotent epidermal stem cells (MESCs) selects the meristemoid mother cell (MMC; green) fate. The MMC is functionally defined as a cell that initiates an “entry” asymmetric division (e) to produce two daughters with distinct fates; the smaller meristemoid (M; red triangle) will eventually produce the stomata itself, while the larger sister cell transiently retains it multipotent state (green). Meristemoids may self-renew through several additional rounds of asymmetric division that “amplify” (a) the stomatal cell lineage. A meristemoid eventually differentiates directly into a guard mother cell (GMC; blue) that divides symmetrically to form two equivalent cells. These will terminally differentiate as guard cells (GC; yellow) surrounding a pore. The larger cells produced by each asymmetric division of the stomatal lineage are known as stomatal lineage ground cells (SLGCs; green) to acknowledge their common descent from a parental MMC. SLGCs, as well as non-related but adjacent MESCs, have the potential to host a new entry division (es) to produce a satellite meristemoid (SM), or to terminally differentiate as an epidermal pavement cell (white). As a result of the residual competence of young SLGCs to divide, new stomatal lineages may be “nested” within older ones, but later stomata may also be produced independently when residual MESCs not derived from MMCs divide asymmetrically.
SPCH and ICE1/SCREAM2 mediate both entry and amplifying asymmetric divisions, while MUTE and ICE1/SCREAM2 mediate the transition from meristemoid to guard mother cell fate. FAMA, FLP/MYB88 and ICE1/SCREAM2 control the GMC to GC transition. Both Ms and GMCs may secrete a positional signal (EPF1; orange field) interpreted by nearby cells. AGL16/miR824 may control the decision of SLGCs to divide asymmetrically to produce a new stomatal lineage or leave the MESC population to differentiate as pavement cells.
Although all four receptors are commonly defined as negative regulators, it is unknown how signaling yields opposite outcomes in different cell types and organs. TMM has been described as having opposing roles due to the absence of stomata on embryonic and adult stems of mutants [10,13], but recent work suggests that the function of TMM may be more universal [14]. Asymmetric divisions occur in tmm stems, but stomata are absent because meristemoids do not transition to GMCs and instead differentiate as pavement cells (Figure 2). This shows that TMM signaling is required to promote or maintain meristemoid fate in stems as well as leaves although the outcome is tissue specific (differentiation as pavement cell vs. precocious GMC).
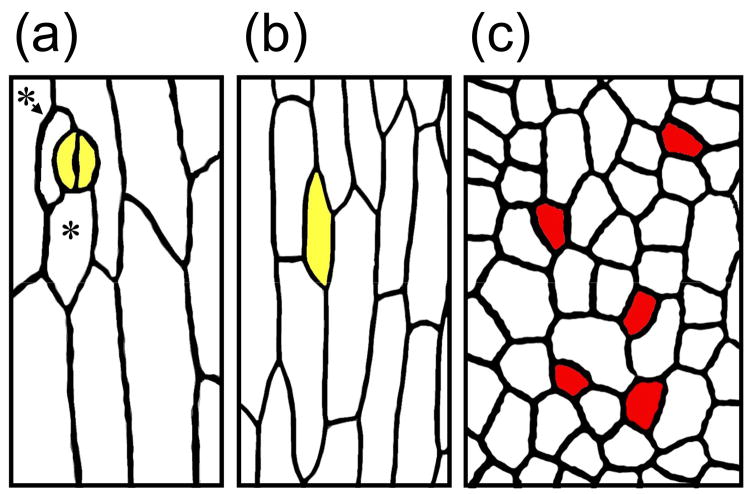
Figure 2.
Mutant tmm stems have no stomata at maturity, but they do execute entry asymmetric divisions. TMM signaling is required to promote meristemoid self-renewal/maintenance in stems, similar to leaves.
(a) Mature wild-type stems have stomata (yellow) with adjacent smaller cells (asterisks) formed by differentiation of SLGCs. (b) In tmm mutant stems, meristemoids do not progress to the GMC stage and instead differentiate as pavement cells (also yellow). (c) Early in stem epidermal development, tmm plants execute entry asymmetric divisions. The smaller cells (red) have the shape and molecular signature of meristemoids.
It is likely that downstream of these receptors is a MAPK cascade, comprised of the MAPKKK YODA (YDA) as well as two MAPKKs (MKK4/MKK5) and two MAPKs (MPK3/MPK6) which also negatively regulate the initiation of stomatal formation or transition to guard mother cell fate [15,16]. Also potential downstream targets of TMM/ERf signaling are key cell cycle regulators that participate in stomatal lineage control [17–19]. Potentially upstream of these receptors or functioning in parallel is the extracellular subtilisin protease STOMATAL DENSITY AND DISTRIBUTION 1 (SDD1) that also negatively regulates stomatal lineage initiation and controls orientation divisions [20,21].
Mobile signals in asymmetric division
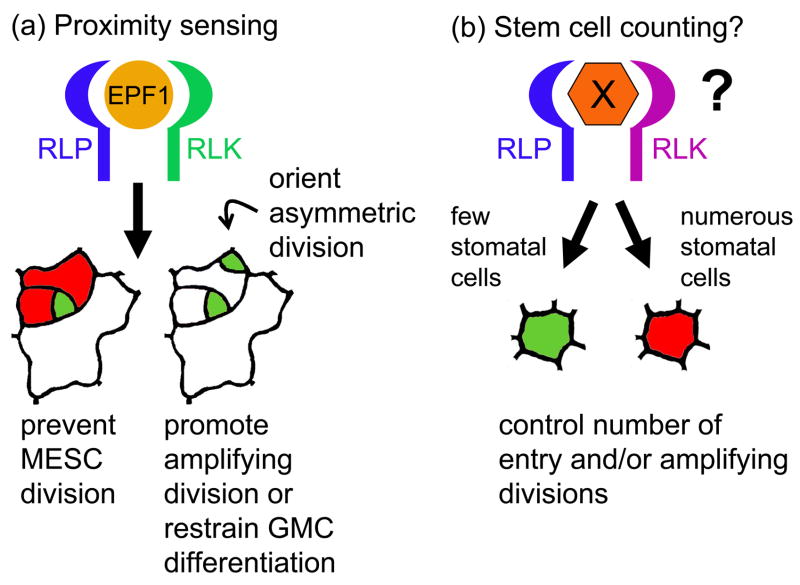
Until recently, positional cues recognized by TMM and ERf cell surface receptors have eluded identification. A potential ligand, EPIDERMAL PATTERNING FACTOR 1 (EPF1), has now been discovered using a large-scale approach to define the roles of small secreted peptides in Arabidopsis [22]. Ectopic overexpression of EPF1 (EPF1-OX) eliminates stomata or dramatically reduces their abundance. Normally, EPF1 is only expressed by precursors that are predicted to produce a signal (M/GMC), and mutation leads to patterning defects qualitatively similar to both tmm and er;erl1;erl2 mutants. EPF1 has the hallmarks of other plant peptides involved in cell communication [23,24]; it is a member of a family of small proteins conserved in only a limited, cysteine-rich C-terminal region. For these reasons, EPF1 is an excellent candidate for a mobile signal that negatively regulates stomatal formation and enforces the orientation of spacing divisions (Figure 3).
Figure 3.
Signals and receptor functions in stomatal biogenesis.
(a) EPF1 acts as a proximity signal potentially recognized by a hetero- or homodimeric TMM or ERf receptor complex (RLK/RLP). Sufficient signal strength is hypothesized to negatively regulate asymmetric divisions of nearby MESCs (red), orient spacing divisions, or promote amplifying divisions of Ms (green) (b) Other types of signals (X) might also be sent and received by stomatal stem cells. One possibility is a mechanism to control the number of cells entering the stomatal pathway in response to precursor/stomatal numbers, perhaps mediated by other EPF family peptides. These hypothetical signals might be recognized by different receptor combinations and integrated with other inputs.
Evidence that EPF1 may be the ligand for TMM and ERf receptors comes from EPF1-OX in mutant backgrounds, where the stomatal patterning defects of tmm, er;erl1;erl2 and also yda mutants are epistatic to the EPF1-OX phenotype. On the other hand, sdd1 patterning defects are not epistatic. This result challenges a previous model that proposed SDD1 might process a secreted ligand to facilitate paracrine signaling between signal sending and receiving cells. However, it remains possible that unprocessed EPF1 retains limited biological activity revealed when produced in excess, or that SDD1 processes another signaling peptide or receptor. Before the function of EPF1 can be fully understood it will be necessary to clarify what stage of stomatal formation is blocked by EPF1-OX, and if EPF1 is directly recognized by TMM or ERf receptors.
Interestingly, the epf1–1 mutant phenotype is less severe than mutation of TMM or the three ERf receptors, and does not seem to encompass the same range of events. A possibility is that other members of the EPF1 family also have overlapping or similar roles in stomatal signaling. At least one other member of the EPF1 family is co-regulated with TMM in microarray expression experiments [25] and is required for correct stomatal patterning (Odapalli and Nadeau, unpublished data).
Intrinsic factors in stomatal cell fate transitions
Stomatal biogenesis involves several clear transitions in cell identity marked by cytological changes [26,27], cell polarization [28], and expression of discrete gene batteries [29–33] but the gene regulatory network was unknown. Recently, three related bHLH transcription factors were discovered that control cell fate progression through the stomatal pathway (Figure 1). The roles of these proteins is strikingly similar to those of animal bHLHs that control sequential cell fate specification during muscle formation [34] or neural development [35]. The earliest-acting bHLH, SPEECHLESS (SPCH), positively regulates asymmetric divisions that form stomata [36,37]. Homozygous spch-1 plants not only fail to execute entry asymmetric divisions but also do not express early markers of stomatal identity, revealing that the pavement cell-only phenotype is a complete deletion of the stomatal lineage. Conversely, mild overexpression of SPCH from its own promotor increases the number of asymmetric divisions and leads to extra stomata in clusters. SPCH also has a later-acting role in promoting amplifying asymmetric divisions revealed in the weaker spch alleles, where some stomatal lineages are initiated but meristemoids divide fewer times. Normal and correctly spaced stomata are produced in the weak allele, ruling out a role for SPCH in later events such as regulating the plane of spacing divisions and GMC to GC fate transition.
SPCH seems to act as a stomatal pathway-specific “switch” for asymmetric divisions in a dividing cell population. It remains to be seen how SPCH activity is regulated, but evidence argues against known signaling receptors because TMM is not expressed in spch-1 plants and spch-1 is epistatic to tmm. On the other hand, SPCH overexpression can restore stomatal formation in locations where tmm plants typically lack stomata, suggesting that a SPCH activity is downstream of TMM in at least some developmental contexts.
The second bHLH protein, MUTE, is required to transition from meristemoid to guard mother cell identity [36–38]. In strong alleles, meristemoids continue to divide asymmetrically in an inward spiral without differentiating as GMCs and MUTE overexpression is sufficient to convert all shoot epidermal cells to GMCs. All GMCs express MUTE but only a subpopulation of meristemoids do, consistent with the hypothesis that it is only present in meristemoids during differentiation. This observation implies MUTE is not meristemoid-specific, but instead is stimulated only after a variable number of amplifying asymmetric divisions have occurred. It not clear how MUTE expression is promoted, but this might entail an intrinsic mechanism to “count” the number of asymmetric cell cycles, or alternatively could result from changes in the strength of extrinsic signaling as distance between precursors increases. It is plausible that positional signals perceived via TMM and ERf receptors might negatively regulate the MUTE-mediated transition to ensure production of adequate numbers of pavement cells, since plants defective in extrinsic signaling show a small but measurable alleviation of the mute block in the M to GMC transition [38]. If so, misexpression of MUTE from an earlier-acting meristemoid promoter would cause premature GMC formation and reduction in SLGC number.
The final fate transition in the stomatal pathway is controlled by the bHLH protein FAMA [39]. Mutation of FAMA, similar to the MYB gene FOUR LIPS (FLP) and its paralog MYB88 [40], leads to reiterative symmetric divisions within a cell lineage derived from a single GMC. FAMA appears to be required to terminate expression of cell cycle genes and for differentiation of guard cells, and ectopic FAMA expression can convert almost any cell to the GC fate independent of prior symmetric or asymmetric division. In contrast, FLP/MYB88 proteins are likely required to regulate division of GMCs. Despite coincident expression in the GMC, evidence from in vivo assays suggests FLP/MYB88 and FAMA proteins do not form heterodimeric transcriptional complexes. The function of these transcription factors awaits identification of their downstream targets and upstream regulators.
Global positive regulators of fate transition
Recently two new bHLH-leucine zipper transcription factors were recognized as playing redundant, global roles in stomatal cell fate transitions [41]. SCREAM1 was first identified as INDUCER OF CBF EXPRESSION 1 (ICE1) because it regulates expression of cold-induced genes [42]. The dual role of ICE1/SCREAM1 (bHLH116) was recognized when it was re-isolated as a dominant allele that converts almost all epidermal cells to stomata, a phenotype almost identical to MUTE overexpression. Expression of early stomatal lineage markers TMM and ERL1 indicate that scrm-D forces epidermal cells (but not other cell layers) to execute ectopic cell divisions and adopt stomatal fate.
The scrm-D and ice1-D phenotypes are caused by the same R to H mutation in a unique “KRAAM” domain outside the bHLH-LZ domains [41]. ICE1 is normally activated by low temperature, yet ice1-D is insensitive to cold and dominantly interferes with activation of CBF expression [42]; it is provocative that the same domain causes scrm-D to be insensitive to and/or interfere with normal stomatal pathway regulation. Regardless, SCRM1 likely acts as a transcription factor because a second mutation in the DNA binding domain eliminates the scrm-D dominant effect. The KRAAM domain is shared with one other closely related paralog, SCRM2 (bHLH33), and the same alteration in SCRM2 produces an identical dominant stomatal phenotype. Both genes are expressed throughout the plant but are preferentially expressed in all cells of the stomatal lineage, except for absence of SCRM2 in mature GCs.
Genetic analysis indicates that ICE1 and SCRM2 are at least partly redundant and required at all three transitions in the stomatal pathway (Figure 1). ICE1/SCRM2 control initiation of asymmetric divisions because double mutants produce only pavement cells, similar to spch-1, and do not express SPCH or later-acting bHLHs. Several lines of evidence suggest SPCH and ICE1/SCRM2 collaborate to activate asymmetric divisions. SPCH, but not late acting bHLHs MUTE or FAMA, is required for scrm-D ectopic divisions, and the relationship between scrm-D and SPCH is dosage dependent. Both ICE1 and SCRM2 proteins interact with SPCH in Bimolecular Fluorescence Complementation (BiFC) assays in a small fraction of transformed epidermal cells. In contrast, scrm-D was able to interact in almost all cells, which hints that dimerization might be cell type-dependent and regulated through the KRAAM domain. ICE1 and SCRM2 also play a role later in the pathway, because ice1/ice1; scrm2/+ plants are able to execute asymmetric divisions but meristemoids arrest. In BiFC assays, MUTE and FAMA interact equally well with ICE1, SCRM2 and scrm-D proteins in most cells, so it seems possible that ICE1/SCRM2 heterodimerize to control both M to GMC and GMC to GC transitions. FAMA also has two other bHLH interaction partners (bHLH71 and bHLH93) that have weak stomatal phenotypes [39] and MUTE can homodimerize, so different combinatorial interactions between the bHLHs may play as yet undefined roles.
Collectively, these findings suggest that ICE1/SCRM2 may have functions similar to the broadly expressed cofactors (e.g. daughterless) that complex with multiple cell-autonomous bHLHs to specify successive fates in neurogenesis and myogenesis [43,44], further extending the analogy between stomatal lineage progression in plants and developmental circuitry in animals. Still missing from the analogy are HLH proteins that interact to form inactive or repressive transcriptional complexes [35].
MicroRNA control of asymmetric division
miRNAs are known to play a regulatory role in numerous developmental processes [45], now including control of stomatal stem cells (Figure 1). Previous surveys showed that the MADS box transcription factor AGL16 is expressed in guard cells [30]. Overexpression of AGL16 has no obvious effect, but this mRNA is the only target for the newly discovered miR824 that directs its cleavage [46]. Plants overexpressing a miRNA-resistant form of AGL16 produce many extra satellite meristemoid lineages while plants that overexpress miR824 produce very few. AGL16 either promotes the division of SLGCs to form satellite meristemoids or restrains departure of SLGCs from the pool of competent cells. AGL16 transcripts are present only in guard cells but miR824 is expressed in recently produced SLGCs, meristemoids, and GMCs. Because AGL16 mRNA was not observed in the SLGCs it presumably regulates, it was proposed that it might be a mobile signal produced by young stomatal cells that transits through plasmodesmata. It seems equally possible that failure to observe AGL16 mRNA reflects a transient requirement for expression, an issue that can be resolved by examining expression of transcriptional reporters. Regardless, additional work will be needed to place AGL16 in the known pathways and clarify the purpose and circumstances of miRNA regulation of this new positive regulator.
Conclusions and perspectives
Current data suggests that fate modulation of stomatal stem cells relies on finely tuned combinations of TMM and ERf receptors that communicate with a MAPK signaling cascade and regulatory transcription factors. One of our next great challenges is to understand the underlying purpose and mechanistic details of the observed complexity in receptor interactions revealed by TMM and ERf mutant combinations. One possibility is that many different receptor combinations are required to detect related ligands that carry subtly different messages to epidermal stem cells. In fact, we should expect that plant stem cells, like animal cells, must integrate a battery of incoming signals to make appropriate developmental decisions. EPF1 serves as a precursor-derived “proximity” signal to regulate adjacent cells, but not before meristemoids are present. A major question is how, at the population level, an appropriate number of MESCs are chosen for entry asymmetric divisions. Since EPF1 has paralogs in Arabidopsis, it is tempting to speculate that these peptides might serve as precursor “abundance” signals to coordinate the overall proportion of cells that enter the stomatal pathway (Figure 3b), or as global promoters or inhibitors of differentiation associated with whole organ development. Determining EPF1 family functions and possible interaction with hypothetical TMM/ER receptor pairings might resolve some of these questions, as would clarification of the connection between receptors, MAPK activity and transcriptional regulation by early- and late-acting bHLHs.
Another major gap in our knowledge is how asymmetric divisions are executed. SPCH may act as a switch, but what machinery actually polarizes stomatal precursor cells prior to asymmetric division [26,28], controls division plane, and ensures that the sister cells are functionally distinct? Despite recent advances, we still know little about what target genes are regulated by the stomatal bHLH, MYB or MADS-box transcription factors to yield unique cellular characteristics. It will also be important to determine precisely how these regulators of stomatal development interface with regulation of the cell cycle, or how stem cells are maintained outside meristems. Ultimately, answers to the questions will be valuable not only for dissecting developmental processes, but because stomata are of central importance to plant productivity and water use efficiency, as well as both ecosystem and global climate processes.
Acknowledgments
The author apologizes to those colleagues whose work could not be discussed in this article due to size limitations. I would like to thank Volker Kern, Fred Sack, and members of my lab for helpful discussions. Research in my lab is funded by the National Institute of Health and the National Science Foundation.
Abbreviations
- MESC
multipotent epidermal stem cell
- SLGC
stomatal lineage ground cell
- MMC
meristemoid mother cell
- M
meristemoid
- GMC
guard mother cell
- GC
guard cell
- AGL
AGAMOUS-like
- bHLH
basic helix-loop-helix transcription factor
- CBF
C-repeat binding factor
- LRR-RLP
leucine-rich repeat containing receptor-like protein
- LRR-RLK
leucine-rich repeat containing receptor kinase
- MAPK
mitogen-activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Geisler MD, Nadeau JA, Sack FD. Oriented asymmetric divisions that generate the stomatal spacing pattern in Arabidopsis are disrupted by the too many mouths mutation. Plant Cell. 2000;12:2075–2086. doi: 10.1105/tpc.12.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadeau JA, Sack F. Stomatal development: cross talk puts mouths in place. Trends Plant Sci. 2003;8:294–299. doi: 10.1016/S1360-1385(03)00102-X. [DOI] [PubMed] [Google Scholar]
- 3.Sachs T. Pattern Formation in Plant Tissues. New York: Cambridge University Press; 1991. [Google Scholar]
- 4.Savaldi-Goldstein S, Chory J. Growth coordination and the shoot epidermis. Curr Opin Plant Biol. 2008;11:42–48. doi: 10.1016/j.pbi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casson SA, Gray JE. Influence of environmental factors on stomatal development. New Phytol. 2008;178:9–23. doi: 10.1111/j.1469-8137.2007.02351.x. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann DC, Sack FD. Stomatal Development. Annu Rev Plant Biol. 2007;58:163–181. doi: 10.1146/annurev.arplant.58.032806.104023. [DOI] [PubMed] [Google Scholar]
- 7.Torii KU. Stomatal Patterning and Guard Cell Differentiation. In: Verma D, Hong Z, editors. Plant Cell Monographs. Springer; 2008. pp. 1861–1370. [Google Scholar]
- 8.Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436:866–870. doi: 10.1038/nature03835. [DOI] [PubMed] [Google Scholar]
- 9.Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal Patterning and Differentiation by Synergistic Interactions of Receptor Kinases. Science. 2005;309:290–293. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Sack FD. The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell. 1995;7:2227–2239. doi: 10.1105/tpc.7.12.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serna L, Fenoll C. Stomatal development and patterning in Arabidopsis leaves. Physiol Plant. 2000;109:351–358. [Google Scholar]
- 12.von Groll U, Altmann T. Stomatal cell biology. Curr Opin Cell Biol. 2001;4:555–560. doi: 10.1016/s1369-5266(00)00215-6. [DOI] [PubMed] [Google Scholar]
- 13.Geisler M, Yang M, Sack FD. Divergent regulation of stomatal initiation and patterning in organ and suborgan regions of the Arabidopsis mutants too many mouths and four lips. Planta. 1998;205:522–530. doi: 10.1007/s004250050351. [DOI] [PubMed] [Google Scholar]
- *14.Bhave N, Veley K, Nadeau J, Lucas J, Bhave S, Sack F. TOO MANY MOUTHS promotes cell fate progression in stomatal development of Arabidopsis stems. Planta. doi: 10.1007/s00425-008-0835-9. in press. This paper provides evidence that TMM controls meristemoid fate progression in stems. The absence of stomata from some organs reflects a fate transition defect that occurs later, rather than a failure to initiate the stomatal cell lineage. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal Development and Patterning Are Regulated by Environmentally Responsive Mitogen-Activated Protein Kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergmann DC, Lukowitz W, Somerville CR. Stomatal Development and Pattern Controlled by a MAPKK Kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- 17.Castellano MdM, Boniotti MB, Caro E, Schnittger A, Gutierrez C. DNA Replication Licensing Affects Cell Proliferation or Endoreplication in a Cell Type-Specific Manner. Plant Cell. 2004;16:2380–2393. doi: 10.1105/tpc.104.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kono A, Umeda-Hara C, Adachi S, Nagata N, Konomi M, Nakagawa T, Uchimiya H, Umeda M. The Arabidopsis D-Type Cyclin CYCD4 Controls Cell Division in the Stomatal Lineage of the Hypocotyl Epidermis. Plant Cell. 2007;19:1265–1277. doi: 10.1105/tpc.106.046763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boudolf V, Barroco R, Engler JdA, Verkest A, Beeckman T, Naudts M, Inze D, De Veylder L. B1-Type Cyclin-Dependent Kinases Are Essential for the Formation of Stomatal Complexes in Arabidopsis thaliana. Plant Cell. 2004;16:945–955. doi: 10.1105/tpc.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger D, Altmann T. A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 2000;14:1119–1131. [PMC free article] [PubMed] [Google Scholar]
- 21.von Groll U, Berger D, Altmann T. The subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during Arabidopsis stomatal development. Plant Cell. 2002;14:1527–1539. doi: 10.1105/tpc.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007;21:1720–1725. doi: 10.1101/gad.1550707. The authors have identified the first candidate for a ligand for TMM and ERECTA family receptor kinases by screening a large population of putative small peptides for overexpression phenotypes. Stomatal precursors produce the EPF1 peptide, which may act as a mobile signal that negatively regulates asymmetric divisions and orients nearby patterning asymmetric divisions. The work provides evidence that TMM, ERf, and YODA are downstream of EPF1 but SDD1 is not. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda H, Hirakawa Y, Sawa S. Peptide signaling in vascular development. Curr Opin Plant Biol. 2008;10:477–482. doi: 10.1016/j.pbi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Franssen H, Bisseling T. Peptide signaling in plants. Proc Natl Acad Sci. 2001;98:12855–12856. doi: 10.1073/pnas.231490798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann J. A gene expression map of Arabidopsis development. Nature Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Sack FD. Ultrastructure of stomatal development in Arabidopsis (Brassicaceae) leaves. Amer J Bot. 1999;86:929–939. [PubMed] [Google Scholar]
- 27.Lucas JR, Nadeau JA, Sack FD. Microtubule arrays and Arabidopsis stomatal development. J Exp Bot. 2006;57:71–79. doi: 10.1093/jxb/erj017. [DOI] [PubMed] [Google Scholar]
- 28.Geisler MJ, Deppong DO, Nadeau JA, Sack FD. Stomatal neighbor cell polarity and division in Arabidopsis. Planta. 2003;216:571–579. doi: 10.1007/s00425-002-0912-4. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura RL, McKendree WL, Jr, Hirsch RE, Sedbrook JC, Gaber RF, Sussmank MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Buylla ER, Liljegren SJ, Pelaz S, Gold SE, Burgeff C, Ditta GS, Vergara-Silva F, Yanofsky MF. MADS-box gene evolution beyond flowers: Expression in pollen, endosperm, guard cells, roots and trichomes. Plant J. 2000;24:457–466. doi: 10.1046/j.1365-313x.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- 31.Terryn N, Arias MB, Engler G, Tire C, Villarroel R, Van Montagu M, Inze D. rha1, a gene encoding a small GTP binding protein from Arabidopsis, is expressed primarily in developing guard cells. Plant Cell. 1993;5:1761–1769. doi: 10.1105/tpc.5.12.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI. Microarray Expression Analyses of Arabidopsis Guard Cells and Isolation of a Recessive Abscisic Acid Hypersensitive Protein Phosphatase 2C Mutant. Plant Cell. 2004;16:596–615. doi: 10.1105/tpc.019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galbiati M, Simoni L, Pavesi G, Cominelli E, Francia P, Vavasseur A, Nelson T, Bevan M, Tonelli C. Gene trap lines identify Arabidopsis genes expressed in stomatal guard cells. Plant J. 2008;53:750–762. doi: 10.1111/j.1365-313X.2007.03371.x. [DOI] [PubMed] [Google Scholar]
- 34.Bryson-Richardson RJ, Currie PD. The genetics of vertebrate myogenesis. Nat Rev Genet. 2008;9:632–646. doi: 10.1038/nrg2369. [DOI] [PubMed] [Google Scholar]
- 35.Quan X-J, Hassan BA. From skin to nerve: flies, vertebrates and the first helix. Cell Molec Life Sci. 2005;62:2036–2049. doi: 10.1007/s00018-005-5124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.MacAlister CA, Ohashi-Ito K, Bergmann DC. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature. 2007;445:537–540. doi: 10.1038/nature05491. This paper and its companion Pillitteri et al. (2007), uncover the role of a family of bHLH transcription factors related to FAMA. Each controls a separate fate specification event in the stomatal lineage and their functions reveal commonalities with animal cell fate specification. This paper focuses on the role of SPCH in controlling entry and amplifying asymmetric divisions. SPCH is expressed in cells that may divide asymmetrically. They also show that FAMA and MUTE cannot replace SPCH activity in controlling entry asymmetric divisions. [DOI] [PubMed] [Google Scholar]
- **37.Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445:501–505. doi: 10.1038/nature05467. This paper and its companion MacAlister et al. (2007), uncover the role of FAMA-like bHLH transcription factors. This paper focuses on the role of MUTE in promoting the fate transition between meristemoid and GMC. Defects in MUTE cause immortalization of meristemoids and failure to transition to the GMC. MUTE is found in nuclei of new GMCs and meristemoids that have undergone some self-renewing divisions, and ectopic MUTE expression is sufficient to convert most epidermal cell types to GMCs that produce GCs. [DOI] [PubMed] [Google Scholar]
- *38.Pillitteri LJ, Bogenschutz NL, Torii KU. The bHLH Protein, MUTE, Controls Differentiation of Stomata and the Hydathode Pore in Arabidopsis. Plant Cell Physiol. 2008;49:934–943. doi: 10.1093/pcp/pcn067. This paper provides more information about the functions of MUTE, including its role in hydathode formation and independence from auxin regulation. It also provides an indication that TMM/ERf signaling might be linked to the transition from meristemoid to GMC via MUTE. [DOI] [PubMed] [Google Scholar]
- **39.Ohashi-Ito K, Bergmann DC. Arabidopsis FAMA Controls the Final Proliferation/Differentiation Switch during Stomatal Development. Plant Cell. 2006;18:2493–2505. doi: 10.1105/tpc.106.046136. This paper describes the function of the bHLH transcription factor FAMA in guard cell differentiation. Mutants undergo reiterative rounds of symmetric division of the GMC, and never produce GCs. FAMA is expressed at the GMC to GC transition and overexpression converts any cell to GC, sometimes without prior division. FAMA seems to be a transcriptional activator that interacts with two other bHLH proteins with weak GC twinning phenotypes, but not the MYB protein FLP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai LB, Nadeau JA, Lucas J, Lee E-K, Nakagawa T, Zhao L, Geisler M, Sack FD. The Arabidopsis R2R3 MYB Proteins FOUR LIPS and MYB88 Restrict Divisions Late in the Stomatal Cell Lineage. Plant Cell. 2005;17:2754–2767. doi: 10.1105/tpc.105.034116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **41.Kanaoka MM, Pillitteri LJ, Fujii H, Yoshida Y, Bogenschutz NL, Takabayashi J, Zhu J-K, Torii KU. SCREAM/ICE1 and SCREAM2 Specify Three Cell-State Transitional Steps Leading to Arabidopsis Stomatal Differentiation. Plant Cell. 2008;20:1775–1785. doi: 10.1105/tpc.108.060848. A pair of new broadly expressed bHLH-LZ proteins, SCREAM1 and SCREAM2, control cell fate transitions in the stomatal lineage most likely through dimerization with MUTE, SPCH and FAMA bHLH proteins. SCREAM1 is identical to ICE1, which controls cold-induced gene expression, revealing an interesting signaling intersection between stomatal biogenesis and environmental responsiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chinnusamy V, Ohta M, Kanrar S, Lee B-h, Hong X, Agarwal M, Zhu J-K. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003;17:1043–1054. doi: 10.1101/gad.1077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabourin L, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- 44.Jan YN, Jan LY. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993;75:827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- 45.Hunter C, Poethig RS. miSSING LINKS: miRNAs and plant development. Curr Opin Gen Devel. 2003;13:371–378. doi: 10.1016/s0959-437x(03)00081-9. [DOI] [PubMed] [Google Scholar]
- **46.Kutter C, Schob H, Stadler M, Meins F, Jr, Si-Ammour A. MicroRNA-Mediated Regulation of Stomatal Development in Arabidopsis. Plant Cell. 2007;19:2417–2429. doi: 10.1105/tpc.107.050377. The MADS box protein AGL16 controls the number of cells that enter the stomatal pathway to form satellite meristemoids. AGL16 is regulated by miR824, revealing for the first time involvement of a recently evolved miRNA in stomatal biogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]