Abstract
The uropathogenic strain Escherichia coli J96 mediates mannose-resistant hemagglutination owing to production of a digalactoside-binding adhesin. A cosmid clone from this strain has been isolated that, when harbored in E. coli K-12, expressed Pap pili and this adhesin (R. Hull et al., Infect. Immun. 33:933-938, 1981). By transposon mutagenesis and by the construction of a number of hybrid plasmid derivatives, we have demonstrated that about 8.5 kilobases of DNA is required to generate a mannose-resistant hemagglutination-positive phenotype in E. coli K-12 strain P678-54. The structural gene for the Pap pili monomer, papA, has been identified and mapped close to the promotor-proximal end of the Pap operon. Although strain P678-54 that harbored a Tn5 insertion within papA showed a mannose-resistant hemagglutination-positive phenotype, it was negative in a competitive enzyme-linked immunosorbent assay with anti-Pap pilus serum. This could mean that a Pap adhesin is encoded by a region on the Pap operon that is distinct from papA.
Full text
PDF
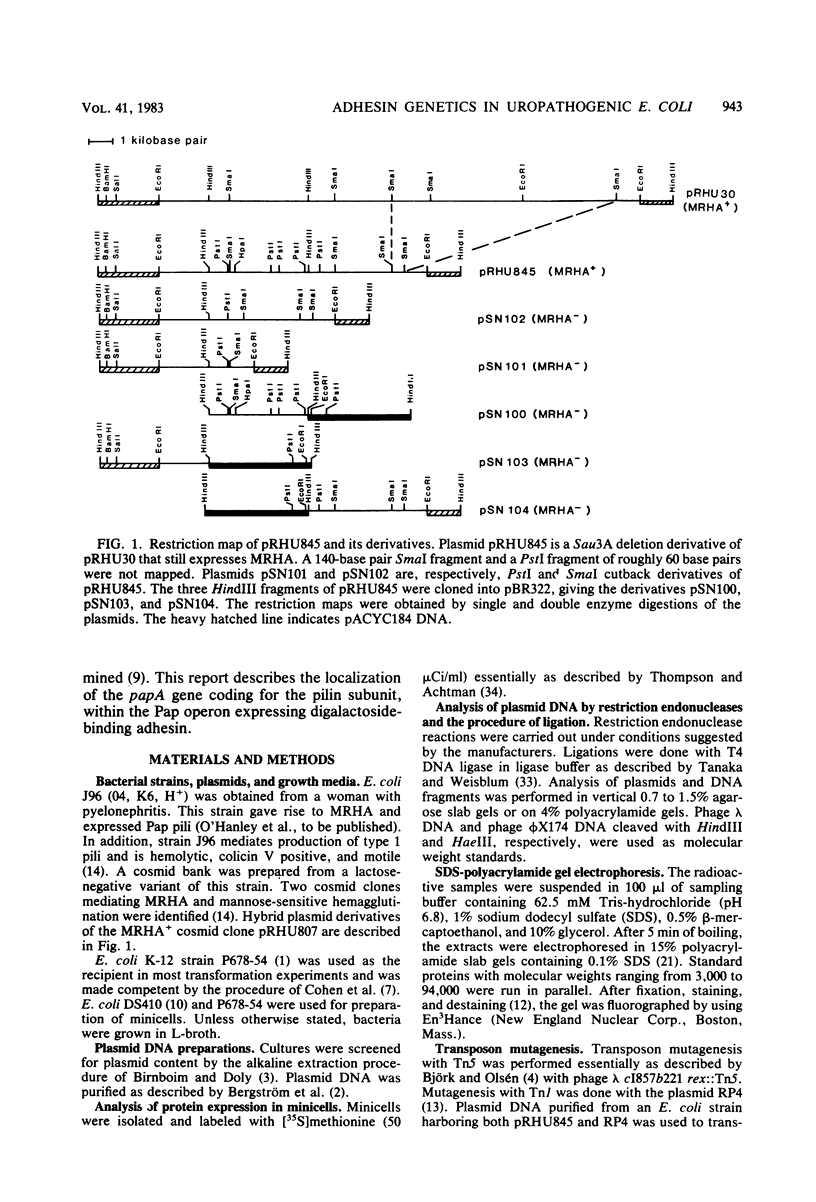

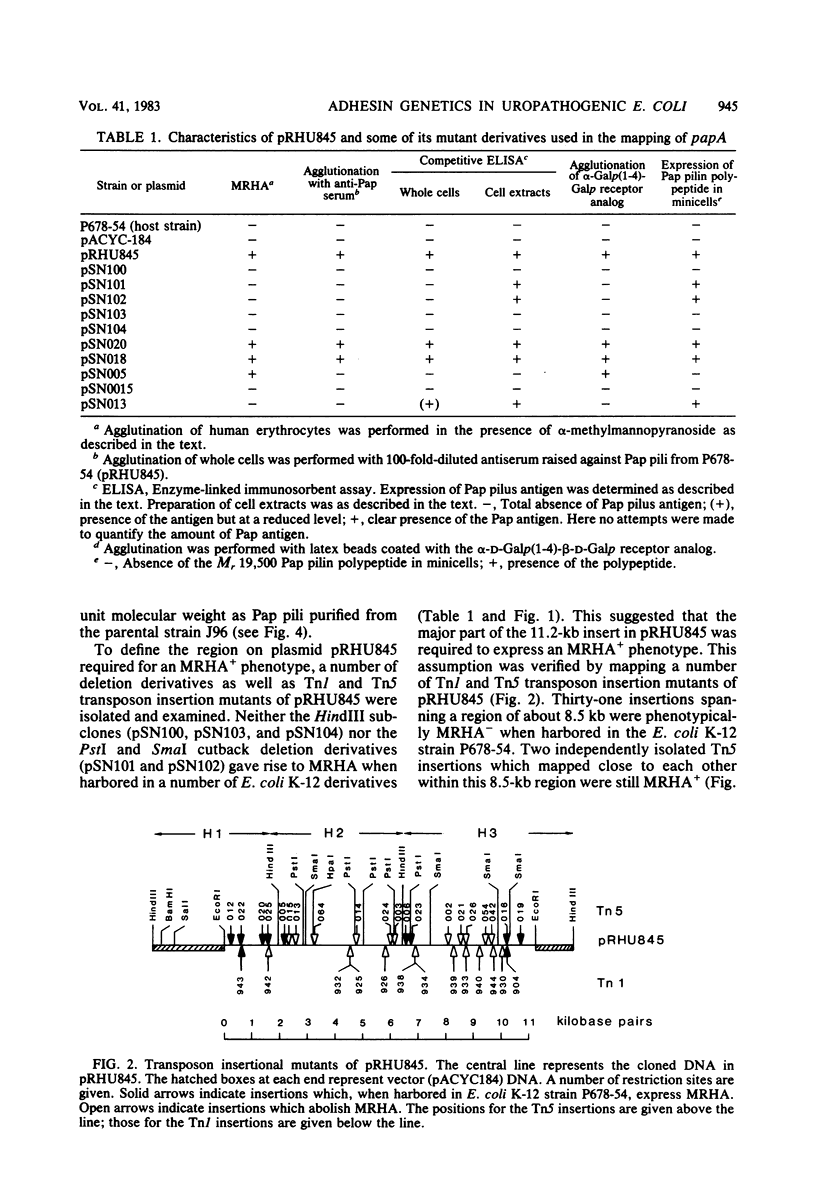
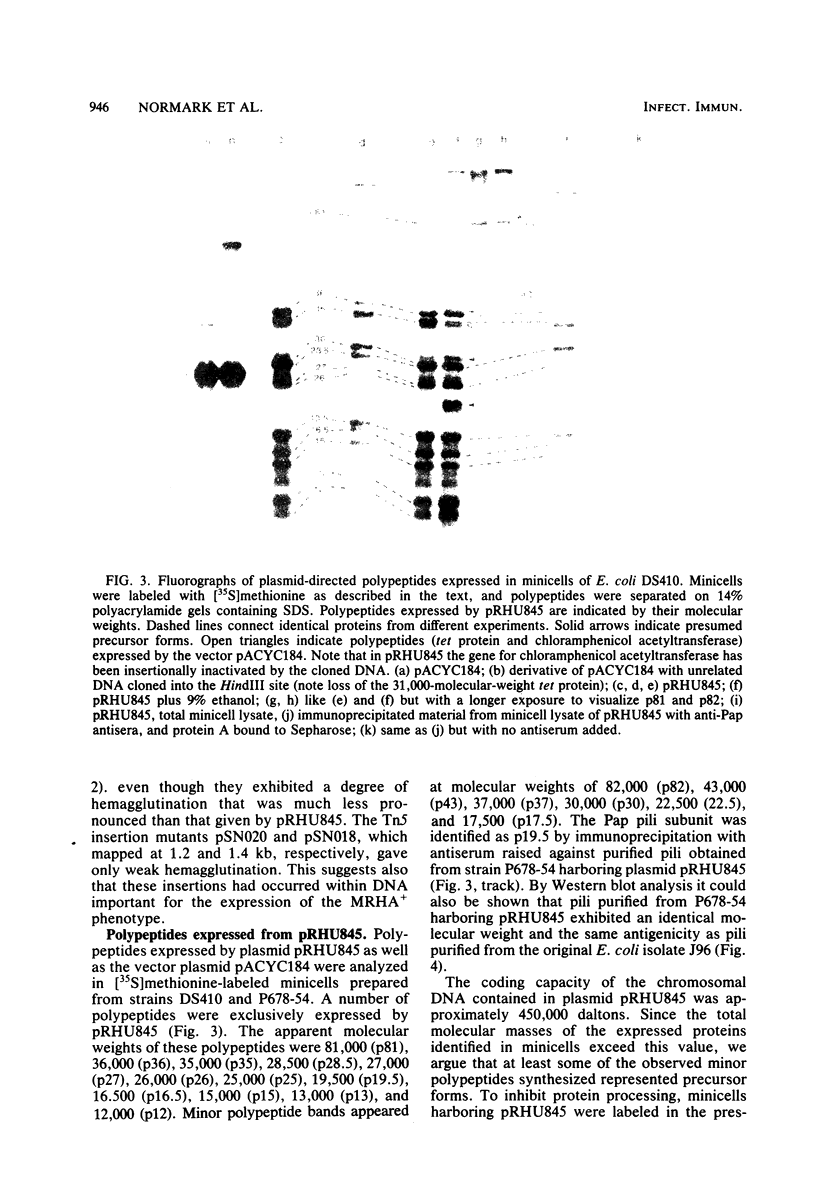


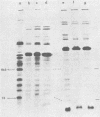
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Olsson O., Normark S. Common evolutionary origin of chromosomal beta-lactamase genes in enterobacteria. J Bacteriol. 1982 May;150(2):528–534. doi: 10.1128/jb.150.2.528-534.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Olsén A. A method for isolation of Escherichia coli mutants with aberrant RNA methylation using translocatable drug resistance elements. Acta Chem Scand B. 1979;33(8):591–593. doi: 10.3891/acta.chem.scand.33b-0591. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. The molecular nature of heat-labile enterotoxin (LT) of escherichia coli. Nature. 1979 Feb 1;277(5695):406–407. doi: 10.1038/277406a0. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. The molecular nature of heat-labile enterotoxin (LT) of escherichia coli. Nature. 1979 Feb 1;277(5695):406–407. doi: 10.1038/277406a0. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundström T., Jaurin B., Edlund T., Normark S. Physical mapping and expression of hybrid plasmids carrying chromosomal beta-lactamase genes of Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1127–1134. doi: 10.1128/jb.143.3.1127-1134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M., Sellwood R., Shipley P., Dougan G. Genetic analysis of K88-mediated adhesion of enterotoxigenic Escherichia coli. Nature. 1981 May 14;291(5811):122–126. doi: 10.1038/291122a0. [DOI] [PubMed] [Google Scholar]
- Källenius G., Svenson S. B., Möllby R., Korhonen T., Winberg J., Cedergren B., Helin I., Hultberg H. Carbohydrate receptor structures recognized by uropathogenic E. coli. Scand J Infect Dis Suppl. 1982;33:52–60. [PubMed] [Google Scholar]
- Källenius G., Svenson S., Möllby R., Cedergren B., Hultberg H., Winberg J. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet. 1981 Sep 19;2(8247):604–606. doi: 10.1016/s0140-6736(81)92743-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Harms N., Bakker D., de Graaf F. K. Organization and expression of genes involved in the production of the K88ab antigen. Infect Immun. 1981 Jun;32(3):1155–1163. doi: 10.1128/iai.32.3.1155-1163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Wouters C., Wijfjes A., de Graaf F. K. Construction and characterization of mutants impaired in the biosynthesis of the K88ab antigen. J Bacteriol. 1982 May;150(2):512–521. doi: 10.1128/jb.150.2.512-521.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Shafferman A. Conditional expression of the vesicular stomatitis virus glycoprotein gene in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6670–6674. doi: 10.1073/pnas.78.11.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheladia V. L., Chambers J. P., Guevara J., Jr, Evans D. J. Isolation, purification, and partial characterization of type V-A hemagglutinin from Escherichia coli GV-12, O1:H-. J Bacteriol. 1982 Nov;152(2):757–761. doi: 10.1128/jb.152.2.757-761.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Källenius G., Möllby R., Hultberg H., Winberg J. Rapid identification of P-fimbriated Escherichia coli by a receptor-specific particle agglutination test. Infection. 1982;10(4):209–214. doi: 10.1007/BF01666912. [DOI] [PubMed] [Google Scholar]
- Swanson J., Mayer L. W., Tam M. R. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982 Nov;38(2):668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]