Abstract
We recently reported that the ferrocene-containing cationic lipid BFDMA [bis(11-ferrocenylundecyl)dimethylammonium bromide] can be used to mediate cell transfection, and that levels of transfection depend critically upon the oxidation state of the ferrocenyl groups of the lipid. Here, we report that the redox activity of BFDMA can be exploited to transform lipoplexes formed from oxidized BFDMA (which do not transfect cells) to lipoplexes that are ‘active’ (and thus mediate high levels of transgene expression) by treatment with the chemical reducing agent glutathione (GSH). We demonstrate that GSH can be used to reduce the ferrocenium groups of oxidized BFDMA rapidly both (i) in solution and (ii) in lipoplexes formed by mixing oxidized BFDMA and DNA. Lipoplexes transformed in this manner mediate levels of cell transfection in vitro that are comparable to levels of transfection mediated by lipoplexes prepared by mixing DNA and reduced BFDMA. We demonstrate further that the chemical reduction of oxidized BFDMA leads to changes in the zeta potentials of these lipoplexes (e.g., from negative to positive). Characterization of lipoplex internalization using confocal microscopy demonstrated that these changes in zeta potential correlate to differences in the extents to which these lipoplexes are internalized by cells. These results provide a framework from which to interpret differences in cell transfection mediated by reduced and oxidized BFDMA. When combined, the results of this study suggest the basis of an approach that could be used to transform lipoplexes actively or ‘on-demand’ and provide spatial and/or temporal control over the transfection of cells in a range of different fundamental and applied contexts.
Introduction
Cationic lipids are used widely as agents for the delivery of DNA to cells because they can interact with DNA to form nanoscale aggregates (or ‘lipoplexes’) with sizes, charges, and other properties that can be used to address several critical barriers to cell transfection (1–6). Numerous past studies have identified a wide range of structural features and physical properties of cationic lipids that are important for the transfer of DNA into cells (1–12). This past work has contributed broadly to the development of cationic lipids and lipid-based formulations that can be used to promote high levels of cell transfection in vitro and in vivo.
Past studies on the development of cationic lipids for DNA delivery have focused, in large measure, on the design of lipids and lipoplex formulations that are (i) stable in extracellular environments (13–15) and/or (ii) unstable in (or designed to be responsive to) conditions found in intracellular environments (16–27). The former goal is often accomplished by the conjugation or incorporation of chemical functionality that resists protein adsorption or prevents the premature disruption of lipid/DNA interactions (13–15). The second goal has been addressed generally by the design of lipids containing functional groups that respond to changes in pH (16–19, 26), redox-potentials (19–23), or enzyme concentrations (19, 24, 25) present in intracellular environments. The broad motivations for the design of lipoplexes having this combination of properties are clear, as effective lipoplex-mediated transfer of DNA into cells will ultimately require lipoplexes that address effectively both extracellular barriers and intracellular barriers to the transport, internalization, and processing of DNA by cells.
In contrast to the work described above, less work has been focused on the development of lipoplexes or other non-viral DNA delivery systems that are designed to be transformed chemically or physically in extracellular environments (27). Lipoplexes that could be transformed in extracellular environments (for example, from states that may be ‘inactive’, and, thus, unable to transfect cells, to states that are ‘active’ and, thus, able to transfect cells efficiently) could be useful in a broad range of fundamental and applied contexts. For example, the ability to exert spatial and/or temporal control over the ‘activation’ of inactive lipoplexes in vitro could lead to new methods for the patterning of cell transfection or the delivery of DNA to defined sub-populations of cells, and could thus contribute broadly to the development of new tools for both basic and applied biomedical research. The ability to exert such control could also prove useful for the localization of DNA delivery in vivo.
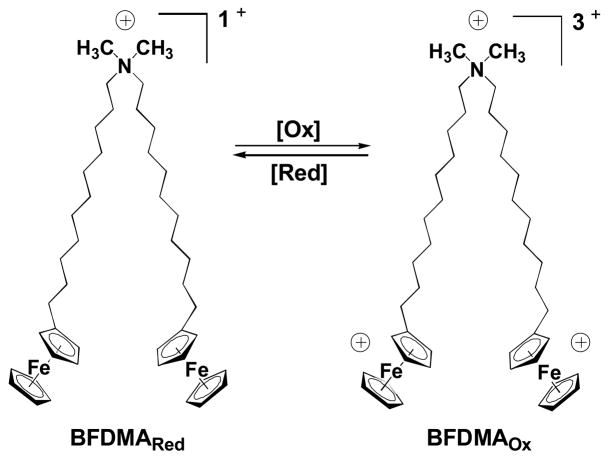
The work reported here builds upon the results of several past studies by our groups (28–42) and others (43–50) demonstrating that redox-active amphiphiles containing ferrocenyl groups can be used to exert spatial and/or temporal control over a broad range of properties of aqueous systems (28–36, 43–50) and interactions with macromolecular species (37), including DNA (38–42). Of particular relevance to the study reported here, we reported recently that a two-tailed, ferrocene-containing cationic lipid [bis-(11-ferrocenylundecyl)dimethylammonium bromide (BFDMA; Figure 1)] (44, 45, 47) can be used to transfect cells (38, 39). One important outcome of these past studies was the observation that the oxidation state of the ferrocenyl groups of the BFDMA used to form lipoplexes with DNA plays a critical role in determining the ability of lipoplexes to promote transgene expression (38, 39). Lipoplexes formed from DNA and reduced BFDMA (which has an overall net charge of +1) can be used to mediate high levels of cell transfection. However, lipoplexes formed using DNA and oxidized BFDMA (which has an overall net charge of +3) generally promote very low levels of cell transfection (38, 39). In subsequent studies aimed at characterizing the physical properties of lipoplexes formed from reduced and oxidized BFDMA, we identified differences in lipoplex sizes (38, 39, 41), internal dynamics (41), zeta potentials (41), and nanostructures (42) that correlated to these large differences in cell transfection. The results of these past studies, when combined, suggest that changes in the physical properties (and thus the transfection behaviors) of lipoplexes formed from reduced and oxidized BFDMA can be understood broadly in terms of changes in the amphiphilicity of BFDMA that occur upon the oxidation or reduction of ferrocene (38, 39, 41, 42). Because the redox state of ferrocenyl surfactants can, in general, be transformed rapidly and reversibly in aqueous environments (e.g., using either chemical or electrochemical methods) (28–\50), these past results also suggest a basis for the development of methods that could permit active or reversible control over the ability (or the inability) of lipoplexes formed from BFDMA to transfect cells.
Figure 1.
Structure of BFDMA, a redox-active cationic lipid. The charge density of BFDMA can be cycled between +1 (reduced) and +3 (oxidized) by oxidation or reduction of the ferrocenyl groups at the end of each hydrophobic tail.
This current investigation sought to determine whether it was possible to exploit the redox activity of ferrocene to transform ‘inactive’ lipoplexes formed from oxidized BFDMA to lipoplexes that are ‘active’ and, thus, able to transfect cells. We report here that it is possible to transform or ‘activate’ lipoplexes formed from DNA and oxidized BFDMA to lipoplexes that mediate high levels of transfection by treatment with a chemical reducing agent (glutathione, GSH). We demonstrate that GSH can be used to reduce the ferrocenium groups of oxidized BFDMA rapidly both (i) in solution and (ii) in lipoplexes formed by mixing oxidized BFDMA and DNA, and that lipoplexes transformed in this manner mediate levels of cell transfection in vitro that are comparable to levels of transfection mediated by lipoplexes prepared by the direct mixing of DNA and reduced BFDMA. We demonstrate further that the chemical reduction of the oxidized BFDMA in these lipoplexes leads to changes in zeta potentials (e.g., from negative values to positive values) and that these changes in zeta potential correlate to significant differences in the extents to which these lipoplexes are internalized by cells.
Our results demonstrate that it is possible to transform both the physicochemical properties and the biological behaviors of nanoscale aggregates of DNA and a redox-active cationic lipid. The results of this study, in combination with the results of our past studies (38, 39, 41, 42), suggest a basis for the development of methods for the transformation of cationic lipids and/or lipoplexes actively or ‘on-demand’ in ways that could be used to provide spatial and/or temporal control over the transfection of cells in vitro or in vivo.
Materials and Methods
Materials
Bis-(11-ferrocenylundecyl)dimethylammonium bromide (BFDMA) was synthesized as previously described (45). Glutathione (GSH) and lithium sulfate monohydrate were purchased from Sigma Aldrich (St. Louis, MO). Dodecyltrimethylammonium bromide (DTAB) was purchased from Acros Organics (Morris Plains, NJ). Plasmid DNA encoding enhanced green fluorescent protein [pEGFP-N1 (4.7 kb), >95% supercoiled] was purchased from the Waisman Clinical Biomanufacturing Facility at the University of Wisconsin – Madison. Plasmid DNA encoding firefly luciferase [pCMV-Luc, >95% supercoiled] was purchased from Elim Biopharmaceuticals, Inc. (San Francisco, CA). Deionized water (18 MΩ) was used to prepare all buffers and salt solutions. Dulbecco’s modified Eagle’s medium (DMEM), OptiMEM cell culture medium, phosphate-buffered saline (PBS), fetal bovine serum (FBS), Lipofectamine 2000, Live/dead viability/cytotoxicity assay kits, calcein AM, and Lysotracker Red were purchased from Invitrogen (Carlsbad, CA). Bicinchoninic acid (BCA) protein assay kits were purchased from Pierce (Rockford, IL). Glo Lysis Buffer and Steady-Glo Luciferase Assay kits were purchased from Promega Corporation (Madison, WI). Cy5 Label-IT nucleic acid labeling kits were purchased from Mirus Bio (Madison, WI). Glass inset dishes used for laser scanning confocal microscopy (LSCM) were purchased from MatTek (Ashland, MA). All commercial materials were used as received without further purification unless otherwise noted.
General Considerations
Oxidation of BFDMA was conducted at 75 °C using a bipotentiostat (Pine Instruments, Grove City, PA) and a three-electrode cell to maintain a constant potential of 500 mV between the working electrode and a Ag/AgCl reference electrode. Platinum mesh (1.0 in2) was used as the working and counter electrodes. The progress of oxidation was followed by monitoring current passed at the working electrode and by UV/visible spectrophotometry, as described previously (38, 39). All UV/visible absorbance values were recorded using a Beckman Coulter DU520 UV/vis Spectrophotometer (Fullerton, CA). All zeta potential measurements were performed using a Zetasizer 2000HS instrument (Malvern Instruments, Worcestershire, UK). Fluorescence microscopy images used to evaluate the expression of enhanced green fluorescent protein (EGFP) in cell transfection experiments were recorded using an Olympus IX70 microscope and were analyzed using the Metavue version 7.1.2.0 software package (Molecular Devices; Toronto, Canada). Fluorescence, luminescence, and absorbance measurements used to characterize cytotoxicity, luciferase expression, and total cell protein were made using a PerkinElmer EnVision multilabel plate reader (Calcein AM: Ex: 492 nm, Em: 535 nm; Ethidium homodimer-1: Ex: 535 nm, Em: 620 nm; Luciferase: Em: 700 nm cutoff; BCA: Abs: 560 nm). For DNA used in LSCM experiments, DNA was labeled using a Label-IT nucleic acid labeling kit according to the manufacturer’s protocol (labeling density ~100 labels per plasmid). Labeled DNA was purified by ethanol precipitation, and labeling densities were determined using a UV/vis spectrophotometer, as described by the manufacturer of the labeling kit. LSCM was performed using a Bio-Rad Radiance 2100 MP Rainbow laser scanning confocal microscope equipped with a multiphoton laser. LSCM images were processed using the LaserSharp 2000 processing kit (Bio-Rad; Hercules, CA), ImageJ 1.38x (National Institutes of Health; Washington, D.C.), and Photoshop CS2 (Adobe Systems; San Jose, CA).
Preparation of Reduced and Oxidized BFDMA Solutions
Solutions of reduced BFDMA were prepared by dissolving a desired mass of reduced BFDMA in aqueous Li2SO4 (1.0 mM) followed by serial dilution with water over the desired concentration range (see text). Solutions of oxidized BFDMA were prepared by electrochemical oxidation of a 1.0 mM Li2SO4 solution containing reduced BFDMA, followed by serial dilution to desired concentrations, as described previously (38, 39).
Preparation of Lipoplexes
Lipoplexes prepared from oxidized and reduced BFDMA were prepared in the following general manner. A solution of plasmid DNA (24 μg/mL in water) was added to a vortexing solution of aqueous Li2SO4 containing an amount of reduced or oxidized BFDMA sufficient to give the final lipid concentrations reported in the text. Total sample volumes for UV/vis absorbance experiments and zeta potential experiments were 1.0 mL. Sample volumes for LSCM and cell transfection experiments were 500 μL and 50 μL, respectively. Lipoplexes were allowed to stand at room temperature for at least thirty minutes before characterization or use in cell transfection experiments. Lipoplexes prepared to characterize the kinetics of reduction of BFDMA upon the addition of GSH were used immediately after preparation.
Characterization of the Transformation of Lipoplexes upon Addition of GSH
Experiments involving the addition of GSH to lipoplexes were performed by adding a negligible volume (0.5–5 μL) of a concentrated solution of GSH (0.5 M) to solutions of lipoplexes in an amount sufficient to yield the GSH:lipid molar ratios described in the text. Time-dependent changes in the oxidation state of BFDMA upon the addition of GSH to lipoplexes were characterized by measuring UV/vis absorbance spectra at wavelengths ranging from 400–800 nm. For these experiments, lipoplexes were prepared at higher lipid concentrations (but at equivalent lipid/DNA charge ratios) compared with lipoplexes prepared for use in transfection experiments to allow characterization of absorbance spectra. Following the acquisition of an initial wavelength scan, a kinetic scan was initiated by monitoring the absorbance of the solution at five second intervals at a wavelength of 630 nm (a wavelength characteristic of the absorbance of oxidized BFDMA). GSH was then added to the lipoplex solution, and the absorbance at 630 nm was monitored until a plateau in the measured value was observed. A second wavelength scan was performed over the range of 400–800 nm at the conclusion of the kinetic experiment. For experiments involving measurements made on solutions of reduced BFDMA, DTAB was added immediately prior to measurement of absorbance to eliminate clouding observed in BFDMA solutions at concentrations greater than 100 μM. Note that DTAB does not absorb light in the visible region of interest of 400–800nm. For experiments for which GSH was added to transform lipoplexes formed using oxidized BFDMA (described above), DTAB was added at the conclusion of each experiment before making a final absorbance measurement.
Characterization of Zeta Potential
Experiments designed to characterize the zeta potentials of lipoplexes after preparation or after exposure to GSH were conducted in the following general manner. For each sample prepared as described above, 1 mL of lipoplex solution was injected into the inlet of a Zetasizer 2000HS instrument, and measurements were made at ambient temperature using an electrical potential of 150V. A minimum of four measurements were recorded for each sample, and the Henry equation was used to calculate zeta potentials from measurements of electrophoretic mobility (51). For this calculation, we assumed the viscosity of the solution to be the same as that of water.
General Protocols for Transfection and Characterization of Gene Expression
COS-7 cells used in transfection experiments were grown in clear or opaque polystyrene 96-well culture plates (for experiments using pEGFP-N1 and pCMV-Luc, respectively) at initial seeding densities of 15,000 cells/well in 200 μL of growth medium (90% Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum, penicillin 100 units/mL, streptomycin 100 μg/mL). After plating, all cells were incubated at 37 °C for 24 hours. At approximately 80% confluence, culture medium was aspirated and replaced with 200 μL of serum-free media (OptiMEM), followed by addition of 50 oL of lipoplex samples. Lipoplexes prepared as described above were added to assigned wells via pipette in replicates of three, and the cells were incubated for four hours at 37°C, at which point lipoplex-containing media was aspirated from all wells and replaced with 200 μL of serum-containing medium. Samples were incubated for an additional 48 hours prior to characterization of transgene expression. For experiments conducted using lipoplexes formed using pEGFP-N1, cell morphology and relative levels of EGFP expression were characterized using phase contrast and fluorescence microscopy. For experiments conducted using lipoplexes formed using pCMV-Luc, quantitative cytotoxicity measurements were conducted using a commercially available fluorescence live/dead assay kit according to the manufacturer’s instructions. Luciferase protein expression was determined using a commercially available luminescence-based luciferease assay kit using the manufacturer’s specified protocol. Samples were compared with signals from control wells and/or normalized against total cell protein in each respective well using a commercially available BCA assay kit (Pierce). Lipoplexes formed using Lipofectamine 2000 were used as a positive control and prepared according to the manufacturer’s instructions.
Characterization of Internalization of Lipoplexes Using LSCM
COS-7 cells were grown in glass inset confocal microscopy dishes at initial seeding densities of 2.4 × 105 cells in 2.0 mL of growth medium [90% (v/v) Dulbecco’s modified Eagle’s medium, 10% (v/v) fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin]. Cells were allowed to grow overnight to approximately 90% confluence. Growth medium was then replaced with 2.0 mL of serum-free culture medium (OptiMEM), and 500 μL of lipoplex solutions (prepared using Cy5-labeled plasmid DNA) were added to sample dishes to achieve the final concentrations described in the text. Cells were incubated for four hours at 37 °C, and media was aspirated and replaced with 2 mL of PBS buffer. Immediately prior to imaging, cells were incubated with solutions of calcein AM (live cell stain) and Lysotracker Red (endosome/lysosome stain) according to the manufacturer’s protocols. Extents of internalization of fluorescently labeled DNA were then evaluated using LSCM. LSCM images were acquired using a 60x/1.40 NA oil-immersion objective. Calcein AM, Lysotracker Red, and Cy5-labeled DNA were excited sequentially using laser lines at 488, 543, and 637 nm, respectively. Fluorescence emission signals were collected for three individual channels using direct scanning mode (N = 1, scan speed = 50 lps) and merged to create three-color images.
Results and Discussion
The results of our past studies demonstrate that the oxidation state of the ferrocene groups in BFDMA influences significantly both the physical properties of BFDMA/DNA aggregates and the ability of these aggregates to transfect cells in vitro (38, 39, 41, 42). Because the oxidation state of ferrocenyl surfactants can be transformed readily using a variety of chemical or electrochemical methods (for example, using electrochemical potentials applied to electrodes or a range of different chemical oxidizing or reducing agents) (28–50), BFDMA offers the potential to formulate lipoplexes with physical properties or behaviors that can be transformed actively or reversibly. This investigation sought to determine (i) whether it was possible to use chemical reducing agents to reduce the ferrocenium groups in lipoplexes formed using DNA and oxidized BFDMA and (ii) whether the reduction of oxidized BFDMA could cause changes in the physical properties of lipoplexes sufficient to alter the ability of these aggregates to transfect cells. In the sections below, we describe the treatment and transformation of lipoplexes formed from oxidized BFDMA using GSH, a naturally-occurring chemical reducing agent found in both extracellular and intracellular environments and involved in numerous biochemical processes and synthetic pathways (52).
Chemical Reduction of Oxidized BFDMA and Oxidized BFDMA/DNA Lipoplexes Using GSH
GSH has been demonstrated in past studies to mediate the chemical reduction of ferrocenium species to ferrocenyl species (53, 54). It was not clear at the outset of this study, however, whether GSH could be used to promote the reduction of oxidized BFDMA or whether the reduction of oxidized BFDMA within lipoplexes could be made to occur at rates, or to extents, that would be useful in the context of cell transfection. We thus conducted a series of initial experiments to characterize the ability of GSH to mediate the reduction of oxidized BFDMA in solution (i.e., in the absence of DNA). Characterization of solutions of oxidized BFDMA in aqueous Li2SO4 using UV/vis spectrophotometry demonstrated that the addition of GSH (at molar excesses ranging from 10-fold to 50-fold) could be used to cause the quantitative reduction of oxidized BFDMA to reduced BFDMA (see Figure S1, Supporting Information). Additional experiments demonstrated that reduction occurred rapidly at high concentrations of GSH (e.g., within seconds to minutes in the presence of a 50-fold excess of GSH; reduction occurred more slowly (e.g., over ~90 min) in the presence of a 10-fold excess of GSH). The results of these initial experiments demonstrated the feasibility of reducing oxidized BFDMA using GSH and provided a baseline for characterization of the reduction of oxidized BFDMA in lipoplexes using UV/vis spectrophotometry.
We next conducted a series of experiments to determine whether it was possible to use GSH to reduce the oxidized BFDMA present in lipoplexes formed by mixing plasmid DNA and oxidized BFDMA. For these experiments, we used lipoplexes formed using oxidized BFDMA and plasmid DNA at a lipid:DNA charge ratio (CR) of 4.1:1. This charge ratio was chosen for these initial experiments on the basis of our past transfection results demonstrating that lipoplexes formed from oxidized BFDMA at a CR of 4.1:1 do not transfect cells, but that lipoplexes formed using reduced BFDMA at a CR of 1.4:1 mediate high levels of cell transfection (39). [We note here, in this context, that lipoplexes formed from DNA and oxidized BFDMA (which has a net charge of +3) at a CR of 4.1:1 contain the same molar ratio of BFDMA and DNA as lipoplexes formed from DNA and reduced BFDMA (which has a net charge of +1) at a CR of 1.4:1. (39)]
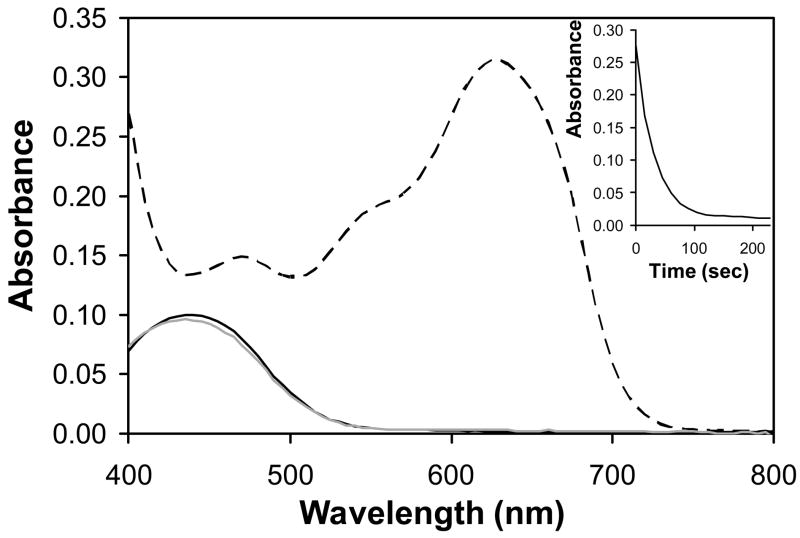
Figure 2 shows the UV/vis absorbance spectrum (over wavelengths ranging from 400 nm to 800 nm) of a solution of lipoplexes formed using oxidized BFDMA (dotted curve). Inspection of these data reveals an overall shape similar to that of solutions of oxidized BFDMA, and an absorbance peak at 630 nm that is diagnostic of oxidized BFDMA (as reported in past studies (38, 39); see also Figure S1, Supporting Information). Figure 2 also shows the UV/vis absorbance spectrum of a solution of lipoplexes of oxidized BFDMA after the addition of a 50-fold molar excess of GSH. Inspection of these data (solid gray curve) reveals large changes in the absorbance spectrum. In particular, we note the loss of the absorbance peak at 630 nm and the appearance of a new maximum in absorbance at 445 nm. The maximum in absorbance at 445 nm indicates the presence of reduced BFDMA (38, 39), and the overall shape of this new absorbance spectrum is identical to that of the absorbance spectrum of lipoplexes formed using reduced BFDMA (at a CR of 1.4:1; see Figure 2, dark solid curve) (38, 39). These observations, when combined, demonstrate that (i) GSH is able to reduce the BFDMA in aggregates formed by the mixing of DNA and oxidized BFDMA and (ii) that the reduction of oxidized BFDMA occurs quantitatively under these conditions (e.g., as evidenced by the complete disappearance of the peak in absorbance at 630 nm). Subsequent investigation of the time-dependence of the decrease in absorbance at 630 nm also demonstrated that the reduction of oxidized BFDMA occurred rapidly at higher concentrations of GSH (e.g., over a period of ~100 seconds in the presence of 50-fold excess GSH; see Figure 2, inset). Further characterization revealed this transformation to occur more slowly at lower concentrations of GSH (e.g., treatment of lipoplexes with a 10-fold molar excess of GSH resulted in the complete reduction of oxidized BFDMA, but this transformation occurred over a period of ~90 minutes; data not shown).
Figure 2.
UV/visible absorbance spectra for solutions of lipoplexes formed from DNA and reduced (dark solid line) or oxidized (dark dashed line) BFDMA. The light solid line corresponds to lipoplexes formed from DNA and oxidized BFDMA and treated with a 50-fold excess of GSH. Inset: Decrease in absorbance vs. time for lipoplexes formed from DNA and oxidized BFDMA upon treatment with a 50-fold excess of GSH. Absorbance was monitored at the absorbance maximum for oxidized BFDMA (630 nm).
Characterization of Levels of Cell Transfection Mediated by Transformed Lipoplexes
The results above demonstrate that GSH can be used to chemically reduce BFDMA in lipoplexes formed from oxidized BFDMA and plasmid DNA. We next performed a series of experiments to determine whether transformation of the redox state of BFDMA in these lipoplexes could change the ability of these lipoplexes to mediate cell transfection in vitro. These experiments were conducted using the COS-7 cell line, a plasmid DNA construct encoding enhanced green fluorescent protein (EGFP), and transfection protocols similar to those used in our past studies (38, 39). To evaluate the general feasibility of this approach, we conducted a series of transfection experiments using lipoplexes at CRs identical to those used for the experiments described above (but at a lower overall concentration of BFDMA of 10 μM). As noted above, lipoplexes prepared using oxidized BFDMA at a CR of 4.1:1 were found to be ‘inactive’ and did not mediate high levels of cell transfection in our past studies, but lipoplexes prepared using reduced BFDMA at a CR of 1.4:1 did mediate high levels of transgene expression (38, 39). This series of initial experiments was thus designed to determine whether the addition of GSH to solutions of lipoplexes formed from oxidized BFDMA could be used to produce solutions of lipoplexes that promote high levels of cell transfection.
Figure 3 shows representative fluorescence microscopy images of cells 48 hours after treatment with lipoplexes prepared using A) reduced BFDMA, B) oxidized BFDMA, or C) lipoplexes prepared using oxidized BFDMA and treated with a 20-fold excess of GSH prior to addition to cells (see Materials and Methods section for additional details of transfection protocols). Inspection of the images in Figures 3A and 3B reveals that lipoplexes prepared using reduced BFDMA mediated high levels of EGFP expression and that lipoplexes prepared using oxidized BFDMA did not. These results are consistent with the results of our past studies (38, 39) and illustrate the significant influence of the oxidation state of BFDMA on levels of cell transfection. Inspection of the image in Figure 3C, however, reveals levels of EGFP expression that are (i) significantly higher than those shown in Figure 3B and (ii) qualitatively similar to the high levels of EGFP expression shown in Figure 3A. We conclude on the basis of these results that the addition of GSH to lipoplexes formed using oxidized BFDMA can be used to ‘activate’ or transform solutions of these ‘inactive’ lipoplexes to lipoplexes that mediate levels of transgene expression that are qualitatively similar to those formed using reduced BFDMA.
Figure 3.

Representative fluorescence micrographs (10X) of EGFP Expression in COS-7 cells treated with lipoplexes formed from A) pEGFP-N1 and 10 μM reduced BFDMA, B) pEGFP-N1 and oxidized BFDMA, and C) pEGFP-N1 and oxidized BFDMA and treated with a 20-fold excess of GSH following lipoplex formation. Images were acquired at 48 h after exposure of cells to lipoplexes in serum-free medium (see text).
To characterize the ability of GSH-treated lipoplexes to mediate cell transfection more quantitatively, and across a greater range of lipid:DNA charge ratios, we conducted a second set of transfection experiments using lipoplexes formed from BFDMA and a plasmid DNA construct encoding firefly luciferase (pCMV-Luc). These transfection experiments were performed in a manner identical to our past experiments used to quantify the ability of reduced and oxidized BFDMA to mediate cell transfection (38, 39). These experiments were conducted using lipoplexes prepared at molar concentrations of BFDMA ranging from 2 μM to 60 μM. These molar concentrations correspond to lipid:DNA CRs ranging from 0.3:1 to 8.3:1 (for lipoplexes prepared using reduced BFDMA) and from 0.8:1 to 24.8:1 (for lipoplexes prepared using oxidized BFDMA). A table comparing lipid:DNA CRs to the molar concentrations of BFDMA used in the experiments described below can be found in a past publication (39).
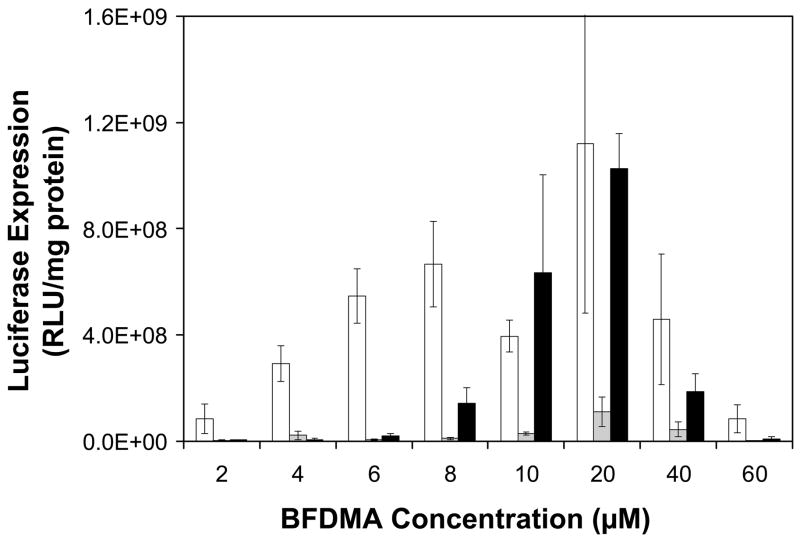
Figure 4 shows quantitative luciferase expression results (expressed as relative light units normalized to total cell protein) for cells treated with lipoplexes prepared using concentrations of reduced BFDMA (white bars) or oxidized BFDMA (gray bars) ranging from 2 μM to 60 μM. Figure 4 also shows levels of luciferase expression for experiments using GSH-treated lipoplexes over this same range of BFDMA concentrations (black bars). The results of experiments using lipoplexes prepared using reduced and oxidized BFDMA (white bars and gray bars) are in general agreement with the results of our past studies (38, 39), and confirm that reduced BFDMA mediates high levels of cell transfection over a broad range of lipid concentrations, while oxidized BFDMA, in general, does not. Inspection of the data corresponding to levels of transfection mediated by GSH-treated lipoplexes reveals that these aggregates are also able to mediate transgene expression over at lipid concentrations ranging from 8 μM to 40 μM at levels that are, in several cases, comparable to the levels of expression mediated by lipoplexes formed from reduced BFDMA. The levels of transfection mediated in control experiments using lipoplexes formed from reduced BFDMA and treated with 20 μM GSH prior to addition to cells were identical (within error) to the levels of luciferase expression mediated by lipoplexes formed from reduced BFDMA alone (data not shown). These results, when combined, provide support for the view that the levels of gene expression mediated by the GSH-treated lipoplexes formed from oxidized BFDMA shown in Figure 4 result from the chemical reduction of oxidized BFDMA and not from any potential general influence of added GSH on cell transfection.
Figure 4.
Normalized luciferase expression in COS-7 cells mediated by lipoplexes formed from reduced BFDMA (white bars), oxidized BFDMA (grey bars), and oxidized lipoplexes treated with a 20-fold excess of GSH prior to addition to cells (black bars). Experiments were performed in Opti-MEM with an exposure time of 4 h.
Inspection of the data in Figure 4 also demonstrates that levels of luciferase expression mediated by reduced BFDMA are a function of lipid concentration: levels of luciferase expression increase up to concentrations of 20 μM and decrease at concentrations higher than 40 μM (at which levels of lipid cytotoxicity become high) (38, 39). Further inspection of the results using GSH-treated lipoplexes of oxidized BFDMA reveals a similar concentration dependence (black bars), but over a more narrow range of concentrations (e.g., from 8 μM to 40 μM). We note that the decrease in luciferase expression at higher lipid concentrations (e.g., from 40 μM to 60 μM) again results, at least in part, from significant increases in the cytotoxicity of BFDMA at these concentrations (as described above; see also Figure S2 in the Supporting Information for results showing levels of cytotoxicity corresponding to transfection experiments in Figure 4). The specific reasons for the much lower levels of luciferase transfection at lower lipid concentrations (e.g., from 2 μM to 8 μM) is not yet completely understood. However, the results shown in Figures 3 and 4, when combined, do demonstrate that the treatment of lipoplexes formed from oxidized BFDMA (at concentrations ranging from 10 μM to 20 μM) with GSH can be transformed or activated in a manner that leads to lipoplexes that transfect cells at levels similar to those mediated by lipoplexes formed from reduced BFDMA. In the sections below, we demonstrate that these large changes in transfection behavior may result, at least in part, from large changes in the zeta potentials of lipoplexes upon treatment with GSH.
Characterization of the Zeta Potentials of Lipoplexes upon Treatment with GSH
We reported previously that the zeta potentials of lipoplexes formed from DNA and reduced BFDMA are negative at low lipid concentrations and positive at the lipid concentrations that promote the highest levels of transfection (e.g., 20 μM) (41). In contrast, lipoplexes formed using oxidized BFDMA were measured to remain negative or near neutral over the broad range of lipid concentrations used in the transfection experiments described above (as measured in both aqueous Li2SO4 and in serum-free cell culture media). Because particles with positive zeta potentials are generally internalized by cells more readily than particles with negative zeta potentials (e.g., by non-specific endocytosis) (55, 56), these results provided a framework from which to interpret and evaluate the large differences in cell transfection mediated by reduced and oxidized BFDMA (e.g., Figure 4) (42). The results shown in Figures 3 and 4 demonstrate clearly that the treatment of lipoplexes of oxidized BFDMA with GSH leads to large changes in luciferase expression. We sought to understand whether these large increases in the transfection behavior of these lipoplexes could also be understood in terms of changes in zeta potentials that occur upon treatment with GSH.
Table 1 shows zeta potentials measured for lipoplexes formed from reduced or oxidized BFDMA either (i) before or (ii) after the addition of GSH. For these experiments, lipoplexes were formed at lipid:DNA CRs of 4.1:1 at total lipid concentrations of either 500 μM or 10 μM. These conditions were evaluated to permit comparison to the results of both UV/vis spectrophotometry experiments shown in Figure 2 ([BFDMA] = 500 μM) and the results of cell transfection experiments shown in Figures 3–5 ([BFDMA] = 10 μM).
Table 1.
Zeta Potentials of BFDMA Lipoplexes
| Sample | 500 μM BFDMAa | 10 μM BFDMAb |
|---|---|---|
| Red BFDMA | 26.5 ± 2.3 mV | 27.6 ± 5.5 mV |
| Red BFDMA + GSH | 26.9 ± 6.3 mV | 26.3 ± 1.9 mV |
| Ox BFDMA | −16.0 ± 0.5 mV | −18.9 ± 3.1 mV |
| Ox BFDMA + GSH | 30.3 ± 1.0 mV | 17.6 ± 0.9 mV |
Concentrations given correspond to concentrations of BFDMA used in
UV/Vis spectrophotometry experiments and
cell transfection and DNA internalization experiments. Note that in all cases, lipid:DNA charge ratios were fixed at 1.4:1 for reduced lipoplexes and 4.1:1 for oxidized lipoplexes (see text).
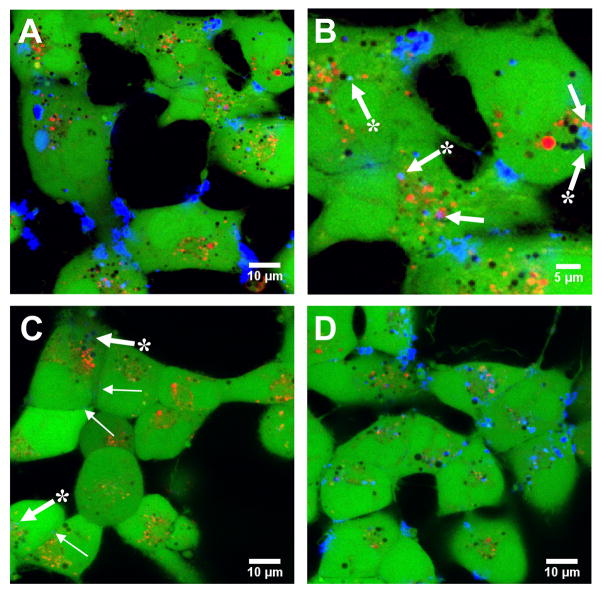
Figure 5.
Representative confocal fluorescence micrographs showing DNA internalization in COS-7 cells treated with lipoplexes formed from A–B) fluorescently-labeled pEGFP-N1 and 10 μM reduced BFDMA, C) fluorescently-labeled pEGFP-N1 and 10 μM oxidized BFDMA, and D) fluorescently-labeled pEGFP-N1 and 10 μM oxidized BFDMA and treated with a 20-fold excess of GSH following lipoplex formation. Images were acquired 4 h after addition of lipoplexes (see Materials and Methods). Fluorescence signals correspond to Cy5 (fluorescently-labeled DNA; blue signal), calcein AM (live cell stain; green signal), and Lysotracker Red (endosome/lysosome stain; red signal).
The results shown in Table 1 demonstrate that the zeta potentials of lipoplexes formed from reduced BFDMA were positive (approximately +27 mV) and that the zeta potentials of lipoplexes formed from oxidized BFDMA were negative (from approximately −16 mV to −18 mV) at both lipid concentrations. These results are in general agreement with the results of our past study (41). The data in Table 1 also demonstrate that the addition of a 20-fold molar excess of GSH to lipoplexes of reduced BFDMA results in no significant changes in zeta potential. We note, however, that the addition of a 20-fold molar excess of GSH to lipoplexes of oxidized BFDMA results in large changes in zeta potential. In particular, the zeta potentials of these lipoplexes were found to change from negative to positive upon treatment with GSH (e.g., values of approximately +30 mV and +18 mV for lipoplexes prepared at 500 μM and 10 μM lipid, respectively). These results, when combined with those shown in Figure 2, provide additional support for the view that the treatment of lipoplexes of oxidized BFDMA with GSH results in chemical reduction of the ferrocenium groups of BFDMA. These results also suggest that these GSH-treated lipoplexes are able to reorganize physically over periods of ~30 minutes in a manner that results in lipoplexes with positive zeta potentials. Additional experiments will be required to characterize changes in the nanostructures of these GSH-transformed lipoplexes and understand the dynamics of this physical transformation more completely. We demonstrate below, however, that these changes in zeta potential are consistent with large differences in the extents to which these lipoplexes are internalized by cells.
Characterization of the Internalization of Lipoplexes Using Confocal Microscopy
The data described above, and the results of our past studies,(41) suggest that the redox state of the ferrocenyl groups of BFDMA could influence transfection, at least in part, by its influence on the zeta potentials of lipoplexes (which could, in turn, result in differences in the rates at which, or the extents to which, lipoplexes are internalized by cells). To characterize the internalization of lipoplexes by cells directly, we conducted a series of experiments using laser scanning confocal microscopy (LSCM) and lipoplexes formed using plasmid DNA labeled with a fluorescent probe (Cy5). Figure 5 shows representative LSCM images corresponding to cells incubated with A–B) lipoplexes formed from reduced BFDMA, C) lipoplexes formed from oxidized BFDMA, or D) lipoplexes formed from oxidized BFDMA and then treated with a 20-fold excess of GSH prior to addition to cells. In these images, Cy5-labeled DNA is false-colored blue; the green and red fluorescence signals in these images correspond to calcein AM (a whole-cell stain) and Lysotracker Red (an endosome/lysosome stain), respectively. These experiments were conducted using lipoplexes prepared at charge ratios and lipid concentrations identical to those used in the transfection experiments shown in Figure 3. Lipoplexes were added to cells and incubated for four hours before acquisition of the fluorescence microscopy images shown in Figure 5 (see Materials and Methods for additional details).
Figure 5A shows a representative image of cells treated with lipoplexes of reduced BFDMA at a magnification of 600X. The image in Figure 5B shows an enlarged view of a portion of the image shown in Figure 5A. Inspection of these two images reveals DNA to be present in three different locations: (i) in large aggregates that appear to be associated with (but not internalized by) cells, (ii) in endosomes and/or lysosomes (e.g., as identified by plain white arrows in Figure 5B), and (iii) in small, punctate blue fluorescent spots in the cytosol that are not co-localized with endosomes or lysosomes (e.g., as identified by starred white arrows in Figure 5B). (We note here for clarity that the endosome/lysosome stain used in this experiment is red, but that the vesicles in these images, in general, appear as yellow or orange/yellow in cases where calcein is also co-localized; stained vesicles that also contain Cy-5 labeled DNA appear as punctate magenta-colored spots in these images).
The images in Figures 5A–B demonstrate that lipoplexes formed from reduced BFDMA are internalized readily during the four-hour period over which they were exposed to cells. These results contrast to the results shown in the image in Figure 5C, which corresponds to cells treated with lipoplexes of oxidized BFDMA. Inspection of this image reveals that these cells do contain areas of punctate blue fluorescence (e.g., as identified by starred white arrows), but that these punctate structures are both smaller in size and are less numerous than those observed in Figure 5B. Inspection of this image also reveals small areas of blue fluorescence that are associated with, but that are apparently not internalized by, these cells (e.g., as identified by thin plain white arrows). The results shown in Figure 5C are representative of the sizes and numbers of punctate structures observed within cells treated with lipoplexes of oxidized BFDMA. We note, however, that considerable variability existed in the amounts of DNA that were associated with cell membranes. A comparison of the results in Figure 5C to those in Figures 5A–B suggests that the redox state of BFDMA influences significantly the internalization of lipoplexes by cells over the four-hour incubation period investigated in this study. We note that these apparent differences in extents of internalization correlate with the results of zeta potential measurements discussed above (Table 1) and provide a basis for interpreting the large differences in cell transfection mediated by reduced and oxidized BFDMA (e.g., Figures 3 and 4). Figure 5D shows images of cells that were incubated in the presence of GSH-treated lipoplexes for four hours and reveals small, punctate blue fluorescent spots in nearly all cells. These results also correlate with our measurements of changes in zeta potential and cell transfection described above, and provide additional support for the view that chemical reduction of oxidized BFDMA by GSH can be used to activate lipoplexes of oxidized BFDMA that are otherwise inactive and do not transfect cells efficiently.
The observation of lipoplexes in Figures 5A–B and 5D that are co-localized with intracellular vesicles is consistent with the internalization of lipoplexes by endocytosis and, in general, with the results of past studies investigating the internalization of polyplexes formed using a range of other lipids (1–6). In this context, the observation of lipoplexes that are not co-localized with endosomes or lysosomes is an interesting result, and suggests that lipoplexes formed from reduced BFDMA (or GSH-treated lipoplexes of oxidized BFDMA) may be able to mediate escape from these intracellular vesicles (or, alternatively, that they could potentially enter cells through a mechanism that does not involve endocytosis). Additional experiments will be required to characterize mechanisms of lipoplex internalization and trafficking quantitatively and determine the ultimate fates of lipoplexes of BFDMA more completely. In the context of this present study, however, we do observe that differences in transfection measured using reduced and oxidized BFDMA appear to correlate with a limited ability of oxidized BFDMA to mediate the transport of DNA into cells as compared to reduced BFDMA.
Summary and Conclusions
We have demonstrated that it is possible to exploit the redox properties of ferrocene to chemically activate or transform ‘inactive’ lipoplexes formed from oxidized BFDMA and DNA to lipoplexes that mediate high levels of cell transfection by treatment with the chemical reducing agent glutathione (GSH). Our results demonstrate that GSH can be used to reduce the ferrocenium groups of oxidized BFDMA rapidly and quantitatively both (i) in solution and (ii) when it is present as a component of a lipoplex. The results of our cell transfection experiments demonstrate that lipoplexes of oxidized BFDMA treated with GSH mediate levels of transgene expression that are, in several cases, comparable to those mediated by lipoplexes prepared by mixing DNA and reduced BFDMA.
We suggested in a previous report (41) that the dependence of cell transfection on the oxidation state of BFDMA could arise from differences in the zeta potentials of lipoplexes formed from either reduced or oxidized BFDMA. Characterization of the zeta potentials of lipoplexes in this present study revealed that the surface charges of lipoplexes of oxidized BFDMA undergo large changes in zeta potential (e.g., from negative to positive) upon treatment with GSH. In addition, the results of our confocal microscopy experiments using lipoplexes formulated using fluorescently labeled DNA suggested that differences exist in the levels at which lipoplexes of reduced BFDMA and lipoplexes of oxidized BFDMA were internalized by cells. These experimental results, when combined, provide experimental support for the proposition that the oxidation state of BFDMA influences cell transfection, at least in part, through its influence on the zeta potentials of lipoplexes formed from reduced and oxidized BFDMA.
In summary, we have demonstrated that it is possible to transform both the physicochemical properties and the biological behaviors of nanoscale aggregates of DNA and a redox-active cationic lipid by treatment with a chemical reducing agent. These results suggest the basis of a general approach that could, with further development, lead to methods for the transformation of cationic lipids and/or lipoplexes actively or ‘on-demand’ in ways that could be used to provide spatial and/or temporal control over the transfection of cells in vitro or in vivo.
Supplementary Material
Results of additional experiments, including characterization of GSH-mediated reduction of oxidized BFDMA and characterization of cytotoxicity. This information is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
Financial support was provided by the National Institutes of Health (1 R21 EB006168) and the National Science Foundation (CTS-0754921). We are grateful to Lance Rodenkirch, Michael Hendrickson, and the W. M. Keck Center for Biological Imaging at the UW for access to and assistance with confocal microscopy facilities. We thank Selin Aytar and John Muller for many helpful discussions. D. M. L. is a Research Fellow of the Alfred P. Sloan Foundation.
References
- 1.Kabanov AV, Felgner PL, Seymour LW. Self-Assembling Complexes for Gene Delivery: From Laboratory to Clinical Trial. John Wiley and Sons; New York: 1998. [Google Scholar]
- 2.Zabner J. Cationic lipids used in gene transfer. Adv Drug Deliv Rev. 1997;27:17–28. doi: 10.1016/s0169-409x(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa M, Huang L. Nonviral vectors in the new millennium: delivery barriers in gene transfer. Hum Gene Ther. 2001;12:861–70. doi: 10.1089/104303401750195836. [DOI] [PubMed] [Google Scholar]
- 4.de Lima MCP, Simoes S, Pires P, Faneca H, Duzgunes N. Cationic lipid-DNA complexes in gene delivery: from biophysics to biological applications. Adv Drug Deliv Rev. 2001;47:277–294. doi: 10.1016/s0169-409x(01)00110-7. [DOI] [PubMed] [Google Scholar]
- 5.Audouy SAL, de Leij LFMH, Hoekstra D, Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm Res. 2002;19:1599–1605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SB, Xu YM, Wang B, Qiao WH, Liu DL, Li ZS. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J Control Release. 2004;100:165–180. doi: 10.1016/j.jconrel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Mahato RI, Rolland A, Tomlinson E. Cationic lipid-based gene delivery systems: Pharmaceutical perspectives. Pharm Res. 1997;14:853–859. doi: 10.1023/a:1012187414126. [DOI] [PubMed] [Google Scholar]
- 8.Miller AD. Cationic Liposomes for Gene Therapy. Angew Chem Int Edit. 1998;37:1768–1785. [Google Scholar]
- 9.Bally MB, Harvie P, Wong FMP, Kong S, Wasan EK, Reimer DL. Biological barriers to cellular delivery of lipid-based DNA carriers. Adv Drug Deliv Rev. 1999;38:291–315. doi: 10.1016/s0169-409x(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 10.Safinya CR. Structures of lipid-DNA complexes: supramolecular assembly and gene delivery. Curr Opin Struc Biol. 2001;11:440–448. doi: 10.1016/s0959-440x(00)00230-x. [DOI] [PubMed] [Google Scholar]
- 11.Martin B, Sainlos M, Aissaoui A, Oudrhiri N, Hauchecorne M, Vigneron JP, Lehn JM, Lehn P. The design of cationic lipids for gene delivery. Curr Pharm Design. 2005;11:375–394. doi: 10.2174/1381612053382133. [DOI] [PubMed] [Google Scholar]
- 12.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JG, Lin KY, Kothavale A, Flanagan WM, Matteucci MD, DePrince RB, Mook RA, Hendren RW, Wagner RW. A serum-resistant cytofectin for cellular delivery of antisense oligodeoxynucleotides and plasmid DNA. Proc Natl Acad Sci U S A. 1996;93:3176–3181. doi: 10.1073/pnas.93.8.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong KL, Zheng WW, Baker A, Papahadjopoulos D. Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. Febs Lett. 1997;400:233–237. doi: 10.1016/s0014-5793(96)01397-x. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler JJ, Palmer L, Ossanlou M, MacLachlan I, Graham RW, Zhang YP, Hope MJ, Scherrer P, Cullis PR. Stabilized plasmid-lipid particles: construction and characterization. Gene Ther. 1999;6:271–281. doi: 10.1038/sj.gt.3300821. [DOI] [PubMed] [Google Scholar]
- 16.Budker V, Gurevich V, Hagstrom JE, Bortzov F, Wolff JA. pH-sensitive, cationic liposomes: A new synthetic virus-like vector. Nat Biotechnol. 1996;14:760–764. doi: 10.1038/nbt0696-760. [DOI] [PubMed] [Google Scholar]
- 17.Demeneix B, Behr J, Boussif O, Zanta MA, Abdallah B, Remy J. Gene transfer with lipospermines and polyethylenimines. Adv Drug Deliv Rev. 1998;30:85–95. doi: 10.1016/s0169-409x(97)00109-9. [DOI] [PubMed] [Google Scholar]
- 18.Gerasimov OV, Boomer JA, Qualls MM, Thompson DH. Cytosolic drug delivery using pH- and light-sensitive liposomes. Adv Drug Deliv Rev. 1999;38:317–338. doi: 10.1016/s0169-409x(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 19.Guo X, Szoka FC. Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Accounts Chem Res. 2003;36:335–341. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 20.Tang FX, Hughes JA. Introduction of a disulfide bond into a cationic lipid enhances transgene expression of plasmid DNA. Biochem Bioph Res Comm. 1998;242:141–145. doi: 10.1006/bbrc.1997.7923. [DOI] [PubMed] [Google Scholar]
- 21.Tang FX, Hughes JA. Use of dithiodiglycolic acid as a tether for cationic lipids decreases the cytotoxicity and increases transgene expression of plasmid DNA in vitro. Bioconjugate Chem. 1999;10:791–796. doi: 10.1021/bc990016i. [DOI] [PubMed] [Google Scholar]
- 22.Ajmani PS, Tang FX, Krishnaswami S, Meyer EM, Sumners C, Hughes JA. Enhanced transgene expression in rat brain cell cultures with a disulfide-containing cationic lipid. Neurosci Lett. 1999;277:141–144. doi: 10.1016/s0304-3940(99)00856-3. [DOI] [PubMed] [Google Scholar]
- 23.Wetzer B, Byk G, Frederic M, Airiau M, Blanche F, Pitard B, Scherman D. Reducible cationic lipids for gene transfer. Biochem J. 2001;356:747–756. doi: 10.1042/0264-6021:3560747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meers P. Enzyme-activated targeting of liposomes. Adv Drug Deliv Rev. 2001;53:265–72. doi: 10.1016/s0169-409x(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 25.Prata CA, Zhao Y, Barthelemy P, Li Y, Luo D, McIntosh TJ, Lee SJ, Grinstaff MW. Charge-reversal amphiphiles for gene delivery. J Am Chem Soc. 2004;126:12196–7. doi: 10.1021/ja0474906. [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, MacKay JA, Szoka FC. Low-pH-sensitive PEG-stabilized plasmid-lipid nanoparticles: Preparation and characterization. Bioconjugate Chem. 2003;14:420–429. doi: 10.1021/bc025625w. [DOI] [PubMed] [Google Scholar]
- 27.Wolff JA, Rozema DB. Breaking the bonds: Non-viral vectors become chemically dynamic. Mol Ther. 2008;16:8–15. doi: 10.1038/sj.mt.6300326. [DOI] [PubMed] [Google Scholar]
- 28.Gallardo BS, Hwa MJ, Abbott NL. In-Situ and Reversible Control of the Surface-Activity of Ferrocenyl Surfactants in Aqueous-Solutions. Langmuir. 1995;11:4209–4212. [Google Scholar]
- 29.Bennett DE, Gallardo BS, Abbott NL. Dispensing surfactants from electrodes: Marangoni phenomenon at the surface of aqueous solutions of (11-ferrocenylundecyl)trimethylammonium bromide. J Am Chem Soc. 1996;118:6499–6505. [Google Scholar]
- 30.Gallardo BS, Metcalfe KL, Abbott NL. Ferrocenyl surfactants at the surface of water: Principles for active control of interfacial properties. Langmuir. 1996;12:4116–4124. [Google Scholar]
- 31.Gallardo BS, Abbott NL. Active control of interfacial properties: A comparison of dimeric and monomeric ferrocenyl surfactants at the surface of aqueous solutions. Langmuir. 1997;13:203–208. [Google Scholar]
- 32.Gallardo BS, Gupta VK, Eagerton FD, Jong LI, Craig VS, Shah RR, Abbott NL. ELectrochemical principles for active control of liquids on submillimeter scales. Science. 1999;283:57–60. doi: 10.1126/science.283.5398.57. [DOI] [PubMed] [Google Scholar]
- 33.Rosslee C, Abbott NL. Active control of interfacial properties. Curr Opin Colloid In. 2000;5:81–87. [Google Scholar]
- 34.Rosslee CA, Abbott NL. Principles for microscale separations based on redox-active surfactants and electrochemical methods. Anal Chem. 2001;73:4808–4814. doi: 10.1021/ac010273s. [DOI] [PubMed] [Google Scholar]
- 35.Luk YY, Abbott NL. Surface-driven switching of liquid-crystals using redox-active groups on electrodes. Science. 2003;301:623–626. doi: 10.1126/science.1084527. [DOI] [PubMed] [Google Scholar]
- 36.Rosslee CA, Khripin C, Foley TMD, Abbott NL. Using “prosurfactants” to enhance rates of delivery of surfactants. Aiche J. 2004;50:708–714. [Google Scholar]
- 37.Hays ME, Abbott NL. Electrochemical control of the interactions of polymers and redox-active surfactants. Langmuir. 2005;21:12007–12015. doi: 10.1021/la051640o. [DOI] [PubMed] [Google Scholar]
- 38.Abbott NL, Jewell CM, Hays ME, Kondo Y, Lynn DM. Ferrocene-Containing Cationic Lipids: Influence of Redox State on Cell Transfection. J Am Chem Soc. 2005;127:11576–11577. doi: 10.1021/ja054038t. [DOI] [PubMed] [Google Scholar]
- 39.Jewell CM, Hays ME, Kondo Y, Abbott NL, Lynn DM. Ferrocene-containing cationic lipids for the delivery of DNA: Oxidation state determines transfection activity. J Control Release. 2006;112:129–138. doi: 10.1016/j.jconrel.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Hays ME, Jewell CM, Lynn DM, Abbott NL. Reversible Condensation of DNA Using a Redox-Active Surfactant. Langmuir. 2007;23:5609–5614. doi: 10.1021/la0700319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hays ME, Jewell CM, Kondo Y, Lynn DM, Abbott NL. Lipoplexes Formed by DNA and Ferrocenyl Lipids: Effect of Lipid Oxidation State on Size, Internal Dynamics and Zeta-Potential. Biophys J. 2007;93:4414–4424. doi: 10.1529/biophysj.107.107094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pizzey CL, Jewell CM, Hays ME, Lynn DM, Abbott NL, Kondo Y, Golan S, Talmon Y. Characterization of the Nanostructure of Complexes Formed by a Redox-Active Cationic Lipid and DNA. J Phys Chem B. 2008;112:5849–5857. doi: 10.1021/jp7103903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saji T, Hoshino K, Aoyagui S. Reversible Formation and Disruption of Micelles by Control of the Redox State of the Head Group. J Am Chem Soc. 1985;107:6865–6868. [Google Scholar]
- 44.Kakizawa Y, Sakai H, Nishiyama K, Abe M, Shoji H, Kondo Y, Yoshino N. Solution properties of double-tailed cationic surfactants having ferrocenyl groups in their hydrophobic moieties. Langmuir. 1996;12:921–924. [Google Scholar]
- 45.Yoshino N, Shoji H, Kondo Y, Kakizawa Y, Sakai H, Abe M. Syntheses of cationic surfactants having two ferrocenylalkyl chains. Journal of the Japanese Oil Chemistry Society. 1996;45:769–775. [Google Scholar]
- 46.Sakai H, Imamura H, Kakizawa Y, Abe M, Kondo Y, Yoshino N, Harwell JH. Active control of vesicle formation using a redox-active surfactant. Denki Kagaku. 1997;65:669–672. [Google Scholar]
- 47.Kakizawa Y, Sakai H, Yamaguchi A, Kondo Y, Yoshino N, Abe M. Electrochemical control of vesicle formation with a double-tailed cationic surfactant bearing ferrocenyl moieties. Langmuir. 2001;17:8044–8048. [Google Scholar]
- 48.Takei T, Sakai H, Kondo Y, Yoshino N, Abe M. Electrochemical control of solubilization using a ferrocene-modified nonionic surfactant. Colloid Surface A. 2001;183:757–765. [Google Scholar]
- 49.Tsuchiya K, Sakai H, Saji T, Abe M. Electrochemical reaction in an aqueous solution of a ferrocene-modified cationic surfactant mixed with an anionic surfactant. Langmuir. 2003;19:9343–9350. [Google Scholar]
- 50.Sakai H, Imamura H, Kondo Y, Yoshino N, Abe M. Reversible control of vesicle formation using electrochemical reaction. Colloid Surface A. 2004;232:221–228. [Google Scholar]
- 51.Hunter RJ. Zeta Potential in Colloid Science. Academic Press; New York, NY: 1981. [Google Scholar]
- 52.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 53.Wring SA, Hart JP, Birch BJ. Voltammetric Behavior of Screen-Printed Carbon Electrodes, Chemically Modified with Selected Mediators, and Their Application as Sensors for the Determination of Reduced Glutathione. Analyst. 1991;116:123–129. [Google Scholar]
- 54.Schreyer SK, Mikkelsen SR. A synthetic cysteine oxidase eased on a ferrocene-cyclodextrin conjugate. Bioconjugate Chem. 1999;10:464–469. doi: 10.1021/bc9801326. [DOI] [PubMed] [Google Scholar]
- 55.Tomlinson E, Rolland AP. Controllable gene therapy. Pharmaceutics of non-viral gene delivery systems. J Control Release. 1996;39:357–372. [Google Scholar]
- 56.Vijayanathan V, Thomas T, Thomas TJ. DNA Nanoparticles and Development of DNA Delivery Vehicles for Gene Therapy. Biochemistry. 2002;41:14085–14094. doi: 10.1021/bi0203987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of additional experiments, including characterization of GSH-mediated reduction of oxidized BFDMA and characterization of cytotoxicity. This information is available free of charge via the Internet at http://pubs.acs.org.