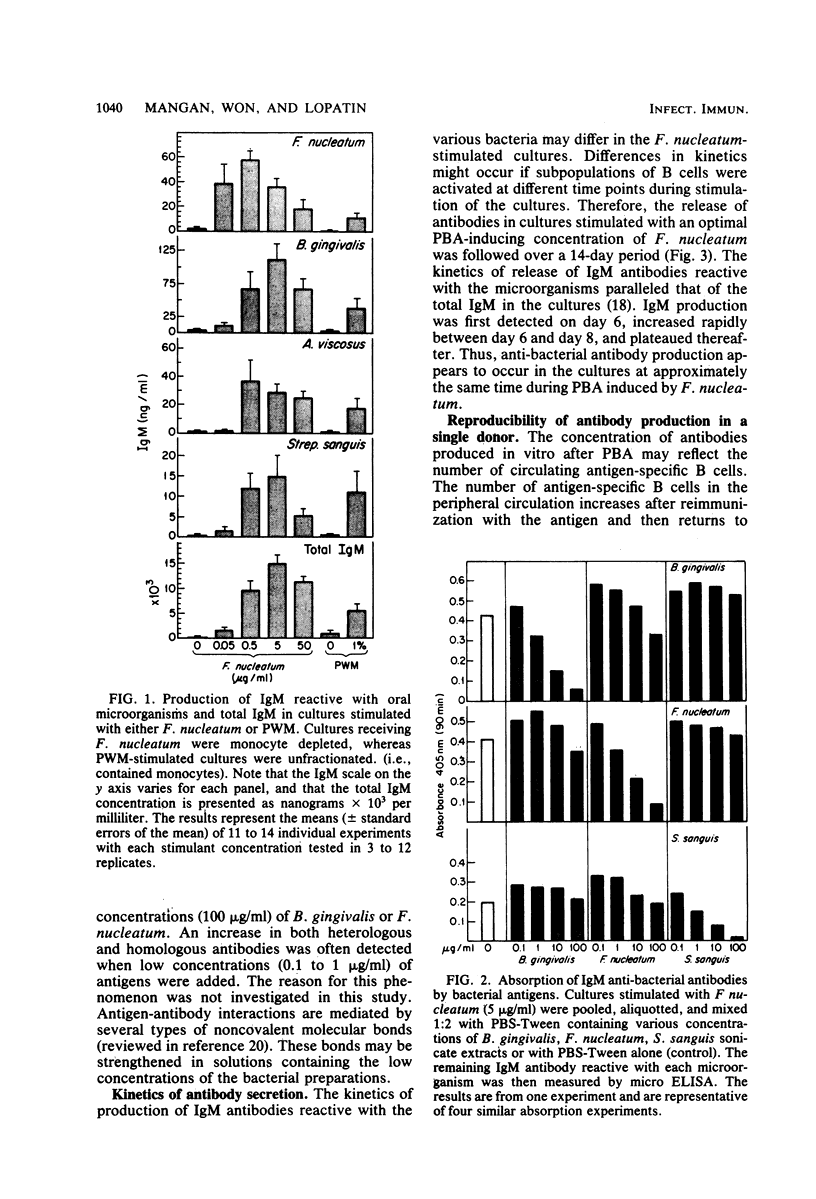
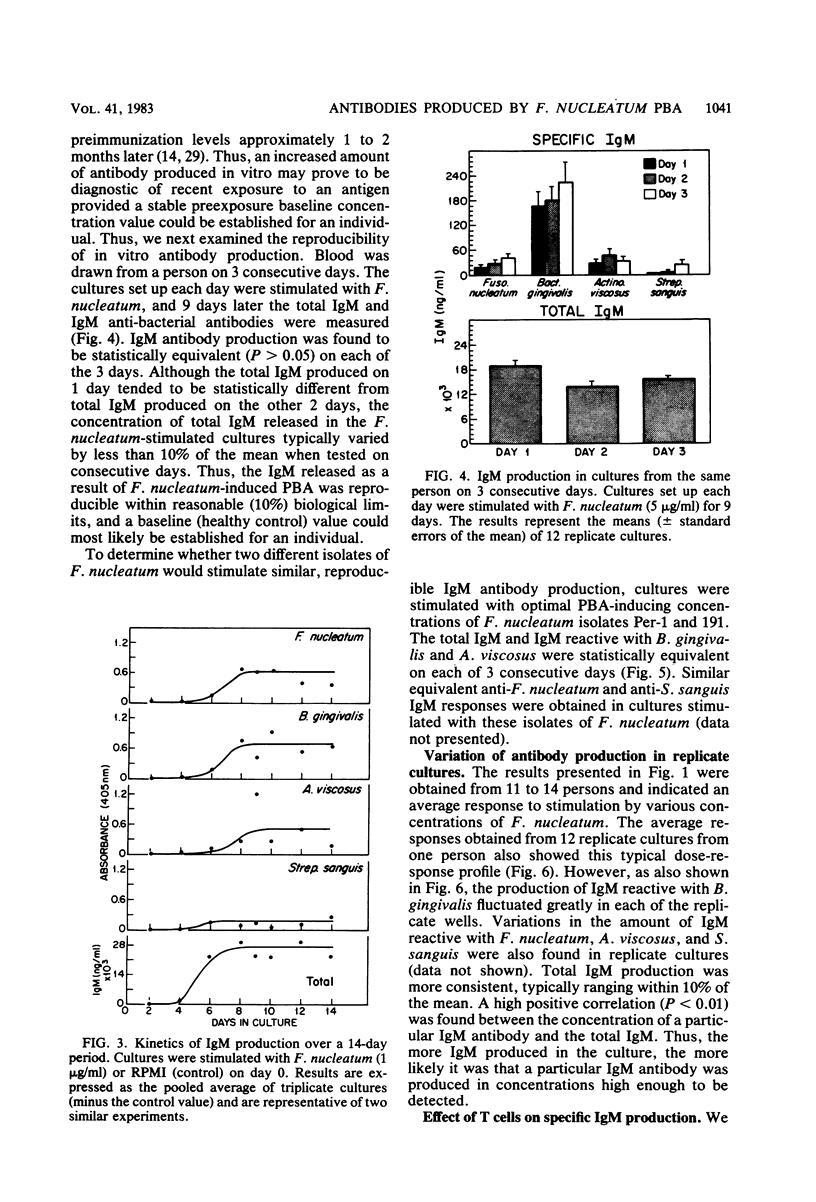
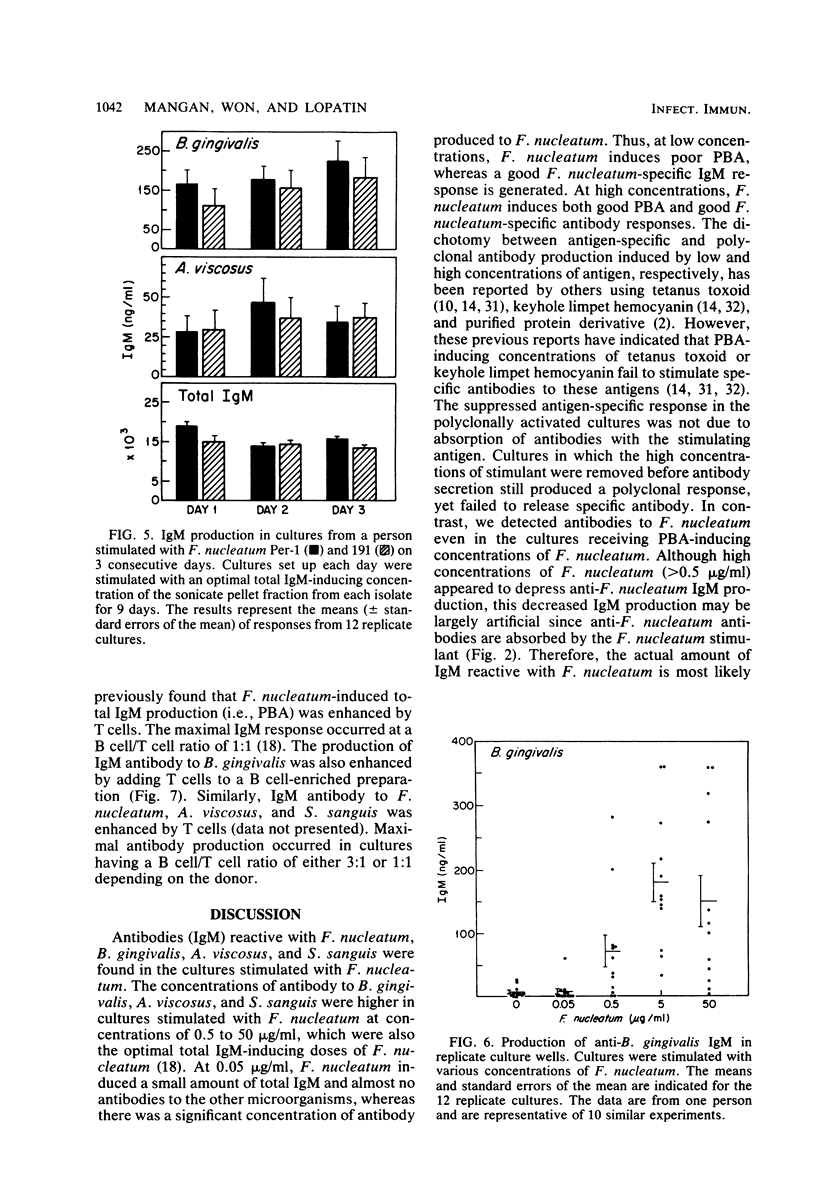
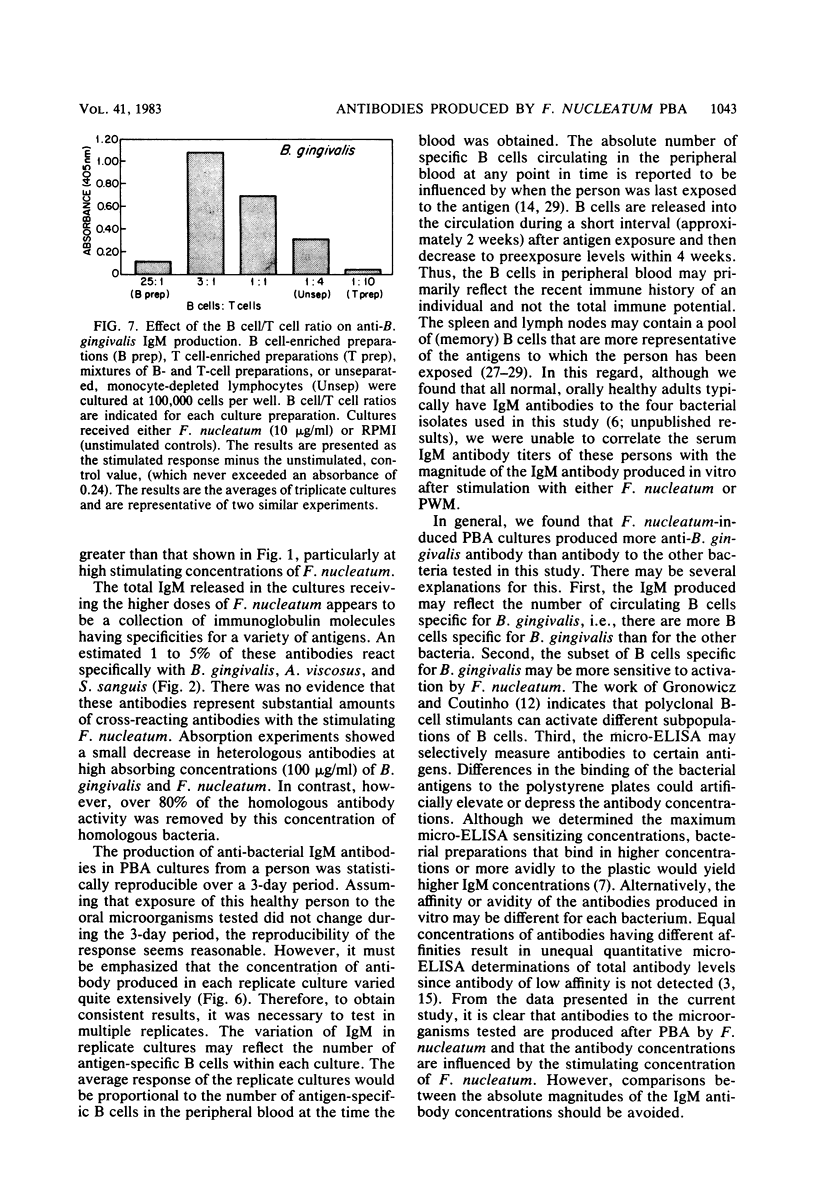
Abstract
The production of antibodies to oral bacteria was determined in lymphocyte cultures stimulated with sonicated Fusobacterium nucleatum, a potent inducer of polyclonal B-cell activation. After 9 days the cultures were examined by a microenzyme-linked immunosorbent assay for immunoglobulin M (IgM) antibodies to F. nucleatum, Bacteroides gingivalis, Actinomyces viscosus, and Streptococcus sanguis. Antibodies to these four bacteria were detected in cultures stimulated with polyclonal B-cell activation-inducing concentrations of F. nucleatum. However, significant concentrations of antibodies to F. nucleatum, but not to the other three microorganisms, were produced in cultures that received suboptimal polyclonal B-cell activation-inducing doses of F. nucleatum. Absorption studies indicated the specificity of the antibodies to each of the bacteria tested. IgM antibody production induced by F. nucleatum was enhanced by the addition of T cells. The production of IgM antibodies to the bacteria was reproducible in cultures from a single person tested on 3 consecutive days. The concentration of antibodies in replicate cultures, however, fluctuated greatly. To obtain consistent responses on successive days, multiple replicate cultures were required. These results suggest that F. nucleatum, which is frequently present in subgingival plaque, could induce the production of antibodies not only to F. nucleatum, but also to other microorganisms associated with periodontal diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bick P. H., Carpenter A. B., Holdeman L. V., Miller G. A., Ranney R. R., Palcanis K. G., Tew J. G. Polyclonal B-cell activation induced by extracts of Gram-negative bacteria isolated from periodontally diseased sites. Infect Immun. 1981 Oct;34(1):43–49. doi: 10.1128/iai.34.1.43-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren H. Role of B cells in the expression of the PPD response of human lymphocytes in vitro. Scand J Immunol. 1975 Sep;4(5-6):499–510. doi: 10.1111/j.1365-3083.1975.tb02655.x. [DOI] [PubMed] [Google Scholar]
- Butler J. E., Feldbush T. L., McGivern P. L., Stewart N. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration or affinity. Immunochemistry. 1978 Feb;15(2):131–136. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- Chen P., Trummel C., Horton J., Baker J. J., Oppenheim J. J. Production of osteoclast-activating factor by normal human peripheral blood rosetting and nonrosetting lymphocytes. Eur J Immunol. 1976 Oct;6(10):732–736. doi: 10.1002/eji.1830061014. [DOI] [PubMed] [Google Scholar]
- Clagett J. A., Engel D. Polyclonal activation: a form of primitive immunity and its possible role in pathogenesis of inflammatory diseases. Dev Comp Immunol. 1978 Apr;2(2):235–241. doi: 10.1016/s0145-305x(78)80066-4. [DOI] [PubMed] [Google Scholar]
- Doty S. L., Lopatin D. E., Syed S. A., Smith F. N. Humoral immune response to oral microorganisms in periodontitis. Infect Immun. 1982 Aug;37(2):499–505. doi: 10.1128/iai.37.2.499-505.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fauci A. S. Human B cell function in a polyclonally induced plaque forming cell system. Cell triggering and immunoregulation. Immunol Rev. 1979;45:93–116. doi: 10.1111/j.1600-065x.1979.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Immunoregulation in autoimmunity. J Allergy Clin Immunol. 1980 Jul;66(1):5–17. doi: 10.1016/0091-6749(80)90132-3. [DOI] [PubMed] [Google Scholar]
- Friedman S. M., Principato M. A., Thompson G. S., Teichman F. Antigen-specific and polyclonal immunoglobulin production induced by a cloned tetanus toxoid-specific T cell line. J Immunol. 1983 Mar;130(3):1164–1170. [PubMed] [Google Scholar]
- Genco R. J., Mashimo P. A., Krygier G., Ellison S. A. Antibody-mediated effects on the periodontium. J Periodontol. 1974 May;45(5):330–337. doi: 10.1902/jop.1974.45.5.330. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Heterogeneity of B cells: direct evidence of selective triggering of distinct subpopulations by polyclonal activators. Scand J Immunol. 1976;5(1-2):55–69. doi: 10.1111/j.1365-3083.1976.tb02992.x. [DOI] [PubMed] [Google Scholar]
- Ivanyi L., Challacombe S. J., Lehner T. The specificity of serum factors in lymphocyte transformation in periodontal disease. Clin Exp Immunol. 1973 Aug;14(4):491–500. [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Volkman D. J., Whalen G., Fauci A. S. In vitro antigen-induced, antigen-specific antibody production in man. Specific and polyclonal components, kinetics, and cellular requirements. J Exp Med. 1981 Oct 1;154(4):1043–1057. doi: 10.1084/jem.154.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen O. P., Eerola E. The effect of different antibody affinities on ELISA absorbance and titer. J Immunol Methods. 1982 Oct 29;54(2):233–240. doi: 10.1016/0022-1759(82)90064-3. [DOI] [PubMed] [Google Scholar]
- Mackler B. F., Frostad K. B., Robertson P. B., Levy B. M. Immunoglobulin bearing lymphocytes and plasma cells in human periodontal disease. J Periodontal Res. 1977 Jan;12(1):37–45. doi: 10.1111/j.1600-0765.1977.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Lopatin D. E. In vitro stimulation of immunoglobulin production from human peripheral blood lymphocytes by a soluble preparation of Actinomyces viscosus. Infect Immun. 1981 Jan;31(1):236–244. doi: 10.1128/iai.31.1.236-244.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Lopatin D. E. Polyclonal activation of human peripheral blood B lymphocytes by Fusobacterium nucleatum. Infect Immun. 1983 Jun;40(3):1104–1111. doi: 10.1128/iai.40.3.1104-1111.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moticka E. J., Streilein J. W. Hypothesis: nonspecific polyclonal activation of memory B cells by antigen as a mechanism for the preservation of long term immunologic anamnesis. Cell Immunol. 1978 Dec;41(2):406–413. doi: 10.1016/0008-8749(78)90237-x. [DOI] [PubMed] [Google Scholar]
- Pearlman D. S. The influence of antibodies on immunologic responses. I. The effect on the response to particulate antigen in the rabbit. J Exp Med. 1967 Jul 1;126(1):127–148. doi: 10.1084/jem.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus C. S., Lamm M. E., Nussenzweig V. Regulation of the immune response: suppressive and enhancing effects of passively administered antibody. J Exp Med. 1971 May 1;133(5):987–1003. doi: 10.1084/jem.133.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primi D., Hammarström L., Smith C. I., Möller G. Characterization of self-reactive B cells by polyclonal B-cell activators. J Exp Med. 1977 Jan 1;145(1):21–30. doi: 10.1084/jem.145.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primi D., Smith C. I., Hammarström L., Möller G. Polyclonal B-cell activators induce immunological response to autologous serum proteins. Cell Immunol. 1977 Dec;34(2):367–375. doi: 10.1016/0008-8749(77)90258-1. [DOI] [PubMed] [Google Scholar]
- Seymour G. J., Greenspan J. S. The phenotypic characterization of lymphocyte subpopulations in established human periodontal disease. J Periodontal Res. 1979 Jan;14(1):39–46. doi: 10.1111/j.1600-0765.1979.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Smith S., Bick P. H., Miller G. A., Ranney R. R., Rice P. L., Lalor J. H., Tew J. G. Polyclonal B-cell activation: severe periodontal disease in young adults. Clin Immunol Immunopathol. 1980 Jul;16(3):354–366. doi: 10.1016/0090-1229(80)90141-5. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Differential synthesis of IgM and IgA anti-tetanus toxoid antibody in vitro following in vivo booster immunization of humans. Cell Immunol. 1979 Jun;45(1):142–150. doi: 10.1016/0008-8749(79)90369-1. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Immunoregulation in humans: control of antitetanus toxoid antibody production after booster immunization. J Clin Invest. 1978 Dec;62(6):1154–1160. doi: 10.1172/JCI109234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]
- Volkman D. J., Allyn S. P., Fauci A. S. Antigen-induced in vitro antibody production in humans: tetanus toxoid-specific antibody synthesis. J Immunol. 1982 Jul;129(1):107–112. [PubMed] [Google Scholar]
- Volkman D. J., Lane H. C., Fauci A. S. Antigen-induced in vitro antibody production in humans: a model for B cell activation and immunoregulation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2528–2531. doi: 10.1073/pnas.78.4.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]


