Abstract
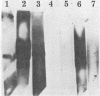
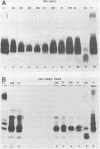
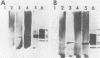
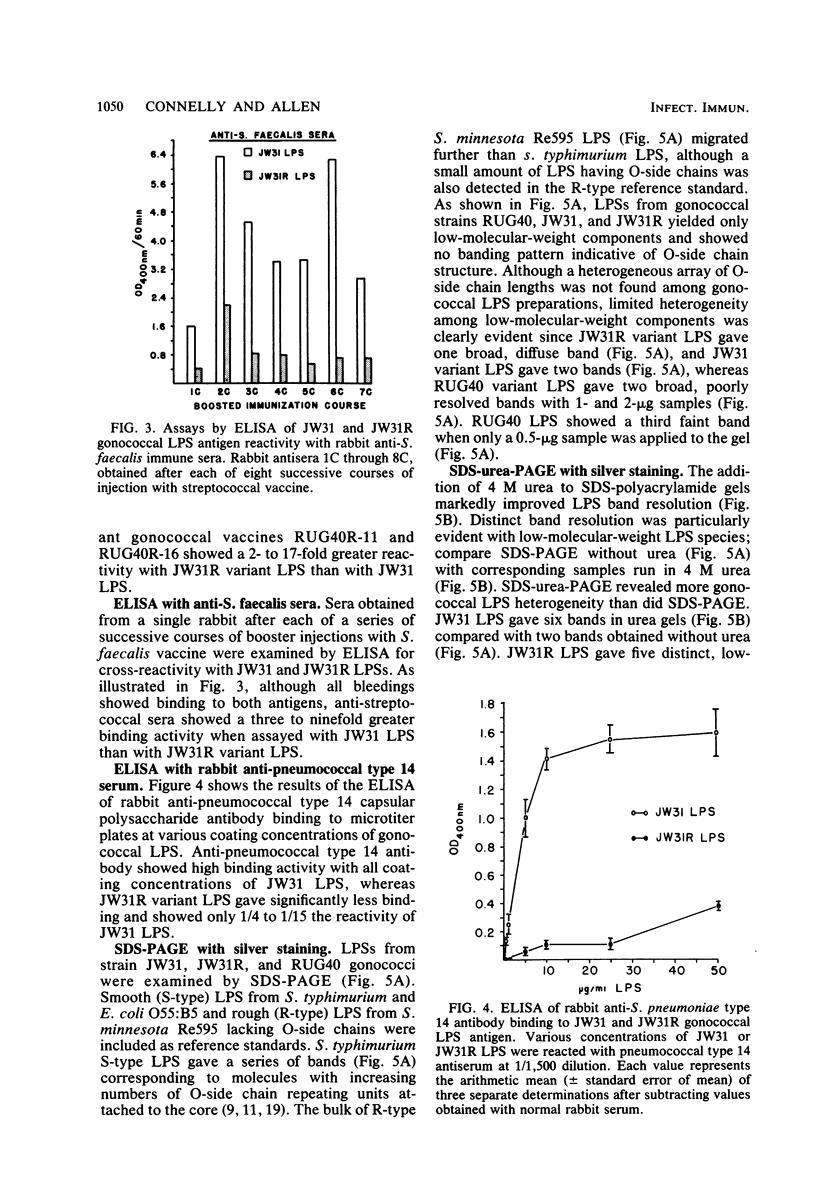
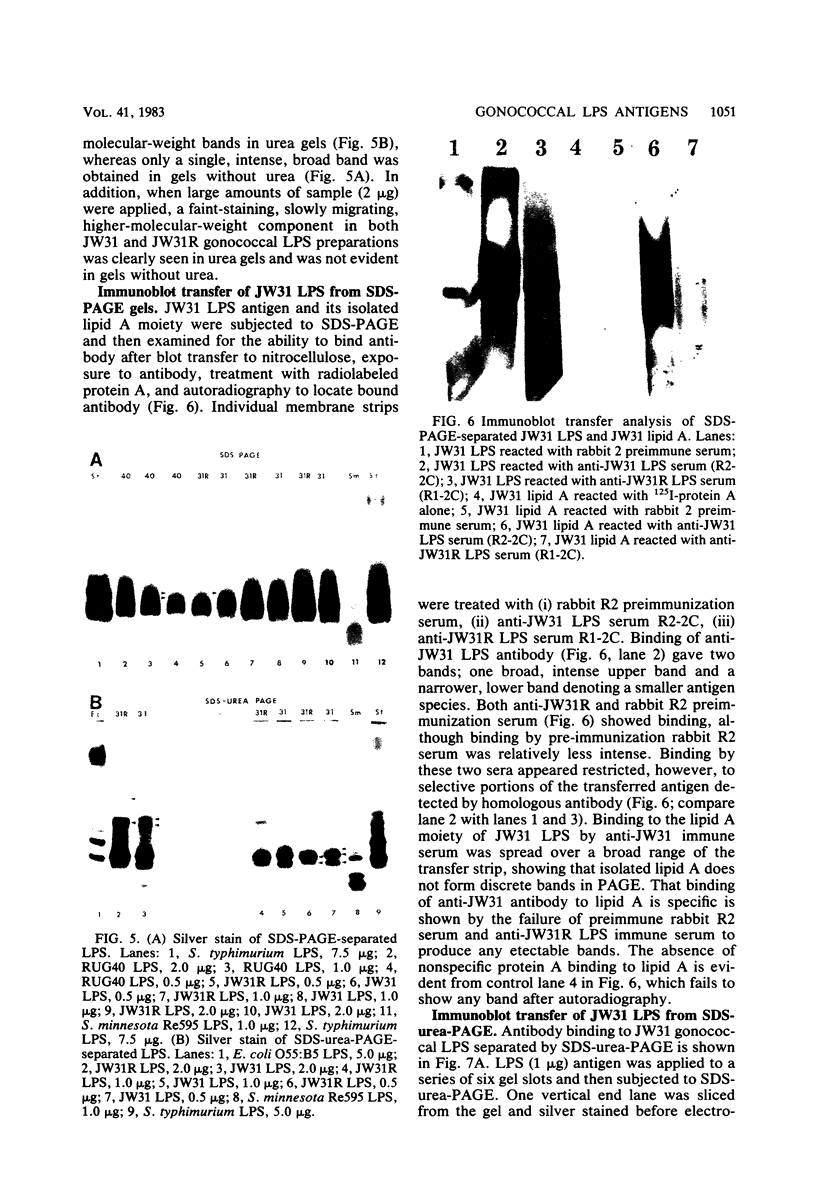
Homologous antisera were raised against lipopolysaccharides (LPSs) isolated from pyocin 103-sensitive JW31 strain Neisseria gonorrhoeae and its isogenic, pyocin-resistant variant, JW31R. Changes in immunochemical reactivity of LPS antigen associated with pyocin-resistance were examined by enzyme-linked immunosorbent assay, employing homologous and heterologous anti-LPS immune sera. The acquisition of pyocin 103 resistance is accompanied by a loss in LPS antigen reactivity with homologous anti-LPS. The variant LPS of pyocin 103-resistant mutants is immunogenic and displays a new, distinct antigenic specificity shared with other pyocin 103-resistant variant gonococcal strains. The acquisition of pyocin 103 resistance by JW31 strain gonococci is also accompanied by a striking loss of LPS cross-reactivity with antistreptococcal polysaccharide reagents having an antibody combining site specificity directed against the chemically defined lactose polymer from Streptococcus faecalis cell wall and pneumococcal type 14 capsular polysaccharide. When examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and sodium dodecyl sulfate-urea-polyacrylamide gel electrophoresis, JW31 and JW31R LPSs show banding patterns characteristic of microheterogeneous, rough-type LPS devoid of O-side chains. Immunoblot transfer analysis of gel-separated gonococcal LPS antigens shows a difference in the pattern of antibody binding by homologous versus cross-reactive anti-LPS, which suggests a heterogeneity in the distribution of cross-reactive determinants among LPS molecules.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. Z., Connelly M. C., Apicella M. A. Interaction of lectins with Neisseria gonorrhoeae. Can J Microbiol. 1980 Apr;26(4):468–474. doi: 10.1139/m80-078. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Gagliardi N. C. Antigenic heterogeneity of the non-serogroup antigen structure of Neisseria gonorrhoeae lipopolysaccharides. Infect Immun. 1979 Dec;26(3):870–874. doi: 10.1128/iai.26.3.870-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Connelly M. C., Stein D. C., Young F. E., Morse S. A., Allen P. Z. Interaction with lectins and differential wheat germ agglutinin binding of pyocin 103-sensitive and -resistant Neisseria gonorrhoeae. J Bacteriol. 1981 Dec;148(3):796–803. doi: 10.1128/jb.148.3.796-803.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Esser M., Shafer W. M. Pyocin-resistant lipopolysaccharide mutans of Neisseria gonorrhoeae: alterations in sensitivity to normal human serum and polymyxin B. Infect Immun. 1982 May;36(2):541–547. doi: 10.1128/iai.36.2.541-547.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert R. E., Hurlbert I. M. Biological and physicochemical properties of the lipopolysaccharide of Chromatium vinosum. Infect Immun. 1977 Jun;16(3):983–994. doi: 10.1128/iai.16.3.983-994.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg B., Lönngren J., Powell D. A. Structural studies on the specific type-14 pneumococcal polysaccharide. Carbohydr Res. 1977 Sep;58(1):177–186. doi: 10.1016/s0008-6215(00)83413-8. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Jones B. V., Lysko P. G. Pyocin inhibition of Neisseria gonorrhoeae: mechanism of action. Antimicrob Agents Chemother. 1980 Sep;18(3):416–423. doi: 10.1128/aac.18.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Pazur J. H., Cepure A., Kane J. A., Hellerquist C. G. Glycans from streptococcal cell walls. The molecular structure of an antigenic diheteroglycan of glucose and galactose from Streptococcus faecalis. J Biol Chem. 1973 Jan 10;248(1):279–284. [PubMed] [Google Scholar]
- Pazur J. H., Forsberg L. S. Determination of the sugar sequences and the glycosidic-bond arrangements of immunogenic heteroglycans. Carbohydr Res. 1978 Jan;60(1):167–178. doi: 10.1016/s0008-6215(00)83474-6. [DOI] [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Stein D. C., Clark V. L., Young F. E. Inhibition of active transport and macromolecular synthesis by pyocin 103 in Neisseria gonorrhoeae. Sex Transm Dis. 1983 Jan-Mar;10(1):7–13. doi: 10.1097/00007435-198301000-00002. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]