Abstract
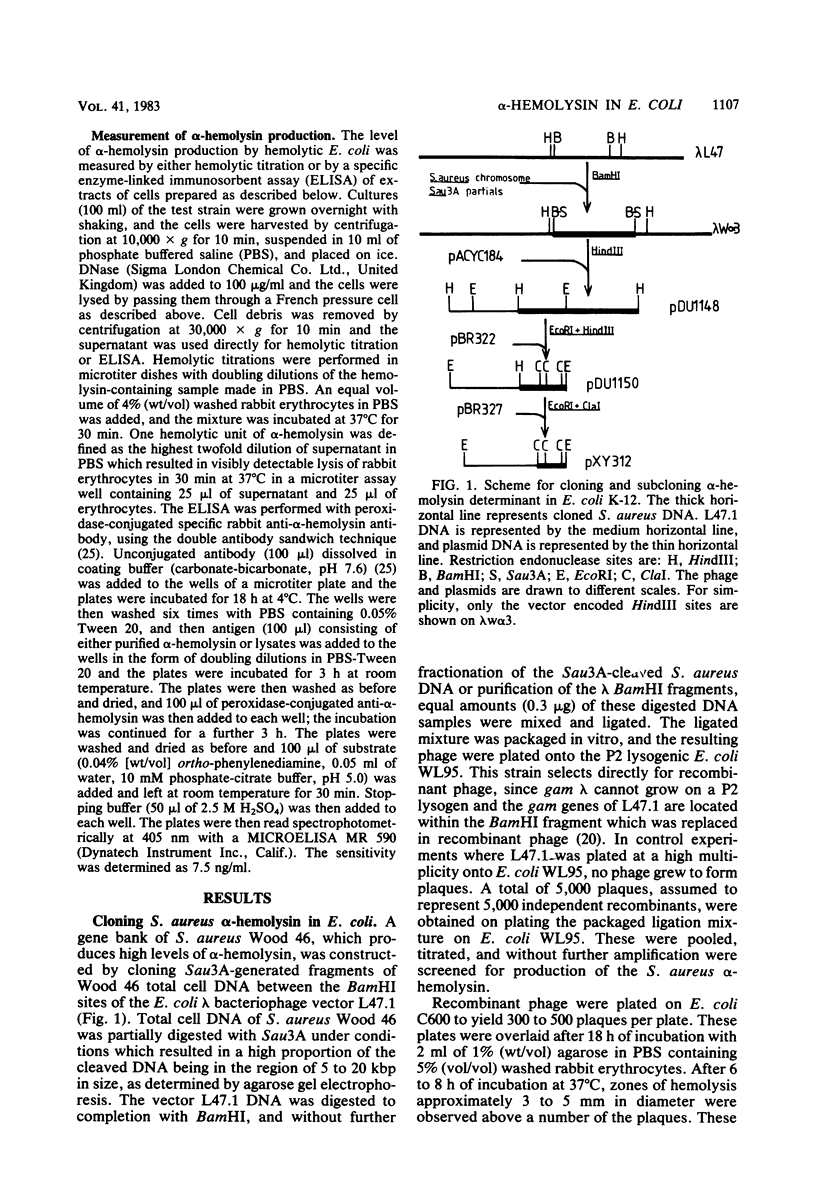
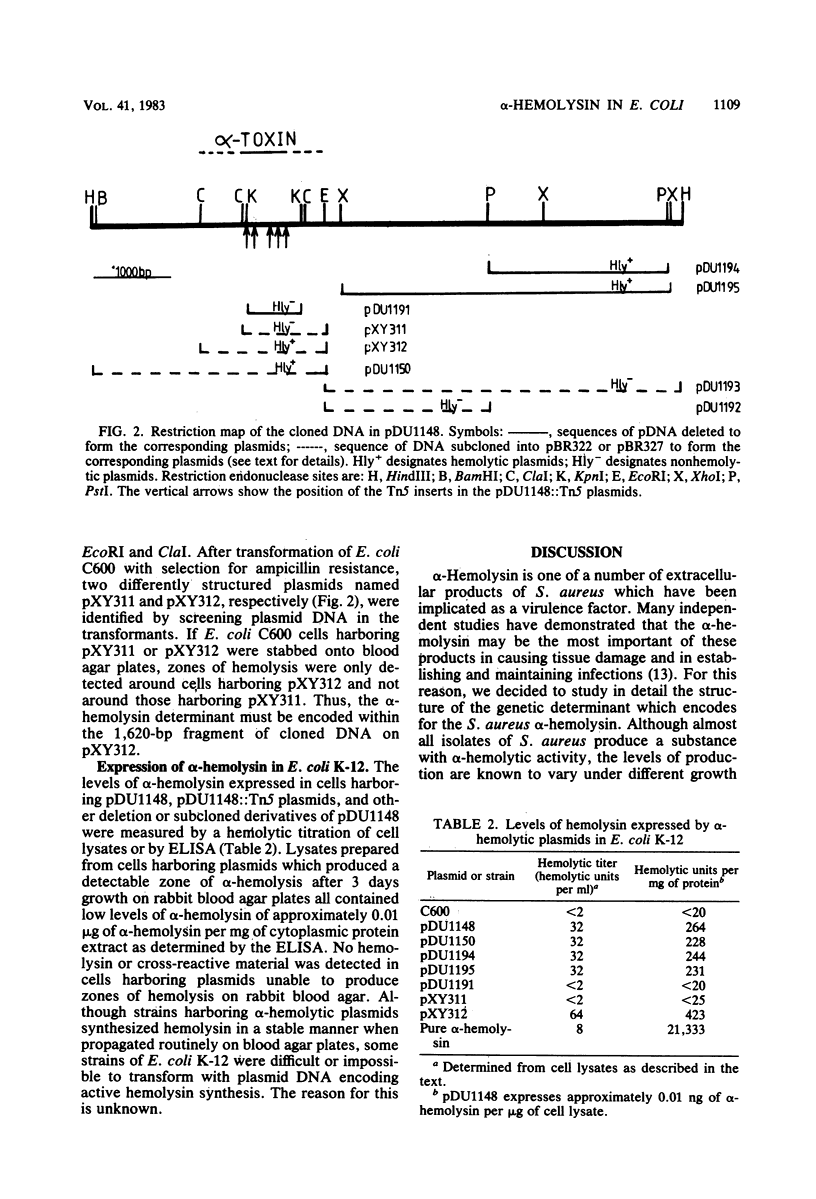
A fragment of Staphylococcus aureus DNA encoding the alpha-hemolysin determinant was cloned from strain Wood 46 by inserting Sau3A-generated genomic DNA fragments between the BamHI sites of the lambda replacement vector L47.1. Phages expressing alpha-hemolysin were detected by overlaying plaques formed from several thousand independent recombinant phage with erythrocytes and looking for zones of hemolysis. One phage expressing alpha-hemolysin was purified and named lambda w alpha 3. This was subsequently shown to contain a 10.2-kilobase pair insert of S. aureus DNA. A 7.6-kilobase pair HindIII fragment encoding the alpha-hemolysin was subcloned from lambda w alpha 3 into the plasmid vector pACYC184 to form the hybrid plasmid pDU1148. Escherichia coli K-12 cells harboring pDU1148 synthesized a low level of alpha-hemolysin which remained associated with the cells and was not secreted into culture supernatants. When the same strain was stabbed onto blood agar plates, no zones of hemolysis were detected after overnight growth at 37 degrees C but hemolysis developed if the plates were left at room temperature for 48 h. By introducing specific deletions or Tn5 insertions into plasmid pDU1148, the alpha-hemolysin gene was mapped to a region within a 3.3-kilobase pair EcoRI-HindIII fragment which was subcloned onto the vector plasmid pBR322. A specific enzyme-linked immunosorbent assay with peroxidase-labeled rabbit anti-alpha-hemolysin antibodies was used to measure the levels of alpha-hemolysin antigen expressed in E. coli K-12 cells harboring pDU1148 or a variety of pDU1148::Tn5 and pDU1148 deletion mutants.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Foster T. J. Analysis of the reduction in expression of tetracycline resistance determined by transposon Tn10 in the multicopy state. Mol Gen Genet. 1981;182(1):171–177. doi: 10.1007/BF00422786. [DOI] [PubMed] [Google Scholar]
- Coleman K., Dougan G., Arbuthnott J. P. Cloning, and expression in Escherichia coli K-12, of the chromosomal hemolysin (phospholipase C) determinant of Pseudomonas aeruginosa. J Bacteriol. 1983 Feb;153(2):909–915. doi: 10.1128/jb.153.2.909-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day S. E., Vasli K. K., Russell R. J., Arbuthnott J. P. A simple method for the study in vivo of bacterial growth and accompanying host response. J Infect. 1980 Mar;2(1):39–51. doi: 10.1016/s0163-4453(80)91773-9. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Fairweather N., Kennedy S., Foster T. J., Kehoe M., Dougan G. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun. 1983 Sep;41(3):1112–1117. doi: 10.1128/iai.41.3.1112-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1982;19(1):55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Kehoe M., Sellwood R., Shipley P., Dougan G. Genetic analysis of K88-mediated adhesion of enterotoxigenic Escherichia coli. Nature. 1981 May 14;291(5811):122–126. doi: 10.1038/291122a0. [DOI] [PubMed] [Google Scholar]
- Kinsman O. S., Arbuthnott J. P. Experimental staphylococcal infections in newborn mice: inhibition of weight gain as an index of virulence. J Med Microbiol. 1980 May;13(2):281–290. doi: 10.1099/00222615-13-2-281. [DOI] [PubMed] [Google Scholar]
- Noegel A., Rdest U., Springer W., Goebel W. Plasmid cistrons controlling synthesis and excretion of the exotoxin alpha-haemolysin of Escherichia coli. Mol Gen Genet. 1979 Oct 1;175(3):343–350. doi: 10.1007/BF00397234. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., Dougan G., Foster T. J., Arbuthnott J. P. Plasmids in epidermolytic strains of Staphylococcus aureus. J Gen Microbiol. 1981 May;124(1):99–107. doi: 10.1099/00221287-124-1-99. [DOI] [PubMed] [Google Scholar]


