Abstract
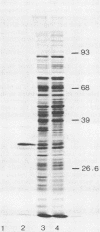
A DNA sequence encoding Staphylococcus aureus alpha-hemolysin, which had been previously cloned and mapped in Escherichia coli K-12, was introduced into Bacillus subtilis BD170 and several strains of S. aureus by using plasmid vectors, some of which could replicate in all three organisms. The determinant was cloned on a 3.3-kilobase pair DNA fragment into B. subtilis by using the vector plasmid pXZ105 to form the hybrid plasmid pXZ111. B. subtilis cells harboring pXZ111 produced large zones of alpha-hemolysis after 18 h of growth at 37 degrees C on rabbit blood agar plates, and alpha-hemolysin activity was detected in supernatants prepared from growing cultures of this strain. The alpha-hemolysin was apparently secreted across the B. subtilis cell envelope. Polypeptides of molecular weights 34,000 and 33,000 were precipitated with anti-alpha-hemolysin serum from lysates prepared from BD170 cells harboring pXZ111. A hybrid replicon which could replicate in both E. coli and S. aureus was constructed in E. coli by ligating a HindIII fragment encoding the replication functions and chloramphenicol resistance genes of S. aureus plasmid pCW59 to the pBR322 alpha-hemolysin hybrid plasmid pDU1150. The DNA of this plasmid, pDU1212, was prepared in E. coli and used to transform protoplasts prepared from a non-alpha-hemolytic, nonrestricting strain of S. aureus RN4220. Some of the transformants contained plasmids which had suffered extensive deletions. Some plasmids, however, were transformed intact into RN4220. Such plasmids were subsequently maintained in a stable manner. pDU1212 DNA was prepared from RN4220 and transformed into alpha-hemolytic S. aureus 8325-4 and two mutant derivatives defective in alpha-hemolysin synthesis. All three strains expressed alpha-hemolysin when harboring pDU1212.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Ward P. D., McCartney A. C., Arbuthnott J. P., Thorley C. M. Effect immunization with highly purified alpha- and beta-toxins on staphylococcal mastitis in rabbits. Infect Immun. 1977 Aug;17(2):250–256. doi: 10.1128/iai.17.2.250-256.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. R., Pattee P. A. Identification of a chromosomal determinant of alpha-toxin production in Staphylococcus aureus. Infect Immun. 1980 Oct;30(1):36–42. doi: 10.1128/iai.30.1.36-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contente S., Dubnau D. Characterization of plasmid transformation in Bacillus subtilis: kinetic properties and the effect of DNA conformation. Mol Gen Genet. 1979 Jan 2;167(3):251–258. doi: 10.1007/BF00267416. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Dowd G., Cafferkey M., Dougan G. Gentamicin and methicillin resistant Staphylococcus aureus in Dublin hospitals: molecular studies. J Med Microbiol. 1983 May;16(2):129–138. doi: 10.1099/00222615-16-2-129. [DOI] [PubMed] [Google Scholar]
- Duncan J. L., Cho G. J. Production of staphylococcal alpha toxin. I. Relationship between cell growth and toxin formation. Infect Immun. 1971 Oct;4(4):456–461. doi: 10.1128/iai.4.4.456-461.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D. DNA cloning in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1433–1436. doi: 10.1073/pnas.75.3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1982;19(1):55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K., Haefeli C. Expression in Escherichia coli of a staphylococcal gene for resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol. 1982 Oct;152(1):524–526. doi: 10.1128/jb.152.1.524-526.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M., Duncan J., Foster T., Fairweather N., Dougan G. Cloning, expression, and mapping of the Staphylococcus aureus alpha-hemolysin determinant in Escherichia coli K-12. Infect Immun. 1983 Sep;41(3):1105–1111. doi: 10.1128/iai.41.3.1105-1111.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- McNiven A. C., Arbuthnott J. P. Cell-associated alpha-toxin from Staphylococcus aureus. J Med Microbiol. 1972 Feb;5(1):123–127. doi: 10.1099/00222615-5-1-123. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., Dougan G., Foster T. J., Arbuthnott J. P. Plasmids in epidermolytic strains of Staphylococcus aureus. J Gen Microbiol. 1981 May;124(1):99–107. doi: 10.1099/00221287-124-1-99. [DOI] [PubMed] [Google Scholar]
- Pattee P. A. Distribution of Tn551 insertion sites responsible for auxotrophy on the Staphylococcus aureus chromosome. J Bacteriol. 1981 Jan;145(1):479–488. doi: 10.1128/jb.145.1.479-488.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A., Neveln D. S. Transformation analysis of three linkage groups in Staphylococcus aureus. J Bacteriol. 1975 Oct;124(1):201–211. doi: 10.1128/jb.124.1.201-211.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley P. L., Dougan G., Falkow S. Identification and cloning of the genetic determinant that encodes for the K88ac adherence antigen. J Bacteriol. 1981 Feb;145(2):920–925. doi: 10.1128/jb.145.2.920-925.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Boyer H. W., Betlach M., Falkow S. Molecular cloning of an Escherichia coli plasmid determinant than encodes for the production of heat-stable enterotoxin. J Bacteriol. 1976 Oct;128(1):463–472. doi: 10.1128/jb.128.1.463-472.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Kato I. Purification and some properties of staphylococcal alpha-toxin. Jpn J Exp Med. 1974 Apr;44(2):165–178. [PubMed] [Google Scholar]
- Wilson C. R., Skinner S. E., Shaw W. V. Analysis of two chloramphenicol resistance plasmids from Staphylococcus aureus: insertional inactivation of Cm resistance, mapping of restriction sites, and construction of cloning vehicles. Plasmid. 1981 May;5(3):245–258. doi: 10.1016/0147-619x(81)90002-0. [DOI] [PubMed] [Google Scholar]