Abstract
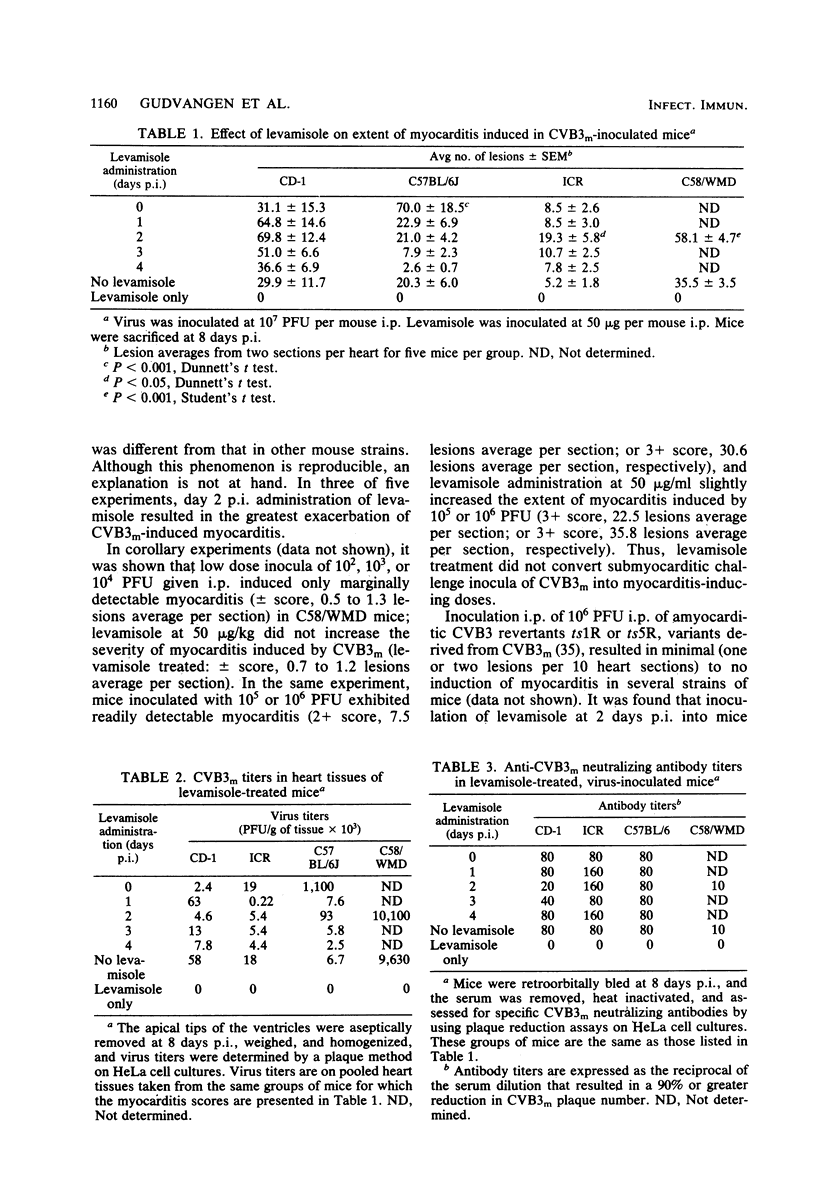
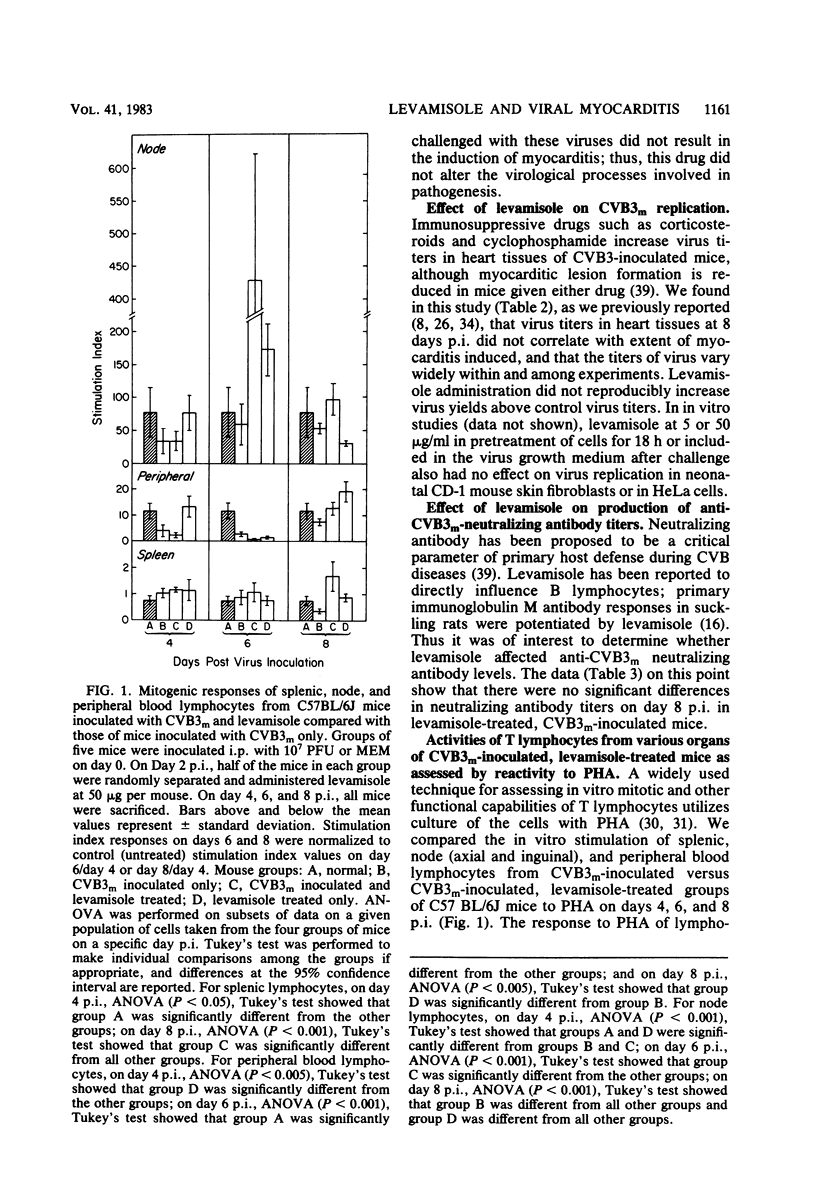
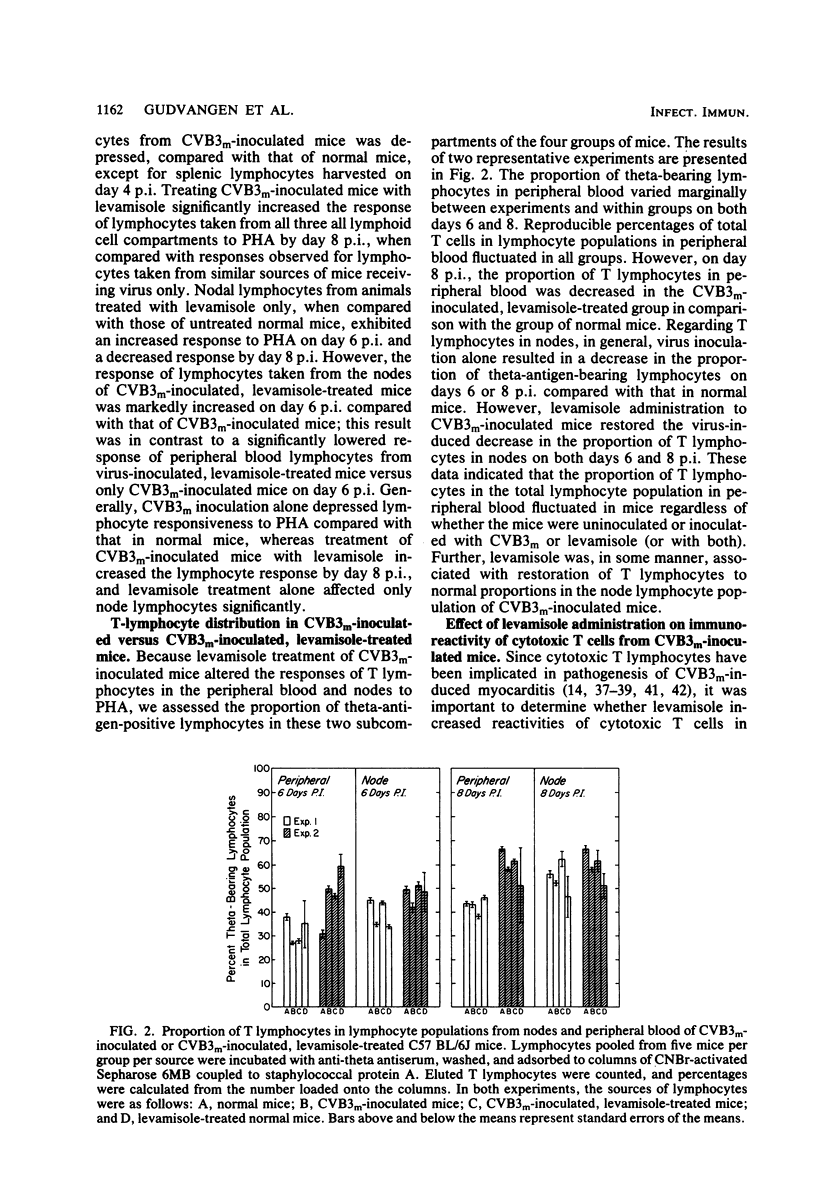
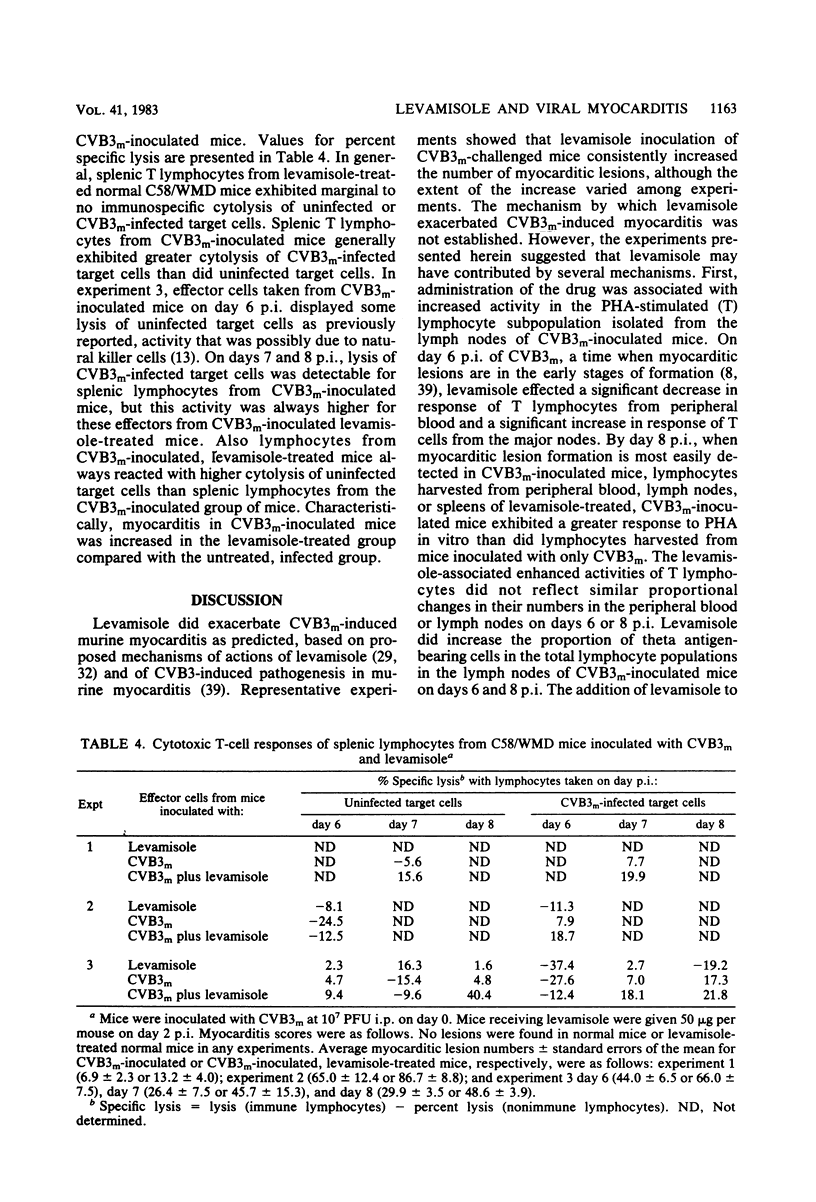
Levamisole administration to several strains of adolescent mice at the time of or up to 4 days post-inoculation (p.i.) with a myocarditic variant of coxsackievirus B3 (CVB3m) increased the number of myocarditic lesions above that found in CVB3m-inoculated mice. Virus replication in heart tissues in vivo was not affected by levamisole administration to the mice, nor was production of neutralizing antibody to CVB3m. Lymphocytes from nodes of virus-inoculated mice treated with levamisole at 2 days p.i. exhibited an increased reactivity to phytohemagglutinin on days 6 and 8 p.i., compared with respective responses by nodal T lymphocytes from CVB3m-inoculated mice. Levamisole treatment of CVB3m-inoculated mice also increased the reactivity of splenic and peripheral blood T lymphocytes to phytohemagglutinin on day 8 p.i., but not day 6 p.i., compared with the respective responses by lymphocytes from CVB3m-inoculated mice. The proportion of theta antigen-bearing lymphocytes in the total lymphocyte population in peripheral blood of CVB3m-inoculated mice was not altered by levamisole treatment. However, CVB3m-induced reduction in this subpopulation of lymphocytes in the nodes was restored to control levels by levamisole treatment. Reactivities of cytotoxic T lymphocytes from CVB3m-inoculated mice were increased against both normal and CVB3m-inoculated target cells after levamisole treatment of these mice. The results suggest that levamisole may contribute to CVB3m induction of myocarditis by several mechanisms, such as increasing the blastogenic activity of the phytohemagglutinin-responding subset of T lymphocytes, by possibly altering T-lymphocyte distribution in the body and by nonspecifically increasing reactivities of cytotoxic T lymphocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelmann W. H. Viral myocarditis and its sequelae. Annu Rev Med. 1973;24:145–152. doi: 10.1146/annurev.me.24.020173.001045. [DOI] [PubMed] [Google Scholar]
- Amery W. K. A hypothesis. The mechanism of action of levamisole: immune restoration through enhanced cell maturation. J Reticuloendothel Soc. 1978 Aug;24(2):187–193. [PubMed] [Google Scholar]
- Amery W. K., Gough D. A. Levamisole and immunotherapy: some theoretic and practical considerations and their relevance to human disease. Oncology. 1981;38(3):168–181. doi: 10.1159/000225546. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Giles T. D. The role of viruses in the production of heart disease. Am J Cardiol. 1972 Feb;29(2):231–240. doi: 10.1016/0002-9149(72)90634-0. [DOI] [PubMed] [Google Scholar]
- Duffey P. S., Drouillard D. L., Barbe C. P. Lymphocyte sorting on albuminated CIBA blue dextran-staphylococcal protein A-conjugated sepharose 6MB affinity columns. J Immunol Methods. 1981;45(2):137–151. doi: 10.1016/0022-1759(81)90208-8. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Paque R. E., Trousdale M. D., Gudvangen R. J., Barr D. T., Lipotich G. J., Nealon T. J., Duffey P. S. Temperature-sensitive mutant of coxsackievirus B3 establishes resistance in neonatal mice that protects them during adolescence against coxsackievirus B3-induced myocarditis. Infect Immun. 1983 Feb;39(2):851–864. doi: 10.1128/iai.39.2.851-864.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Trousdale M. D., LaBadie D. R., Paque R. E., Nealon T. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol. 1979;3(3):207–220. doi: 10.1002/jmv.1890030307. [DOI] [PubMed] [Google Scholar]
- Gomez M. P., Reyes M. P., Smith F., Ho L. K., Lerner A. M. Coxsackievirus B3-positive mononuclear leukocytes in peripheral blood of Swiss and athymic mice during infection. Proc Soc Exp Biol Med. 1980 Oct;165(1):107–113. doi: 10.3181/00379727-165-40942. [DOI] [PubMed] [Google Scholar]
- Han T., Nemoto T., Ledesma E. J., Bruno S., Amery W. K. Monocyte-mediated suppression of T lymphocyte proliferative response in breast and colorectal cancer patients: specific action of levamisole on suppressor monocytes. Int J Immunopharmacol. 1981;3(1):103–111. doi: 10.1016/0192-0561(81)90050-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto I., Komatsu T. Myocardial changes after infection with Coxsackie virus B3 in nude mice. Br J Exp Pathol. 1978 Feb;59(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Auld K. R., Woodruff J. F. Sex-related differences in the rapid production of cytotoxic spleen cells active against uninfected myofibers during Coxsackievirus B-3 infection. J Immunol. 1981 Apr;126(4):1336–1340. [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. Lysis of infected myofibers by coxsackievirus B-3-immune T lymphocytes. Am J Pathol. 1980 Mar;98(3):681–694. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. Sex-related differences in the pattern of coxsackievirus B-3-induced immune spleen cell cytotoxicity against virus-infected myofibers. Infect Immun. 1981 Apr;32(1):68–73. doi: 10.1128/iai.32.1.68-73.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Fischer G. W., Sayles P. C., Strickland G. T. Levamisole: potentiation of primary immunoglobulin M antibody responses in suckling rats. Immunopharmacology. 1981 Jun;3(2):117–127. doi: 10.1016/0162-3109(81)90013-8. [DOI] [PubMed] [Google Scholar]
- Kantor F. S. Infection, anergy and cell-mediated immunity. N Engl J Med. 1975 Mar 20;292(12):629–634. doi: 10.1056/NEJM197503202921210. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Lewes D. Viral myocarditis. Practitioner. 1976 Mar;216(1293):281–287. [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Otterness I. G., Bliven M. L., Holden H. E., Jr Effect of levamisole on the mitosis of murine thymocytes in culture. Immunopharmacology. 1979 Jun;1(3):245–254. doi: 10.1016/0162-3109(79)90041-9. [DOI] [PubMed] [Google Scholar]
- Otterness I. G., Lachman L. B., Bliven M. L. Effects of levamisole on the proliferation of thymic lymphocyte subpopulations. Immunopharmacology. 1981 Feb;3(1):61–69. doi: 10.1016/0162-3109(81)90040-0. [DOI] [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J. Assessment of cell-mediated immunity against coxsackievirus B3-induced myocarditis in a primate model (Papio papio). Infect Immun. 1981 Jan;31(1):470–479. doi: 10.1128/iai.31.1.470-479.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paque R. E., Gauntt C. J., Nealon T. J., Trousdale M. D. Assessment of cell-mediated hypersensitivity against coxsackievirus B3 viral-induced myocarditis utilizing hypertonic salt extracts of cardiac tissue. J Immunol. 1978 May;120(5):1672–1678. [PubMed] [Google Scholar]
- Paque R. E., Straus D. C., Nealon T. J., Gauntt C. J. Fractionation and immunologic assessment of KCl-extracted cardiac antigens in coxsackievirus B3 virus-induced myocarditis. J Immunol. 1979 Jul;123(1):358–364. [PubMed] [Google Scholar]
- Rabson A., Blank S., Lomnitzer R. Effects of levamisole on in vitro suppressosr cell function in normal humans and patients with systemic lupus erythematosus. Immunopharmacology. 1980 Apr;2(2):103–108. doi: 10.1016/0162-3109(80)90002-8. [DOI] [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Symoens J., Rosenthal M. Levamisole in the modulation of the immune response: the current experimental and clinical state. J Reticuloendothel Soc. 1977 Mar;21(3):175–221. [PubMed] [Google Scholar]
- Thienpont D., Vanparijs O. F., Raeymaekers A. H., Vandenberk J., Demoen J. A., Allewijn F. T., Marsboom R. P., Niemegeers C. J., Schellekens K. H., Janssen P. A. Tetramisole (R 8299), a new, potent broad spectrum anthelmintic. Nature. 1966 Mar 12;209(5028):1084–1086. doi: 10.1038/2091084a0. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Gauntt C. J. Isolation of Coxsackievirus B3 temperture-sensitive mutants and their assignment to complementation groups. Biochem Biophys Res Commun. 1976 May 23;76(2):368–375. doi: 10.1016/0006-291x(77)90734-3. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Nealon T., Gauntt C. J. Assessment of coxsackievirus B3 ts mutants for induction of myocarditis in a murine model. Infect Immun. 1979 Feb;23(2):486–495. doi: 10.1128/iai.23.2.486-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock E. F., Toy S. T. Participation of lymphocytes in viral infections. Adv Immunol. 1973;16:123–184. doi: 10.1016/s0065-2776(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. I. Model and viral specificity1. J Immunol. 1977 Apr;118(4):1159–1164. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus tb-3 infection. II. Characterization of effector cells and demonstration cytotoxicity against viral-infected myofibers1. J Immunol. 1977 Apr;118(4):1165–1169. [PubMed] [Google Scholar]
- Woodruff J. F., Kilbourne E. D. The influence of quantitated post-weaning undernutrition on coxsackievirus B3 infection of adult mice. I. Viral persistence and increased severity of lesions. J Infect Dis. 1970 Feb;121(2):137–163. doi: 10.1093/infdis/121.2.137. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. T lymphocyte interaction with viruses and virus-infected tissues. Prog Med Virol. 1975;19:120–160. [PubMed] [Google Scholar]


