SUMMARY
Costimulatory signals are critical to T cell activation, but how their effects are mediated remains incompletely characterized. Here, we demonstrate that locally produced C5a and C3a anaphylatoxins interacting with their G protein-coupled receptors (GPCRs), C5aR and C3aR, on APCs and T cells both upstream and downstream of CD28 and CD40L signaling are integrally involved in T cell proliferation and differentiation. Disabling these interactions reduced MHC class II and costimulatory-molecule expression and dramatically diminished T cell responses. Importantly, impaired T cell activation by Cd80−/− Cd86−/− and Cd40−/− APCs was reconstituted by added C5a or C3a. C5aR and C3aR mediated their effects via PI-3 kinase-γ-dependent AKT phosphorylation, providing a link between GPCR signaling, CD28 costimulation, and T cell survival. These local paracrine and autocrine interactions thus operate constitutively in naive T cells to maintain viability, and their amplification by cognate APC partners thus is critical to T cell costimulation.
INTRODUCTION
Adaptive immune responses must not only be strong enough for host defense but must also avoid autoreactivity and maintain homeostasis. Consequently, antigen-induced expansion and differentiation of T cells must be tightly controlled. Important in this control is the requirement for costimulation. Initially, this involves the dependence of T cell proliferation on the engagement of antigen-presenting cell (APC) B7 and CD40 by T cell CD28 and CD40 ligand (CD40L) (“Signal 2”). Subsequently, it involves the dependence of T cell differentiation on the elaboration by APC partners of IL-12 and IL-23 and other cytokines (“Signal 3”). How these receptor-counterreceptor engagements mediate these two processes remains incompletely characterized.
The complement system is thought to be integral to the innate immune system and function in adaptive immunity only in humoral immune responses (Janeway et al., 2005). Because of this, data implicating complement as impacting adaptive T cell responses have been attributed to “crosstalk” effects of complement activation fragments deriving from serum complement acting on APCs or T cells exogenously. Among these data are findings that antiviral T cell responses are attenuated in C3−/− mice (Kopf et al., 2002) and T cell-mediated renal injury is depressed in C5a receptor (C5ar1)−/− MRL/lpr mice (Wenderfer et al., 2005).
In recent studies (Heeger et al., 2005), we found that T cell responses are markedly augmented in mice targeted in the homolog (Daf1) (Lin et al., 2001) of the gene that encodes human decay-accelerating factor (DAF or CD55), a surface C3 and C5 convertase inhibitor that prevents complement activation on self-cell surfaces (Medof et al., 1984). To understand the basis for this augmentation, we studied wild-type (WT) cells and found that when WT T cells interact with WT APCs bearing peptide specific for their T cell receptor (TCR), both cognate partners locally synthesize the alternative-pathway components C3, factor B (fB), and factor D (fD). These three components spontaneously activate on foreign targets but not on self cells that express C3 and C5 convertase inhibitors. Although we had no evidence indicating that the locally generated components, in fact, activate, we found that concomitant with the local C3, fB and fD synthesis, WT APCs downregulate surface DAF expression lowering local C3 and C5 convertase inhibitory activity. We additionally found that the augmented T cell responses did not occur when Cd55−/− Cfd−/− double-knockout APCs were used and that administration of anti-C5 mAb inhibited the generation of IFNγ-producing cells in vivo, whereas addition of C5a augmented it in vitro. These findings raised the possibility that in WT cells, complement activation products locally produced at the APC-T cell interface might indeed be involved in the T cell activation process.
In view of this, we initiated studies to determine whether C5a and/or another activation fragment i.e., C3a, generated from complement endogenously produced by cognate APC-T cell partners, participate(s) in T cell differentiation into IFNγ+ effector cells. C5a and C3a are ~10 kDa anaphylatoxins able to ligate the C5a receptor (C5aR) and the C3a receptor (C3aR) that are G protein-coupled receptors (GPCRs) generally expressed on APCs and reported under some conditions to be detectable on T cells (Soruri et al., 2003). We performed studies with primary APCs and T cells by using two independent approaches, genetic deficiency and pharmacological blockade, to assess whether and, if so, how the local complement production relates to physiological T cell responses. After finding that these GPCR engagements indeed are integrally involved in the T cell activation process physiologically, we investigated the mechanism and unexpectedly found that their signaling not only functions integrally in costimulation but also operates constitutively in naive T cells to sustain their viability.
RESULTS
Cognate APC-T Cell Partners Locally Generate C5a and C3a and Upregulate C5aR and C3aR
Our previous studies (Heeger et al., 2005) revealed that APC-T cell cognate partners locally synthesize C3, fB, and fD, that added C5a influences the strength of the induced T cell response, and that DAF, which inhibits the generation of C3a as well as C5a, is downregulated on APCs. To establish whether these findings are relevant to T cell activation physiologically and provide mechanistic insight, we tested whether APCs and T cells additionally synthesize C5 as well as C5aR and C3aR that could result in local C5a-C5aR and/or C3a-C3aR engagements.
Initially, we incubated OT-II TCR transgenic T cells with OVA323–339 peptide plus bone-marrow-derived dendritic cells (DCs) as APCs. At various time points, we flow separated the APCs and T cells, isolated their RNA, and assayed complement-gene-expression patterns in the two partners by qPCR. These assays showed that in addition to upregulating C3, fB, and fD, both the APCs and T cells indeed upregulated mRNAs for C5 as well as C5aR and C3aR (Figure 1A). Notably, although both partners produced complement, on the basis of a quantitative analysis of per-cell copy number with an internal standard (data not shown), C3 mRNA was produced in ~1000-fold excess by the APCs compared to the T cells both at rest and after activation. The analyses also showed that during cognate interactions, T cells as well as the APCs concurrently downregulated Daf1 mRNA (Figures 1A and 1B), thereby further lowering restraint on local C3, fB, fD, and C5 activation.
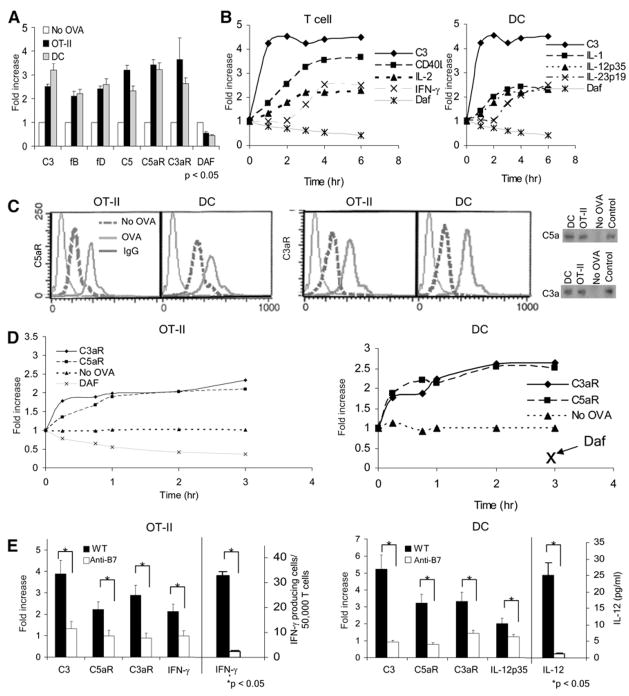
Figure 1. APC-T Cell Partners Upregulate Complement mRNAs and the RNAs Produce Proteins.
(A) OT-II T cells were incubated for 1 hr with WT DCs ± 0.1 μM OVA323–339 and flow separated (with anti-CD3 and anti-CD11c,) and complement mRNA expression in each partner was measured by qPCR.
(B) OT-II cells and DCs were flow separated at increasing times, and complement IL-2, IFN-γ, IL-12, and IL-23 gene expression was measured by qPCR.
(C) The left side shows representative (rep) histograms (four exps; linear scales) depicting C5aR or C3aR on OT-II cells and DCs before (no OVA) and after 1 hr interaction with OVA. The right side shows that after 24 hr of interaction of DCs with OT-II cells ± OVA, flow-separated cells were cultured for 4 hr, and supernatants were blotted for C3a and C5a; stds = 2 ng.
(D) Kinetics of C5aR, C3aR, and DAF protein expression on OT-II T cells and DCs during interaction with ova. Fold increase is relative to no OVA cultures. DAF levels on the DCs were low at all time points.
(E) After interaction of OT-II cells with DCs ± ova for 18 hr with 4 μg/ml anti-B7.1 and anti-B7.2 mAbs or control IgG, mRNAs in flow-separated cells were assayed for C3, C3aR, C5aR, and IFNγ gene expression by qPCR. In parallel cultures, IFNγ was assessed by ELISPOT. No complement or cytokine upregulation occurred without T cells. Data are normalized to no OVA. Each experiment is representative of two to four replicate studies. *p < 0.05 versus controls. All error bars are ± SD.
Kinetic analyses (Figure 1B) revealed that the complement up-regulation in T cells preceded the well-established, activation-induced upregulation of CD40L mRNA expression (Diehl et al., 2000), and that both preceded IL-2 mRNA expression. In the DCs, C3 mRNA upregulation occurred much earlier than upregulation of IL-1, IL-12p35, and IL-23p19 mRNAs known to influence T cell differentiation. As expected, the upregulation of IL-12p35 mRNA by the DCs (2-fold at 2 hr) preceded the upregulation of IFNγ mRNA in the OT-II cells (2-fold >3 hr).
To determine whether the changes in mRNA translated into differences in protein production, we performed flow-cytometric analyses (Figures 1C and 1D). These assays confirmed upregulated expression of C5aR and C3aR levels on both the T cells and APCs. The upregulated surface C5aR and C3aR on both partners persisted in the presence but not absence of OVA peptide (Figure 1D), documenting antigen dependence. Immunoblottings performed on the serum-free culture supernatants showed the ~10 kB C5a and C3a ligands for C5aR and C3aR (Figure 1C, right), indicating that the locally produced components underwent spontaneous alternative-pathway activation. The generation of C5a and C3a (which signal at 10−13 M) and augmentation of C5aR+C3aR on the cell surfaces continued over the ensuing 3 hr and thereafter in the APC-peptide-T cell mixture (Figure 1D). Concurrently, surface Daf protein expression progressively declined on the T cells as well as on the APCs and was well below baseline at 3 hr. Thus, interacting APC and T cell partners both generate C5a and C3a and upregulate C5aR and C3aR.
APC-T Cell Complement Component and Receptor Inductions Are Dependent on CD28-B7 and CD40-40L Couplings
To address mechanisms underlying the observed APC-T cell complement component and receptor upregulations, we next tested the impact of costimulation on these processes. Upon addition of blocking B71/2 mAbs, T cell differentiation and cytokine production was diminished (Figure 1E), APC IL-12 upregulation was prevented, C3, C3aR, and C5aR gene expression on both partners was abrogated, C5aR and C3aR surface upregulation did not occur, and substantially lower amounts C3a and C5a were detected in culture supernatants (not shown). Similarly, after the addition of the blocking anti-CD40L mAb MR-1 but not a control IgG (Figure S1A available online), IFNγ production as well as complement-component and receptor-gene and protein upregulation by the OT-II T cells was prevented. Notably, the abrogation of complement upregulation by the loss of either B7-CD28 or CD40-CD154 interactions was ~70%, but if the two costimulatory pairs were blocked simultaneously, the complement-gene expression was suppressed below control values (Figure S1B). Similar results were obtained with an independent Marilyn TCR transgenic T cell system specific for HYDby plus I-Ab (not shown).
Thus, the data show that cognate T cell-APC interactions along with costimulatory signals (delivered through CD80- and CD86-CD28 and CD40-40L) induce the sustained local production of C5a and C3a, downregulation of cell-surface expression of DAF, and persistent upregulation C5aR and C3aR. All of these events occur prior to T cell proliferation and cytokine secretion.
Disabling C5aR+C3aR Signaling Prevents T Cell Responses In Vitro
In view of the findings that APC and T cell partners sustain local generation of C5a+C3a together with augmenting their C5aR+ C3aR surface expression during cognate interactions, we tested whether signaling through C5aR and/or C3aR by the locally generated C5a and C3a ligands is involved in activation of T cells by APCs.
To do this, we performed studies with OT-II cells, OVA323–339 peptide, and WT DCs in which we blocked C5a-C5aR+C3a-C3aR engagements on the C5ar1+/+C3ar1+/+ OT-II T cells and APCs by the addition of a C5aR antagonist (C5aR-A), a cyclic peptide C5a analog that binds to C5aR and prevents C5aR signaling (Morikis and Lambris, 2002), and a C3aR antagonist (C3aR-A) that, like the C5aR-A, binds to but does not signal through C3aR. Both antagonists block their target receptors with high specificity (Figures S2A and S2B). The two antagonists added together prevented upregulation of IFNγ mRNA in the responding T cells (Figure 2A, left), as well as production of IL-12p35 and IL-23p19 mRNA in the DCs (Figure 2A, middle). A similar result was obtained with anti-CD3+anti-CD28 stimulation of WT T cells (Figure 2A, right).
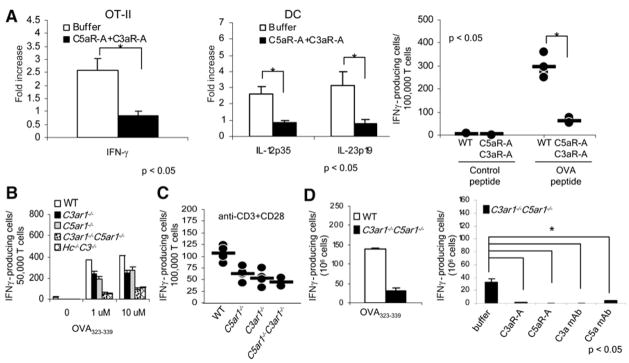
Figure 2. Disabling C3aR and C5aR Prevents T Cell Immunity In Vitro.
(A) OT-II T cells were incubated for 48 hr with WT DCs and OVA323–339 ± 10 ng/ml C5aR-A and 10 ng/ml C3aR-A and either flow separated and assayed for mRNA expression by qPCR (left and middle) or assayed for IFNγ+ cells by ELISPOT (right; dots represent overlapping replicates, n = 5 per group).
(B) OT-II T cells were incubated for 48 hr at 37°C with WT, C3ar1−/−, C5ar1−/−, C5ar1−/−C3ar1−/−, or C3−/−Hc−/− DCs + OVA323–339 and assayed for IFNγ+ cells by ELISPOT.
(C) Purified WT, C3ar1−/−, C5ar1−/−, or C5ar1−/− C3ar1−/− T cells (>96% CD3+) were stimulated with 1 μg/ml anti-CD3 + 1 μg/ml anti-CD28 for 3 hr and assayed for IFNγ+ cells (n = 5 each group; some dots overlap.
(D) OT-II T cells were incubated for 48 hr at 37°C with WT or C3ar1−/−C5ar1−/− DCs in the presence of 10 ng/ml C5aR-A and C3aR-A or 10 μg/ml anti-C5a+C3a mAbs, after which IFN-γ producing cells were assayed by ELISPOT. Each experiment was repeated at least twice with comparable results. *p < 0.05. All error bars are ± SD.
To assess the contribution of C5aR and C3aR function on APCs in mediating T cell reactivity, we mixed WT OT-II T cells with DCs from WT, C5ar1−/−, C3ar1−/−, and C3ar1−/−C5ar1−/− mice. The absence of either receptor from the APC diminished T cell IFNγ production by 30%–50% (Figure 2B). The impact of the absence of both APC C3aR and C5aR was greater than the absence of either receptor alone in that T cell IFNγ production was inhibited by greater than 75% (Figure 2B). A comparable result was obtained if APCs from mice lacking C5 and C3 (Hc−/− C3−/−) were used (Figure 2B). The response of the OT-II T cells to C3ar1−/− C5ar1−/− or Hc−/− C3−/− APCs was markedly lower than the previously reported inhibition (Peng et al., 2006) induced by the absence of APC C3.
To address the impact of C5aR and C3aR on T cells, we used anti-CD3+anti-CD28 stimulation (Figure 2C). Whereas this stimulation induced IFNγ production by WT T cells, stimulation of C5ar1−/− C3ar1−/− T cells yielded a markedly reduced response. Similarly, when we employed anti-C5a and anti-C3a mAbs together with C5ar1−/− C3ar1−/− DCs in the DC-OVA323–339-OT-II system to block OT-II C3aR and C5aR signaling, it had the same effect (Figure 2D).
C3aR and C5aR Control T Cell Immunity In Vivo
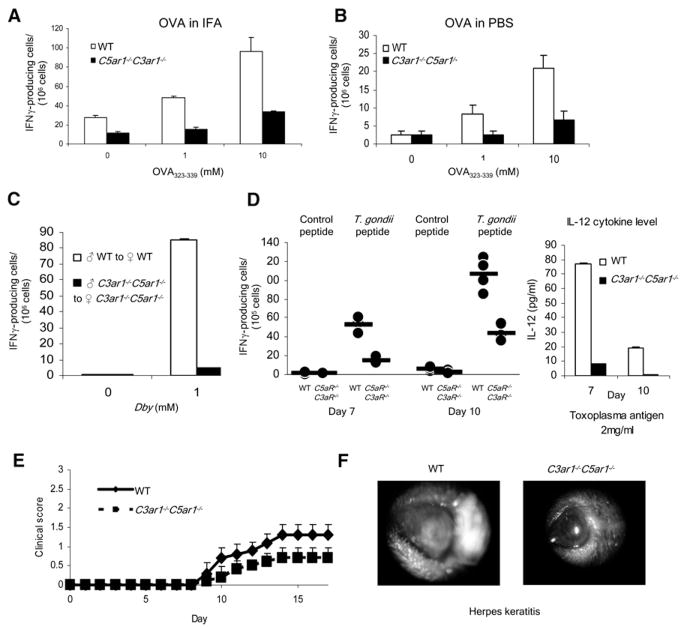
To test the impact of APC-T cell C5a-C5aR+C3a-C3aR signaling in vivo, we performed multiple complementary experiments. After immunization of WT or C5ar1−/−C3ar1−/− mice with ovalbumin protein mixed in incomplete Freund’s adjuvant (IFA), we found that recall responses by ELISPOT assay on day 10 were reduced by 50% in C5ar1−/− C3ar1−/− mice compared to WTs (Figure 3A). To assess immunity in the absence of adjuvant, we immunized mice with ovalbumin in PBS. As anticipated, the responses (Figure 3B) were lower, but again the frequency of specific IFNγ-producing T cells was reduced by 80% in C5ar1−/− C3ar1−/− mice. With another approach, we injected male cells into syngeneic H-2b WT or H-2b C5ar1−/−C3ar1−/− mice. Whereas HYDby responses to the HYDby (I-Ab-restricted determinant) 2 weeks later were detected after the WT male transfers, none were detected with the male C5ar1−/−C3ar1−/− transfers (Figure 3C). In another model system, 40% of CFSE-labeled OT-II T cells underwent at least one round of proliferation after adoptive transfer into WT mice primed 24 hr previously with OVA323–339 in CFA, but <5% underwent at least one round of proliferation in identically primed C5ar1−/−C3ar1−/− mice (p < 0.05 versus WT, n = 2 per group, not shown). Similar results were obtained in an allo model in which CFSE-labeled Balb/c T cells were adoptively transferred to WT and Hc−/−C3−/− mice (20% versus <2% undergoing one or more rounds of proliferation, n = 2, not shown). In another adoptive-transfer model, IFN-γ producing cells generated by C5ar1−/−C3ar1−/− C57BL/6 female Mar T cells into C5ar1−/− male recipients were reduced by 33% of those generated by WT female Mar T cells injected into male C57BL/6 recipients (not shown).
Figure 3. The Absence of C3aR and C5aR Prevents T Cell Immunity In Vivo.
(A) WT or C5ar1−/−C3ar1−/− mice (n = 3 per group) were immunized with ovalbumin protein mixed in IFA, and spleen cells on day 10 were assayed for responses to OVA323–339 by ELISPOT. No response occurred with control peptides or naive mice.
(B) Analogous to the experiment in (A), animals were injected s.c. with ovalbumin mixed in PBS, and responses to OVA323–339 were assayed on day 10.
(C) Syngeneic WT and C5ar1−/−C3ar1−/− male spleen cells were injected i.v. into WT or C5ar1−/− C3ar1−/− B6 females, respectively, and 10 days later, recipient spleen cells were assayed for responses to class II-restricted Dby peptide.
(D) WT or C5ar1−/−C3ar1−/− mice were infected with T gondii. All C5ar1−/−C3ar1−/− mice died by day 12, whereas WT animals survived for >50 days. Spleen cells from C5ar1−/−C3ar1−/− and WT animals isolated on day 7 or 10 were stimulated with 1 μg of Toxoplasma gondii antigen and assayed for IFNγ by ELISPOT (left) or for IL-12 by ELISA (right) (n = 5 per group each time; some dots overlap). *p < 0.05 versus controls.
(E) Clinical scores in WT and C5ar1−/−C3ar1−/− mice in which EAE was induced by immunization s.c. with 200 μg MOG35–55 in CFA and 200 ng of pertussis toxin. (n = 5 each group, p < 0.05).
(F) Globes from WT and C5ar1−/−C3ar1−/− mice 15 days after inoculation of scratched corneas with KDS strain of herpes simplex virus (n = 5 each group). All error bars are ± SD.
To evaluate the impact of C3aR and C5aR in clinically relevant disease models, we infected WT and C5ar1−/−C3ar1−/− mice with T. gondii (ME49 strain), a system in which disease protection is IL-12 and T cell dependent (Liu et al., 2006). The C5ar1−/−C3ar1−/− mice survived for only 12 days, whereas all simultaneously infected WT mice appeared clinically healthy on day 12 (n = 5 per group) and survived for > 50 days (not shown). Spleen cells from the WT animals sacrificed on both day 7 and 10 postinfection prior to appearing ill on day 12 responded strongly to T. gondii antigens as assessed by IL-12 levels in culture supernatants and a high frequency of antigen-specific IFNγ producers (Figure 3D, left). In contrast, at both time points, spleen cells from the infected C5ar1−/−C3ar1−/− mice contained 70% fewer IFNγ-producing cells (p < 0.05) together with a complete lack of IL-12 secretion (Figure 3D, right). In the T cell-dependent MOG35–55-induced experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis, WTs showed characteristic weakness (clinical score >1.5), whereas C5ar1−/− C3ar1−/− mice showed less weakness (clinical score 0.5) that persisted over time (n = 4 per group, p < 0.05). In a herpes keratitis model, in which inflammation is due to host T cell responses against virally infected corneal cells rather than the virus itself, corneas of WT mice completely opacified at day 14, whereas corneas of C5ar1−/−C3ar1−/− mice showed little or no change (n = 5, p < 0.005; Figure 3F). Thus in all systems, both in vitro and in vivo, T cell immunity in the absence of C5aR or C3aR on APCs or T cells was markedly impaired.
C5a-C5aR+C3a-C3aR Engagements Function as Autocrine Regulators
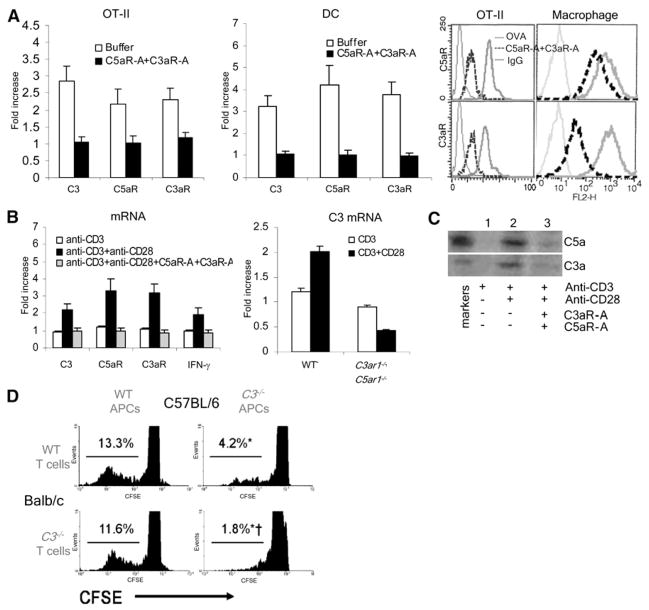
The finding that complement upregulation was maintained for prolonged periods upon initial antigen stimulation (Figure 1) suggested the possibility of an autocrine feedback loop in which cognate interactions induce complement production and initiate activation and locally produced C3a and C5a maintain upregulated production of these components in both partners by virtue of signaling through their C5aR+C3aR. Consistent with this, blockade of C3aR+C5aR signaling with the specific antagonists not only prevented OT-II T cell proliferation and cytokine secretion (Figure 2) but also prevented the induced upregulation of complement components and receptors in both partners (Figure 4A). Moreover, anti-CD3+anti-CD28 stimulation of OT-II cells induced complement-component-gene upregulation in WT T cells (Figure 4B), but the same treatment in the presence of the C3aR-A and C5aR-A or upon stimulation of C5ar1−/−C3ar1−/− T cells had no effect (Figure 4B). Immunoblots of culture supernatants of anti-CD3+anti-CD28-stimulated OT-II cells showed both C5a and C3a (Figure 4C), but >90% reduced C5a+C3a generation after 2 hr of incubation with C5aR-A and C3aR-A. Overexposures of unstimulated T cells in medium alone showed low-level C5a and C3a production (lane 1), an intriguing result that will be addressed below (see Figure 7). Analogously, APCs deficient in both C3aR and C5aR did not upregulate gene expression for complement components during cognate interactions with TCR tg T cells (Figure S3). The C5ar1−/−C3ar1−/− DCs showed no differences from WTs in cell numbers or viability (not shown).
Figure 4. Locally Produced C5a and C3a Interact with C5aR and C3aR in an Autocrine and Paracrine Fashion to Augment T Cell Immunity.
(A) OT-II T cells were incubated for 1 hr with WT DCs and 0.1 μM OVA323–339 ± 10 ng/ml C5aR-A and 10 ng/ml C3aR-A and either flow separated and assayed for mRNA expression (C3, C5aR, C3aR; left and middle) or analyzed (after 24 hr) for C3aR and C5aR by flow cytometry (right); C3aR and C5aR levels on peritoneal macrophages are 14- and 13-fold higher.
(B) The left side shows that complement-gene expression was assessed in purified WT T cells after 1 hr stimulation with 1 μg/ml anti-CD3 + 1 μg/ml anti-CD28 ± 10 ng/ml of C5aR-A and C3aR-A. The right side shows that C3 expression was compared between purified WT and C5ar1−/−C3ar1−/− T cells stimulated with 1 μg/ml each of anti-CD3+anti-CD28.
(C) Supernatants from (B) were assayed for C5a+C3a by immunoblotting (std = 2ng).
(D) CFSE-labeled WT or C3−/− H-2d Balb/c T cells stimulated in vitro for 4 days with WT or C3−/− BL/6 H-2b macrophages. Percent cells within gated (C3−/− versus WT cells, *p < 0.05). Each experiment was repeated at least once with similar results. All error bars are ± SD.
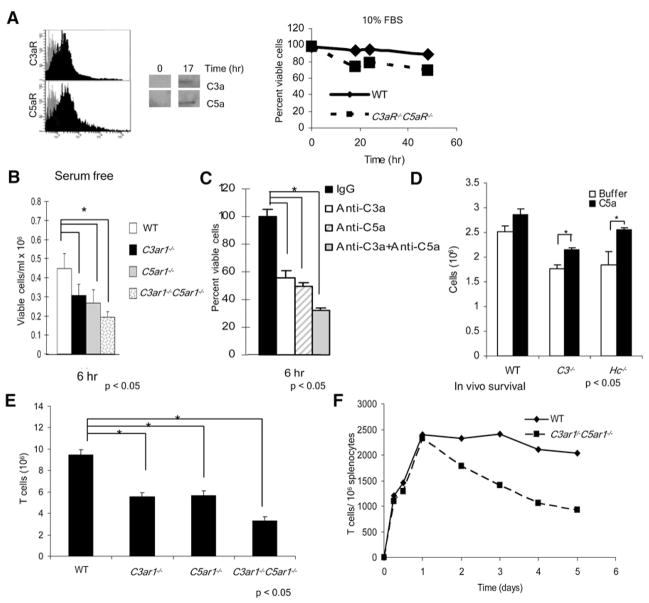
Figure 7. Constitutive C5a and C3a Production and Signaling via the C5aR and C3aR GPCRs Influences Cell Viability In Vitro and In Vivo.
(A) The left side shows that WT T cells were stained with Alexa647-labeled Ab for detection of C3aR and C5aR expression in resting T cells. The center shows that WT T cells were incubated at 37°C for 17 hr, supernatants were concentrated 10-fold, and C3a or C5a were immunoblotted. The right side shows that WT and C5ar1−/−C3ar1−/− T cells were incubated in complete RPMI 1640 and viability assessed as described in the Experimental Procedures (representative of three experiments).
(B) WT and C5ar1−/−, C3ar1−/−, and C5ar1−/− C3ar1−/− T cells were incubated for 6 hr in serum-free HL-1 medium and viability assayed.
(C) WT T cells were incubated at 37°C for 6 hr in HL-1 medium ± anti-C3a, anti-C5a, or anti-C3a+anti-C5a and viability assessed.
(D) C3−/− and C5-deficient T cells were incubated for 6 hr in HL-1 medium ± 300 ng/ml C5a added at time 0 and 90 min.
(E) Spleens from naive WT, C3ar1−/−, C5ar1−/−, and C3ar1−/−C5ar1−/− mice were isolated (three to five animals per group), total cell numbers were counted, and the CD3+ fraction was determined by flow cytometry (WT = 10%, C5ar1−/− = 5.4%, C3ar1−/− = 6.3, and C5ar1−/−C3ar1−/− = 4.1%).
(F) Immediately after isolation and labeling, 106 CFSE–labeled WT T cells and CellTracker Red CMTPX-labeled C5ar1−/−C3ar1−/− T cells were adoptively cotransferred into the same SCID mice (n = 4 mice per group for each time point). Surviving cells numbers were assayed at increasing times. Repeated experiments switching the dyes gave the same results (data not shown). All experiments were performed at least twice with comparable results. *p < 0.05 WT versus C5ar1−/−C3ar1−/− cells. All error bars are ± SD.
To further assess autocrine effects of C5a-C5aR+C3a-C3aR, we evaluated T cell responses in a system in which either the T cell or the APC, or both, were C5aR+C3aR disabled. As shown in Figure 2B, the generation of IFNγ-producing cells was reduced to 20% when C5ar1−/−C3ar1−/− DCs were used, whereas it was reduced to <5% if the C5aR-A and C3aR-A or anti-C5a/anti-C3a mAbs were added to block C5aR+C3aR signals in the OT-II cells. Similarly, the WT Balb/c T cell proliferation in response to B6 C3−/− APCs was reduced to 50% of B6 WT APCs, and C3−/− Balb/c T cell proliferation was reduced to 50% in response to C3−/− than WT APCs (Figure 4D), indicating that C3 production by the T cell as well as the APC participates in proliferation. The findings, taken together with those above, indicate that C3, fB, fD, and C5 produced by either partner during cognate interactions can activate (i.e., generate C3a and C5a) and in turn function in both a paracrine and autocrine manner to perpetuate local complement production and drive T cell responses. In the absence of C5aR+C3aR from either partner (in this serum-free in vitro system), no C5a or C3a is produced, preventing T cell proliferation and differentiation (Figure S4).
Disabling C5aR+C3aR Signaling Reduces Costimulatory-Molecule Expression
To clarify how disabled C5aR+C3aR signaling diminishes T cell responses, we examined costimulatory-molecule expression patterns by flow cytometry (Figure 5A). These analyses showed decreased surface expression of B7, CD40, and class II MHC (not shown) on peritoneal macrophages from C5ar1−/− and C3ar1−/− mice and essentially no staining on C5ar1−/−C3ar1−/− DCs. Parallel analyses of C5aR-A- and C3aR-A-treated WT macrophages showed similar reductions in costimulatory-molecule expression (Figure 5B). Consistent with this, in the absence of C3aR and C5aR on the APC, the cognate T cell APC interaction failed to induce upregulation of IL-1, IL-23, and IL-12 (Figure S5).
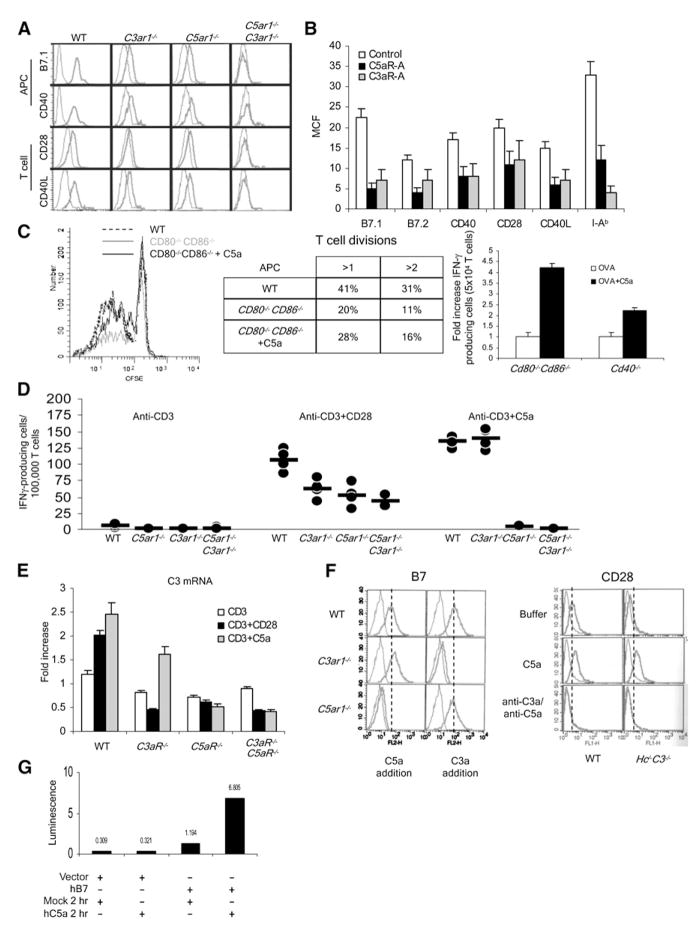
Figure 5. Locally Produced C5a and C3a Influence APC-T Cell Interactions through Regulating Costimulatory-Molecule Expression Levels.
(A) Peritoneal macrophages and CD4+ T cells from naive WT, C3−/−, C3ar1−/−, and C5ar1−/− mice were assayed for B7.1, B7.2, CD40, or CD28, and anti-CD3+anti-CD28-stimulated CD4+ T cells were assayed for CD40L expression levels by flow cytometry. (I-Ab surface expression was also decreased [not shown].)
(B) WT T cells and DCs were incubated for 1 hr at 37°C with 100 ng/ml C5aR-A or C3aR-A, and costimulatory molecule or MHC class II expression was assayed by flow cytometry.
(C) The left side shows that CFSE-labeled Mar T cells were incubated with WT or Cd80−/−Cd86−/− DC and 1 μg/ml Dby peptide ± 100 ng/ml of C5a, and proliferation was assessed by CFSE dilution. In a second experiment (middle), the percentage of cells undergoing greater than one or greater than two divisions was quantified. The right side shows that OT-II T cells were incubated for 18 hr with Cd80−/−Cd86−/− or CD40−/− DCs ± 300 ng/ ml C5a, and IFNγ+ T cells were quantitated by ELISPOT (representative of two experiments; WT versus Cd80−/−Cd86−/−, p < 0.05). C5a was added once at time 0 rather than continuously as occurs with CD28 signaling.
(D) WT, C3ar1−/−, C5ar1−/−, and C5ar1−/−C3ar1−/− T cells were incubated in complete RPMI with anti-CD3 (left), anti-CD3+anti-CD28 (middle), or anti-CD3 + 300 ng/ml C5a (right) and IFNγ+ cells assayed at 48 hr by ELISPOT (n = 5 each group; some dots overlap).
(E) WT, C3ar1−/−, or C5ar1−/− T cells were incubated with buffer, anti-CD3+anti-CD28, or anti-CD3 + 300 ng/ml C5a and complement (C3) mRNA transcripts measured by qPCR (representative of three experiments).
(F) The left side shows that WT, C3ar1−/−, or C5ar1−/− DCs were incubated for 4 hr ± 300 ng/ ml C5a, and Cd80 as well as Cd86 expression was measured by flow cytometry. Similar results were obtained in assays for I-Ab, CD40, with both DCs and macrophages and for CD40L and CD28 with T cells (data not shown). The right side shows that WT or C3−/−Hc−/− T cells were incubated with buffer, C5a, or anti-C3a+anti-C5a mAbs, and CD28 was measured as above.
(G) Base pairs −991 to +72 of the human B7.1 promoter were inserted into a luciferase reporter vector GL4 and transfected into THP-1 cells by electroporation. After overnight incubation in complete medium, the cells were treated with 300 nM of C5a for 2 hr in serum-free media, and luciferase activity was measured with a Lmax Luminometer (Molecular Devices). Each experiment was repeated at least once with similar results. *p < 0.05. All error bars are ± SD.
We also examined CD28 and CD40L expression on WT and C5ar1−/−C3ar1−/− T cells by flow cytometry (Figure 5A). T cell surface expression of CD28 was reduced by >90% on resting C5ar1−/−C3ar1−/− than on WT T cells, and CD40L levels were similarly reduced after anti-CD3+anti-CD28 stimulation. Parallel analyses of C5aR-A- and C3aR-A-treated WT T cells showed similar reductions (Figure 5B).
Costimulatory-Molecule Expression Is Dependent upon C5aR+C3aR Signaling
Costimulatory-molecule interactions are essential for optimal APC induction of T cell responses (Lenschow et al., 1995). To establish whether locally produced C5a+C3a and upregulated expression of C5aR+C3aR (Figure 1) by APC-T cell partners and costimulatory-molecule expression are mechanistically linked, we tested whether abrogation of T cell responsiveness in the absence of costimulatory-molecule signaling can be reversed by C3a+C5a addition. Consistent with this, diminished proliferation of Mar CD4+ T cells (Dby plus I-Ab) in response to Cd80−/− Cd86−/− DCs was reversed upon addition of C5a (Figure 5C), added C5a reconstituted diminished IFNγ production by OT-II cells stimulated with CD40−/− APCs (Figure 5C), and addition of anti-CD3 plus exogenous C5a led to a response equal that of anti-CD3+anti-CD28 (Figure 5D). Consistent with the above implicated autocrine loop (Figure 4), the exogenous C5a also induced upregulation of local complement synthesis comparably to that measured after anti-CD3+anti-CD28 stimulation and greater than that detected after anti-CD3 stimulation alone (Figure 5E). Whereas C3ar1−/− cells responded to exogenous C5a, C5ar1−/− T cells did not respond, demonstrating that the effects are mediated through this receptor (as opposed to the alternative C5a receptor, C5L2 [Cain and Monk, 2002]).
Strong support for a link between costimulation, T cell reactivity, local complement production, and C5aR+C3aR GPCR signaling derives from the observation that diminished B7 and CD28 expression on C3ar1−/− (but not C5ar1−/−) DCs and T cells, respectively (Figure 5A), was restored to WT values after either C5a or C3a addition (Figure 5F left). Likewise, C5a addition to C3−/−Hc−/− T cells restored CD28 expression whereas anti-C5a+anti-C3a addition to WT T cells decreased CD28 expression (Figure 5F right). To directly confirm that both APC and T cell costimulatory-molecule expression is controlled by C5aR and C3aR GPCR signaling, we added human C5a to C5aR-expressing THP1 cells transfected with a luciferase reporter driven by the promoter of B7.1. The added C5a induced a luciferase signal indicative of C5aR dependent regulation (Figure 5G). Overall, these data show that CD80, CD86 and CD40 costimulation yield local complement production and subsequent generation of C5a+C3a. The resultant C5a and C3a fragments signal through their GCPRs to upregulate costimulatory-molecule expression and directly drive T cell proliferation and differentiation.
C5a-C5aR+C3a-C3aR Signaling Is Linked to T Cell Activation through PI3-Kγ-Induced Phosphorylation of AKT
Studies with Jurkat cells (Stein et al., 1994) have shown that B7-CD28 ligation signals in T cells through phosphorylation of Y170 residue in the YMNM motif in CD28’s cytoplasmic tail, permitting SH2-dependent binding and activation of PI-3 kinase p85αp110α (PI-3Kα). The activated PI-3Kα increases the amount of internal leaflet-associated phosphatidylinositol 3,4,5 trisphosphate [PtdIns (3,4,5)P3], causing the recruitment of PDK1, PDK2, and AKT via their pleckstrin homology (PH) domains [which enable them to bind PtdIns (3,4,5)P3]. This juxtaposition on PtdIns(3,4,5)P3 allows PDK1+PDK2 to produce dually Thr308Ser473 phosphorylated AKT that is centrally implicated in CD28 costimulatory signaling (Kane et al., 2001).
To test whether abrogated CD28 costimulation in the absence or blockade of C5aR+C3aR (Figures 2 and 3) relates to a requirement for these GCPR signals for optimal AKT phosphorylation, we stimulated WT T cells with anti-CD3+anti-CD28 ± C5aR-A and C3aR-A and assessed AKT phosphorylation by immunoblotting (Figure S6A) and Luminex assay (Figure 6A). Notably, whereas addition of the C5aR-A diminished AKT phosphorylation, both antagonists together virtually abolished it. Moreover, significantly less phosphorylated AKT was detectable upon anti-CD3+anti-CD28 stimulation of C5ar1−/−C3ar1−/− T cells at all time points tested (Figure 6B).
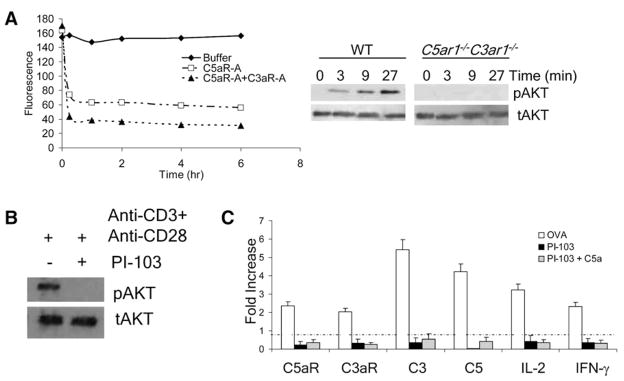
Figure 6. C5aR and C3aR Ligation Activates PI3-Kγ, which in turn Promotes AKT Phosphorylation.
(A) The left side shows that WT T cells were activated with anti-CD3+anti-CD28, and at progressively increasing times, buffer, C5aR-A, or C5aR-A+C3aR-A were added; extracts were analyzed for phospho-Ser473 AKT by Luminex assays (representative of two experiments). (p < 0.05). The right side shows that naive WT and C5ar1−/− C3ar1−/− T cells were incubated with 1 μg/ml of anti-CD3+anti-CD28 at 37°C and phospho-Ser473 AKT assessed at increasing times.
(B) WT T cells were incubated at 37°C with anti-CD3+anti-CD28 (1 μg/ml each) for 3 min after which the cells were incubated for 20 min with buffer or 0.1 μM PI-3Kγ-specific inhibitor PI-103. Extracts were immunoblotted with anti-phospho-Ser473 AKT or total AKT mAb (representative of five experiments).
(C) OT-II T cells were incubated with 0.1 μM OVA323–339, WT DCs, and 0.1 μM PI-103 ± 10 ng/ml C5a. Complement, IL-2, and IFNγ mRNAs were quantitated by qPCR. All error bars are ± SD.
AKT is one product resulting from the activity of the p110 catalytic subunit of PI-3 kinase p101γp110γ (PI-3Kγ), and PI-3Kγ has been tied to GCPR signal transduction in neutrophils and macrophages (Sasaki et al., 2000). To test whether this signaling pathway is operational in T cells, we incubated WT T cells with anti-CD3+anti-CD28 plus a specific inhibitor (PI-103) (Kung et al., 2006) of PI-3Kγ. Strikingly, addition of the inhibitor (1) abrogated anti-CD3+anti-CD28-induced phosphorylation of AKT (Figure 6B), (2) prevented the upregulation of complement-gene expression, and (3) eliminated upregulation of IL-2 and IFNγ mRNA expression (Figure 6C). Consistent with PI-3Kγ activation being mediated through C5aR ligation, these suppressive effects could not be overcome by added C5a (Figure 6C). Thus, C5aR- and C3aR-induced PI-3Kγ activation is necessary for AKT phosphorylation and resultant T cell activation.
C5aR+C3aR Signaling Is Essential for Sustaining Naive T Cell Viability
In our experiments examining whether C5a-C5aR+C3a-C3aR interactions in T cells operate in an autocrine fashion, on overexposed blots (not shown) we detected low levels of C5a+C3a in culture supernatants in the absence of stimulation (Figure 4B), a result raising the possibility that C5a+C3a is generated constitutively and is important for T cell function. Moreover, we observed reduced S473 AKT phosphorylation at early time points after preincubation of mouse T cells with the C5aR-A and C3aR-A (Figure 6A and Figure S6A), further implicating C5a-C5aR+C3a-C3aR interactions as operating tonically in naive T cells. The two results would be consistent with preexisting C5a+C3a feeding back on C5aR+C3aR to trigger ongoing basal GPCR activation. In support of this, C5aR and C3aR could be detected on unstimulated T cells with an ultrabright chromophore, WT but not C5ar1−/−C3ar1−/− T cells produced C5a and C3a at rest (Figure 7A), and as noted in Figure 6A, basal phospho S473 AKT was readily detectable in WT T cells but was markedly reduced in C5ar1−/−C3ar1−/− T cells (Figure 6B). Because of the known association of loss of phospho S473 AKT with induction of programmed cell death (PCD [Goruppi et al., 1997]), this connection of disabled C5aR+C3aR GPCR signaling in naive unstimulated cells with reduced phospho S473 AKT suggested the intriguing possibility that in naive T cells, constitutive C5a-C5aR+C3a-C3aR interactions play a role in maintaining viability.
To test this possibility, we compared the survival of WT and C5ar1−/−C3ar1−/− T cells in vitro after 48 hr of incubation in 10% heat-inactivated FCS. These analyses showed that in contrast to ~5% loss of WT T cells, 20%–30% loss of C5ar1−/− C3ar1−/− T cells occurred (Figure 7A). To eliminate effects of exogenous complement, growth factors, and other agents in serum, we repeated the survival studies with serum-free medium. Fewer surviving C5ar1−/−C3ar1−/− T cells were detected at 6 hr, confirming that disrupted autocrine signaling through both the C5aR and C3aR contributed to the decline (Figure 7B). To confirm that these effects were not reflective of other processes, we incubated naive WT T cells in serum-free medium containing anti-C3a and/or anti-C5a mAbs against the C5aR+ C3aR ligands rather than blocking the receptors themselves (Figure 7C). This caused a diminution in cell numbers similar to that observed with C5ar1−/−C3ar1−/− cells. As yet another test, we added C5a to C5-deficient or C3−/− T cells. This augmented 6 hr viability of both knockouts by ~25%, close to the viability of WT cells (Figure 7D). Thus, tonic C5a-C5aR+C3a-C3aR signal transduction is necessary for maintaining T cells in a viable state.
The In Vivo Half-Lives of Naive T Cells Are Shorter in the Absence of C5aR+C3aR Signaling
To document that the relationship between C5a-C5aR+C3a-C3aR interactions and viability is relevant physiologically, we compared the number of CD3+ T cells in spleens of naive C5ar1−/−, C3ar1−/−, and C5ar1−/−C3ar1−/− mice to those in spleens of WT mice. The cell counts revealed 2-, 2-, and 3-fold fewer C5ar1−/−, C3ar1−/−, and C5ar1−/−C3ar1−/− T cells, respectively, than WT CD3+ T cells per spleen (Figure 7E). Because total cell number at steady state is a reflection of production and destruction, we performed the more direct test of in vivo cell survival. We coadoptively transferred equal numbers of naive CellTracker Red CMTPX-labeled C5ar1−/−C3ar1−/− and CFSE-labeled WT T cells together into SCID mice and determined the number of viable cells in spleens after the transfer (Figure 7F). Similar numbers of each T cell population were detectable 8–24 hr, indicating that the cells equally migrated to spleens after the injection. However, significantly fewer C5ar1−/−C3ar1−/− T cells were detectable in the recipient spleens on days 2–5, consistent with the conclusion that viability is reduced. Control studies in which the cell-membrane labels were switched yielded the same results (data not shown). These studies thus documents that tonic C5a-C5aR and C3a-C3aR signal transduction functions in vivo to maintain the viability of naive T cells.
DISCUSSION
The data in this study provide several important insights. First, complement that is locally produced by APCs and T cells during cognate interactions is integrally involved in the T cell activation process. Specifically, C5a and C3a generated from this endogenous production interact with C5aR and C3aR on both APCs and T cells, and these engagements participate in activation and cytokine production by both partners. Previous studies have regarded complement as being separate from T cells and APCs, attributing complement’s effects on either cell coming from serum complement rather than from the interacting APCs and T cells themselves, i.e., from the outside-in rather than from the inside of APCs and T cells themselves. Second, this study surprisingly shows that in the naive state, T cells synthesize complement components constitutively, and the resultant C5a and C3a signaling through the C5aR and C3aR GPCRs participates in maintaining their viability. If this process is interrupted, the generation of phosphorylated AKT (PKB), a T cell activation intermediate that suppresses apoptosis, is reduced. That such a process operates in naive T cells has not previously been recognized.
That the absence or blockade of C5aR+C3aR leads to lower class II MHC and costimulatory-molecule expression is consistent with previous work showing that MHC class II expression is diminished on C3 deficient APCs (Peng et al., 2006). This documented C5a-C5aR+C3a-C3aR regulation of class II and costimulatory-molecule expression supports the conclusion that one mechanism through which this GPCR signaling influences T cell responsiveness is through regulating antigen presentation and B7-CD28 and CD40-CD40L costimulation. How it affects other APC-T cell costimulatory-molecule expression and signaling or T cell development remains to be determined.
Our data demonstrate that C5aR and C3aR exhibit overlapping but not fully redundant functions because inhibition or deficiency of both has a significantly more profound effect than the absence or blockade of either alone. These experimental results in conjunction with the recent description of C3 bypass pathways for C5 cleavage (Huber-Lang et al., 2006; Kang et al., 2006) explain why T cell immunity is diminished but not as fully abrogated in the absence of C3, C5, C3aR, or C5aR individually.
The findings in this study establish that C5a-C5aR+C3a-C3aR interactions function via both CD28 activation-dependent and -independent mechanisms. CD28 dependence is supported by the observation that CD28 and CD40L engagements upregulate complement and downregulate DAF and that C5a can compensate for CD28 and CD40L blockade or absence. They are consistent with C5a acting both to upregulate costimulatory molecules and to substitute for C5aR+C3aR signaling induced by B7 and CD40 ligation of T cell CD28 and CD40L. APC deficiency of C3, C5aR, and C3aR (each of which leads to less C5a+C3a) markedly limits T cell proliferation and differentiation both in vitro and in vivo. That these GPCR interactions additionally function independently of CD28 is shown by the findings that they are needed to maintain sufficient AKT phosphorylation to support T cell survival: Naive T cells constitutively generate C5a and C3a, C5ar1−/−C3ar1−/− double-deficient cells exhibit reduced viability in vitro and in vivo, addition of C5aR-A+C3aR-A or anti-C5a+anti-C3a mAbs reduces viability of WT T cells, and added C5a promotes viability of Hc−/− and C3−/− T cells. Studies with bone-marrow chimeras (P.N.L., M.G.S., Min Yang, Feng Lin, M.E.M., and P.S.H., unpublished data) showing that the absence of C5aR from bone-marrow-derived cells but not parenchymal cells limits responses of T cells in chimeric hosts support the interpretation that C5a+C3a endogenously produced by APC-T cell partners underlies each of the above results.
That deficient C5aR+C3aR expression suppresses IL-2 and IFNγ generation establishes an important role of amplified and sustained C5aR+C3aR GPCR signaling in the initial costimulatory pathways of T cells. That disabled C5aR+C3aR GPCR signaling in APCs suppresses IL-1, IL-12p35, and IL-23p19 production also centrally implicates this signaling in elaboration of these “Signal 3” cytokines. The findings regarding local APC-T cell C5a-C5aR+C3a-C3aR interactions thus identify previously uncovered GPCR steps that participate in both the in both T cell and APC cytokine signaling pathways. They additionally identify an unrecognized process that operates in naive T cells to maintain their viability. Models of initial T cell costimulation incorporating these findings and of the C5a-C5aR+C3a-C3aR loop are depicted in Figures S7A and S7B.
The linkage of bidirectional C5a+C3a signaling with B7-CD28 and CD40-CD40L costimulation during cognate APC-T cell interactions provides a mechanistic basis for many previously unexplained observations concerning effects of complement-gene deletions or added activation fragments on APC or T cell cytokine production. Importantly, it explains why signaling via “unidentified GCPR(s)” has repeatedly been postulated to be involved and PI-3Kγ activation in T cell activation has been implicated (Alcazar et al., 2007; Deane and Fruman, 2004). The findings thus have mechanistic as well as clinical relevance to adaptive immunity and disease processes that are T cell mediated. Beyond this, they highlight an unrecognized insight that complement functions endogenously in T cells to maintain viability.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Murine C5a was from Cell Sciences (Canton, ME). Mouse C3a and C5a mAbs were from R&D Systems (Minneapolis, MN). Mouse IL-4, GM-CSF, and M-CSF were from Peprotech (Rockyhill, NJ). Antibodies against mouse B7-1 and B7-2 were from BD PharMingen (San Diego, CA). Anti-CD40L mAb was from Bio Express (West Lebanon, NH). Anti-C5aR and anti-C3aR were purchased from Santa Cruz Biotech (Santa Cruz, CA). The PI-3K inhibitors were provided by Dr. Kevin Shokat. Peptides were synthesized by Research Genetics as described (Heeger et al., 2005).
Animals
C57BL/6, OT-II (specific for OVA323–339 plus I-Ab), Cd80−/−Cd86−/−, C3−/−, C5-deficient, and CD40−/− mice were from Jackson labs (Bar Harbor, ME). C3−/− mice and C3ar1−/− and C5ar1−/− were gifts of Dr. Michael Carroll and Dr. Craig Gerard (Harvard Medical School and Children’s Hospital, Boston, MA). Marilyn (MAR) transgenic was a gift of Polly Matzinger, Ghost Lab, NIH. We generated Hc−/−C3−/− mice by crossing C5-deficient B10.2 mice with C57BL/6 congenic C3−/− mice. C5+/+C3+/+ littermates used as controls displayed comparable results to the studies with C57BL/6 mice as controls. All studies were approved by the Case Western Reserve University Institutional Animal Care and Use Center (IACUC).
RNA Purification, cDNA Synthesis, and qPCR
Cells were purified for 5 min at 20°C with Trizol (Invitrogen, Carlsbad, CA)according to the manufacturer’s instructions. When C3aR and C5aR mRNAs were analyzed, preparations were treated with DNase I (standard protocol) for removal of genomic DNA. We synthesized cDNAs by incubating 20 ul of mRNAs in Sprint PowerScript Single Shots (Clontech, Mountain View, CA). A total of 10 ul of diluted cDNA were mixed with 2 ul of primer and 10 ul SYBR green master mix (Applied Biosystems, Foster City, CA) and assayed intriplicate on an ABI prism 7000 cycler. In allassays, fold increases are relative to each basal level and standardized to Actin.
Murine DCs and T Cells
Bone-marrow cells were grown in RPMI 1640/10% FBS containing 10 ug/ml IL-4 + 10 ug/ml GM-CSF. Fresh media with the same cytokines was added on day 3, 10 ug/ml IL-4 and 5 ug/ml GM-CSF were added on day 5, and cells were used on day 6. T cells harvested from spleens were purified with T cell enrichment columns (R&D Systems).
Immunizations, ELISPOT Assays and CFSE Proliferation, and Flow Cytometry
Mice were immunized s.c. with OVA323–339 peptide as described (Heeger et al., 2005). ELSPOT and proliferation assays were performed as described (Heeger et al., 2005). All antibodies were purchased from BD PharMingen (San Diego, CA), and stained cells were analyzed on a Becton Dickinson LSR II.
Anti-CD3 and Anti-CD28 Stimulations
Cells were stimulated for 1 hr with 1 μg/ml anti-CD3 and/or anti-CD28 (BD Biosciences) in serum-free RPMI 1640 for qPCR analyses and for 72 hr for IFNγ ELISPOT assays.
Immunoprecipitations
Cells were washed twice with PBS and extracted on ice for 10 min with 1% NP-40, 150 mM NaCl, 1 mM PMSF, 0.4 mM EDTA, and a protease-inhibitor cocktail (Complete Mini, Roche, Mannheim, Germany). After centrifugation of extracts for 10 min at 13,000 × g, supernatants were incubated for 1 hr at 4°C with appropriate antibody, after which Sepharose A beads were added and the mixture incubated overnight at 4°C. Centrifuged pellets were washed 5×, SDS sample buffer was added, and boiled samples were loaded onto SDS-PAGE gels.
Immunoblotting
All blots were performed by standard procedure as described (Lin et al., 2001) with HRP-conjugated secondary antibody and an ECL enhancer (GE Healthcare, Buckinghamshire, UK).
Quantitation of Murine C3 mRNA
A cDNA library was made from a C57BL/6 liver. The C3 standard was amplified with the qPCR primer for C3 via conventional PCR and diluted to 106 copies/ μL. A standard curve was created with 10-fold dilutions of the C3 standard and assayed by qPCR as above alongside with the cDNA libraries from total RNA isolated from the T cells and DCs. A standard curve was constructed from the CT values of the C3 standard, and the copies/μL of the samples were determined. We used the amount of total RNA from each sample to determine the amount of copies/cell.
Luminex Assay
Cells were stimulated for increasing times with 1 μg/ml anti-CD3+anti-CD28 mAbs. After stimulation, cells were assayed for pAKT and tAKT with Upstate’s Beadlyte assay according to the manufacturer’s instructions (Upstate, NY). In brief, cells were placed on ice immediately after incubation, centrifuged at 4°C, lysed in the buffer provided by the company, incubated with the capture beads and then the detection beads, washed, and assayed on the Bioplex 2200 (Biorad, Hercules, CA).
Luciferase Activity Assay
The base pairs +72 to −991 of the human B7.1 promoter were inserted into a luciferase reporter vector (GL4) then transfected into THP-1 cells by electroporation (6 × 106 cells in 200 μl OptiMEM at 250 V and 950 μF). Cells were incubated overnight in RPMI 1640 and 10% FBS, after incubation at 37°C for 2 hr with 300 nM C5a in serum-free RPMI 1640; luciferase activity was measured with a Lmax Luminometer (Molecular Devices).
In Vitro Cell Viability
Mouse T cells purified by EasySep magnetic bead cocktails (StemCell Technologies, British Columbia) were cultured in 96-well plates in serum-free HL-1 media containing L-glutamine and penicillin+streptomycin for the indicated times or were cultured in complete RPMI 1640 (5% FBS, L-glutamine, penn/ strep). In some experiments, live and dead cells were counted with trypan blue (Invitrogen, Carlsbad, CA). In others, cells were stained with Cy5-anti-CD4/CD8, FITC-anti-CD44, and propidium iodide, mixed with Flow-Check Fluorsperes (Beckman Coulter, Miami, FL), and analyzed on a LSR II flow cytometer. Samples were normalized to 1000 Flow Check bead events.
In Vivo Cell Viability
CD4+ T cells from WT mice were labeled with CFSE (Invitrogen), and C5ar1−/− C3ar1−/− mice were labeled CellTracker Red CMTPX (Invitrogen); afterward, 2 × 106 of each type was injected via tail vein into SCID mice. At various time points, two mice from each group were sacrificed, and total spleen cells were assayed for percentage of labeled cells by flow cytometry.
Toxoplasmosis Infections
WT or C5ar1−/−C3ar1−/− mice were infected i.p. with 20 cysts of T. gondii (ME49 strain; n = 5). The C5ar1−/−C3ar1−/− mice and a parallel set of WT animals (n = 5 per group) were killed on day 10–12 (just before death), and spleen cells were isolated, stimulated with toxoplasma gondii antigen for 48 hr, and tested for IL-12 production by ELISA or IFNγ by ELISPOT.
Statistics
Statistical significance for all experimental data was determined by the Student’s t test.
Supplementary Material
Seven figures are available at http://www.immunity.com/cgi/content/full/28/3/▪▪▪/DC1/.
Acknowledgments
This work was supported by NIH grants AI23598 and EY11288 (M.E.M.) and AI43578 (P.S.H.) and an AHA predoctoral fellowship (P.N.L.). The authors thank M. Carroll for C3−/− mice, C. Gerard for C5ar1−/− and C3ar1−/− mice, J. Lambris for C5aR-A, L. Mayo for help on phospho-AKT, K. Shokat for PI-3 kinase inhibitors, A. Larner for helpful discussion, F. Lin for assisting with tail-vein injections, C. Subauste for help with T. gondii injection, J. Arth and K. Thomas for help with the bacterial keratitis model, and D. Kaplan, M. Lamm, and M. Sy for critical reading.
References
- Alcazar I, Marques M, Kumar A, Hirsch E, Wymann M, Carrera AC, Barber DF. Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation. J Exp Med. 2007;204:2977–2987. doi: 10.1084/jem.20070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SA, Monk PN. The orphan receptor C5L2 has high affinity binding sites for complement fragments C5a and C5a des-Arg(74) J Biol Chem. 2002;277:7165–7169. doi: 10.1074/jbc.C100714200. [DOI] [PubMed] [Google Scholar]
- Deane JA, Fruman DA. Phosphoinositide 3-kinase: Diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- Diehl L, Den Boer AT, van der Voort EI, Melief CJ, Offringa R, Toes RE. The role of CD40 in peripheral T cell tolerance and immunity. J Mol Med. 2000;78:363–371. doi: 10.1007/s001090000126. [DOI] [PubMed] [Google Scholar]
- Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–4453. doi: 10.1128/mcb.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005;201:1523–1530. doi: 10.1084/jem.20041967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, et al. Generation of C5a in the absence of C3: A new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Travers P, Walport M, Shlomchik M. The Immune System in Health and Disease. 6. New York: Garland Publishing; 2005. Immunobiology. [Google Scholar]
- Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM, Steinman RM, Park CG. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Kopf M, Abel B, Gallimore A, Carroll MC, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–378. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- Kung C, Kenski DM, Krukenberg K, Madhani HD, Shokat KM. Selective kinase inhibition by exploiting differential pathway sensitivity. Chem Biol. 2006;13:399–407. doi: 10.1016/j.chembiol.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ, Zeng Y, Hathcock KS, Zuckerman LA, Freeman G, Thistlethwaite JR, Gray GS, Hodes RJ, Bluestone JA. Inhibition of transplant rejection following treatment with anti-B7–2 and anti-B7–1 antibodies. Transplantation. 1995;60:1171–1178. doi: 10.1097/00007890-199511270-00019. [DOI] [PubMed] [Google Scholar]
- Lin F, Fukuoka Y, Spicer A, Ohta R, Okada N, Harris CL, Emancipator SN, Medof ME. Tissue distribution of products of the mouse decay-accelerating factor (DAF) genes. Exploitation of a Daf1 knock-out mouse and site-specific monoclonal antibodies. Immunology. 2001;104:215–225. doi: 10.1046/j.0019-2805.2001.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, Machado FS, Aliberti J. Cutting edge: Dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikis D, Lambris JD. Structural aspects and design of low-molecular-mass complement inhibitors. Biochem Soc Trans. 2002;30:1026–1036. doi: 10.1042/bst0301026. [DOI] [PubMed] [Google Scholar]
- Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol. 2006;176:3330–3341. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- Soruri A, Kim S, Kiafard Z, Zwirner J. Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody. Immunol Lett. 2003;88:47–52. doi: 10.1016/s0165-2478(03)00052-x. [DOI] [PubMed] [Google Scholar]
- Stein PH, Fraser JD, Weiss A. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3′-kinase. Mol Cell Biol. 1994;14:3392–3402. doi: 10.1128/mcb.14.5.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC. C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol. 2005;16:3572–3582. doi: 10.1681/ASN.2005040373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seven figures are available at http://www.immunity.com/cgi/content/full/28/3/▪▪▪/DC1/.